To describe the clinical characteristics and laboratory findings of patients over 15 years old, diagnosed with Systemic Lupus Erythematosus (SLE) that were hospitalized with fever and with a final diagnosis of infection, lupus flare, or both (disease activity and infection).
MethodsA retrospective, desriptive study was conducted, including patients with a diagnosis of SLE, who presented with fever and were admitted to the Emergency Department of Hospital Universitario Clinica San Rafael. Clinical and paraclinical variables were analyzed and the patients were divided into three groups: patients with disease flare-up, infection, or both, in accordance with the final diagnosis upon discharge. Clinical and laboratory variables were analyzed, and a description of the population was submitted based on the 3 presentations.
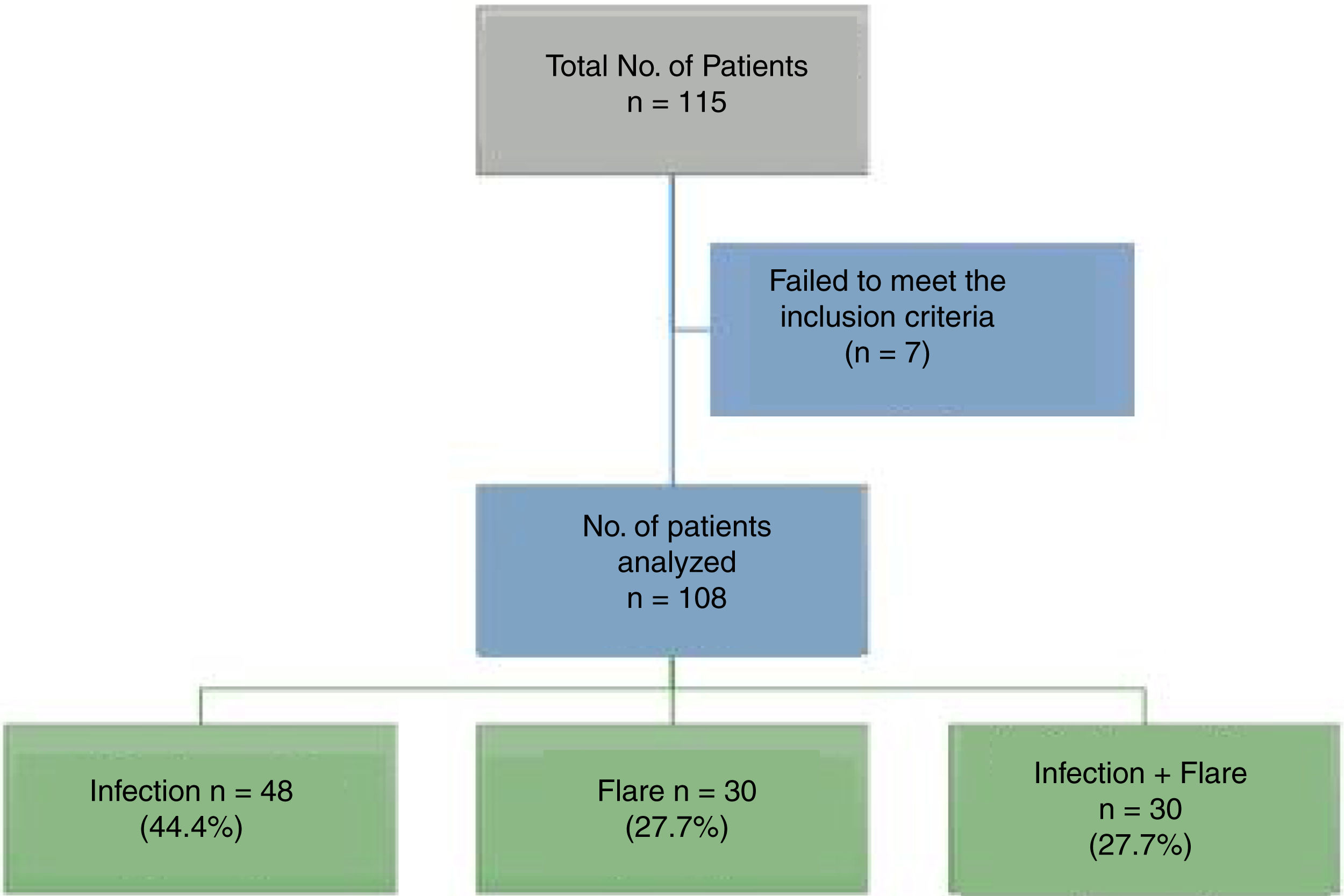
ResultsA total of 115 patients were assesed, and 108 were included in the final analysis. The mean age was 36 years old and 86% were females. The median SLEDAI (Systemic Lupus Erythematous Disease Activity Index) was 6 (R 1-15) for the entire population. In patients with both infection and disease activity, the median score was 9.5 (R 6-15). There were no significant differences betweent the clinical symptoms and the laboratory findings in the various groups. The use of prednisolone during the last 3 months was higher in the infection group (p=0.001), but with no significant differences as compared against other immonusupressive therapies.
ConclusionThe use of steroids over the last 3 months, the SLEDAI score, and the time elapsed sinde the SLE diagnosis, could be helpful variables to discriminate between infection and disease activity in patients with a history of SLE and fever. The clinical and paraclinical findings fail to discriminate between these two conditions.
Describir las características clínicas y de laboratorio en pacientes mayores de 15 años con diagnóstico de lupus eritematoso sistémico (LES) hospitalizados por fiebre, cuyo diagnóstico final fue infección, actividad lúpica o ambas (actividad e infección).
MétodosSe realizó un estudio descriptivo retrospectivo en el que se incluyeron pacientes con diagnóstico de LES admitidos por fiebre en el servicio de urgencias del Hospital Univer-sitario Clínica San Rafael; se estudiaron variables clínicas y paraclínicas, dividiéndose en 3 grupos de interés: pacientes con actividad de la enfermedad, de la infección o de ambas, de acuerdo con el diagnóstico definitivo una vez se daba el alta hospitalaria. Se estudiaron variables clínicas y de laboratorio, realizándose una descripción de la población en los 3 estados.
ResultadosSe evaluaron en total 115 pacientes incluyéndose en el análisis final 108 pacientes. La mediana de edad fue de 36 años y el 86% fueron mujeres. La mediana del puntaje de SLEDAI (Systemic Lupus Erythematosus Disease Activity Index) en toda la población fue de 6 (R 1–15) en los pacientes con infección y actividad el puntaje mostró una mediana de 9,5 (R 6–15). No hubo diferencias significativas entre los síntomas clínicos y los hallazgos de laboratorio en los diferentes grupos. El uso de prednisolona en los últimos 3 meses fue mayor en el grupo de infección (p = 0,001) pero sin diferencias significativas entre las otras terapias de inmunosupresión.
ConclusiónEl uso de esteroides en los últimos 3 meses, el puntaje de la escala SLEDAI y el tiempo transcurrido desde el diagnóstico de LES podrían ser variables que pueden ayudar a discriminar entre infección y actividad en pacientes con historia de LES y fiebre, los hallazgos clínicos y paraclínicos no discriminan entre estas condiciones de enfermedad.
Systemic Lupus Erythematous (SLE) is an autoimmune, chronic disease, compromising multiple systems and with a broad range of clinical manifestations.1,2 This condition is more susceptible to the development of infections as a result of the disruption of the innate and adaptative immune system, and due to the use of immunosuppressants.3 Infections are one of the primary causes for hospitalization in patients with SLE, following disease flare-ups, and involve a high mortality.4,5 Bacterial etiology has been described as the most usual cause of infections, followed by viruses and fungi.6 Respiratory, urinary and soft tissue infections prevail. However, one of diagnostic challenges is to differentiate between flares and infection in patients with SLE that present with fever, since both conditions may manifest in a similar manner. Fever may be present in 36–96% of the patients, both infected and non-infected, in addition to sharing other similar manifestations secondary to the inflammatory process itself, which is common to both infection and inflammation. Hence, the search for differentiators between these two conditions is a constant endeavor in an attempt to make this distinction promptly.7
In the absence of a specific marker, different variables have been suggested to improve diagnostic accuracy. These variables include higher disease activity, complement factor used, leucopenia, anti-DNA antibodies, and the use of immunosuppressive medications such as steroids or cyclophosphamide.8,9 One or several predictive markers that are specific and sensitive should be selected, in order to support the diagnosis and establishing the cause for the febrile episode. The intent of this descriptive study is to determine the behavior of both, the clinical manifestations and laboratory tests, and the previous use of different immunosuppressive drugs in both situations (lupus flare and infection), in order to establish potential diagnostic markers that could be used either alone or in combination, to establish whether it is a lupus flare or an infection, or if both conditions coexist.
MethodsA descriptive, retrospective study was conducted reviewing the medical records of adult patients with SLE, who had been admitted with fever to the emergency department of the Hospital Universitario Clínica San Rafael, between January 2005 and December 2014. The data were collected between January and March 2017. The patients included had a confirmed diagnosis of lupus pursuant to the SLICC classification criteria and the assessment of a rheumatologist; they were over 15 years old and presented with fever (defined as an axillary temperature above 37.5 °C). The first hospital admission with fever was considered as an event, and any further relapsing events in the same patient were disregarded. Patients with other concomitant autoimmune diseases were excluded - except for anti-phospholipid syndrome – as well as those diagnosed with drug-induced lupus. This trial was approved by the ethics committee of the hospital and was conducted pursuant to the protocol of good clinical practices and the principles of the Declaration of Helsinki. The variables were collected in an electronic record, in accordance with a standardized protocol. The information recorded included any previously affected organs and any type of immunosuppressive medications received prior and during hospital admission. The activity of the disease was measured using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). Likewise, general and specific laboratory data were collected, such as immune tests, blood cultures, urinalyses, and others, as the case required. The following test results were obtained during the first 3 days of admission: erythrocyte sedimentation rate (ESR – normal value: < 20 mm/h; C-reactive protein (CRP – normal value: < 1 mg/dl); procalcitonin (< 0.5 ng/mL); anti-double stranded deoxyribonucleic acid antibodies - anti dsDNA), absolute positive or negative value; hemoglobin (normal value 12–17 g/dl); ferritin (normal value: 18–160 mg/ml); HDL (normal value 250–450 UI/l); complement C3 (normal value: 76–181 mg/dl); complement C4 (normal value: 12–52 mg/dl); leukocytes (normal value: 4,600–10,200/μl); neutrophils (normal value: 3,700–8,000/μl); lymphocytes (normal value: 1,000–5,000/μl). Positive cultures: in urine, blood, sputum: none. The definitions for lupus flare, general and specific infection, and relapses are shown in the Appendix A.
After entering the data into the electronic form, the information was analyzed using the SPSS 20 software licensed by the Universidad de La Sabana. The continuous variables were summarized as averages and standard deviation and their distribution was normal; however, in terms of the median and interquartile range, their distribution was non-normal. The qualitative variables were summarized as frequencies and percentages. The general description was for the population as a whole, and then it was separated into three groups: flare, infection, and both flare + infection. Further exploration was done contrasting the clinical and the complete paraclinical variables against the disease status; the qualitative variables were analyzed using chi-square and Fisher’s test; if the quantitative variables exhibited a normal distribution, they were analyzed using the Student-t test, but if the distribution was non-normal, the Mann Whitney U test was used. For more than 2 groups, Kruskal Wallis was used, considering a p < 0.05 as significant. All ethical considerations and data protection measures were followed.
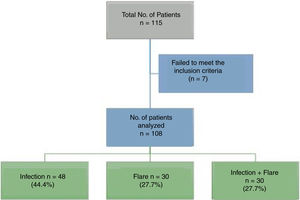
ResultsA total of 115 patients were admitted. Seven patients were excluded because the final diagnosis was not consistent with infection or flare, so in the end 108 patients participated. Fig. 1 shows the diagram of the patients excluded.
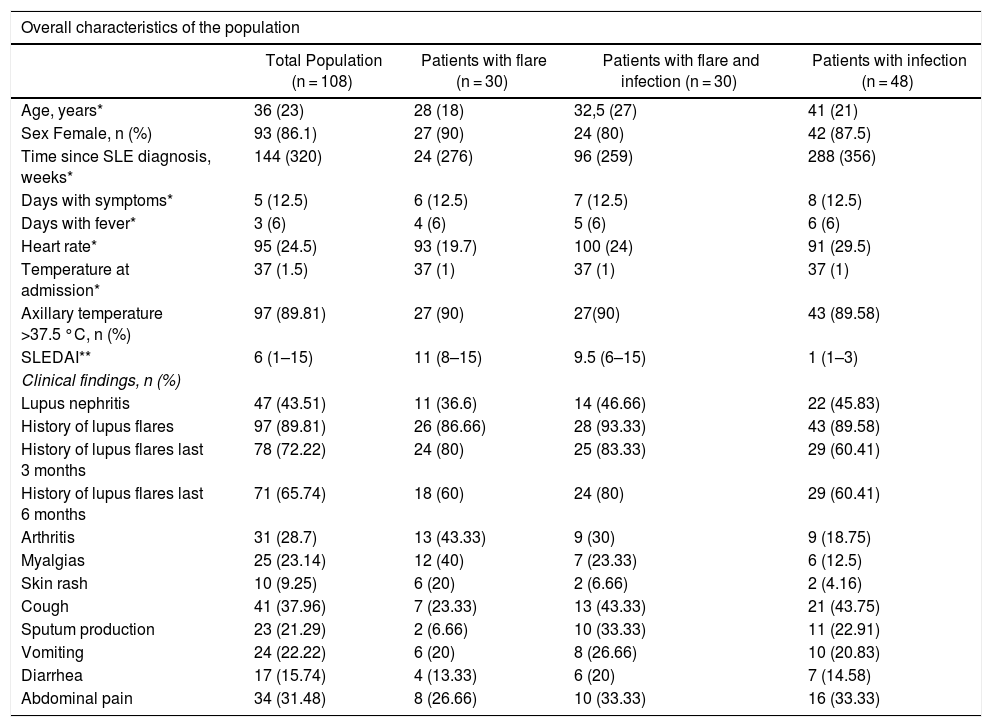
The median age of the patients in the analysis was 36 years old and 86% (93/108) were females. The mean age of the group of patients with infections was 42.3 ± 14.6 years, and this group had a higher age average than the flare group (31.5 ± 12.9 years) or the group with both, infection and flare (37.4 ± 18.2 years) (p = 0.017). Table 1 shows the general characteristics of the population, including the SLE-associated clinical manifestations. Table 2 differentiates between the types of SLE-related involvement present at the time of admission to the emergency department, for the total and for each study subgroup.
Overall characteristics of the population and clinical findings.
| Overall characteristics of the population | ||||
|---|---|---|---|---|
| Total Population (n = 108) | Patients with flare (n = 30) | Patients with flare and infection (n = 30) | Patients with infection (n = 48) | |
| Age, years* | 36 (23) | 28 (18) | 32,5 (27) | 41 (21) |
| Sex Female, n (%) | 93 (86.1) | 27 (90) | 24 (80) | 42 (87.5) |
| Time since SLE diagnosis, weeks* | 144 (320) | 24 (276) | 96 (259) | 288 (356) |
| Days with symptoms* | 5 (12.5) | 6 (12.5) | 7 (12.5) | 8 (12.5) |
| Days with fever* | 3 (6) | 4 (6) | 5 (6) | 6 (6) |
| Heart rate* | 95 (24.5) | 93 (19.7) | 100 (24) | 91 (29.5) |
| Temperature at admission* | 37 (1.5) | 37 (1) | 37 (1) | 37 (1) |
| Axillary temperature >37.5 °C, n (%) | 97 (89.81) | 27 (90) | 27(90) | 43 (89.58) |
| SLEDAI** | 6 (1–15) | 11 (8–15) | 9.5 (6–15) | 1 (1–3) |
| Clinical findings, n (%) | ||||
| Lupus nephritis | 47 (43.51) | 11 (36.6) | 14 (46.66) | 22 (45.83) |
| History of lupus flares | 97 (89.81) | 26 (86.66) | 28 (93.33) | 43 (89.58) |
| History of lupus flares last 3 months | 78 (72.22) | 24 (80) | 25 (83.33) | 29 (60.41) |
| History of lupus flares last 6 months | 71 (65.74) | 18 (60) | 24 (80) | 29 (60.41) |
| Arthritis | 31 (28.7) | 13 (43.33) | 9 (30) | 9 (18.75) |
| Myalgias | 25 (23.14) | 12 (40) | 7 (23.33) | 6 (12.5) |
| Skin rash | 10 (9.25) | 6 (20) | 2 (6.66) | 2 (4.16) |
| Cough | 41 (37.96) | 7 (23.33) | 13 (43.33) | 21 (43.75) |
| Sputum production | 23 (21.29) | 2 (6.66) | 10 (33.33) | 11 (22.91) |
| Vomiting | 24 (22.22) | 6 (20) | 8 (26.66) | 10 (20.83) |
| Diarrhea | 17 (15.74) | 4 (13.33) | 6 (20) | 7 (14.58) |
| Abdominal pain | 34 (31.48) | 8 (26.66) | 10 (33.33) | 16 (33.33) |
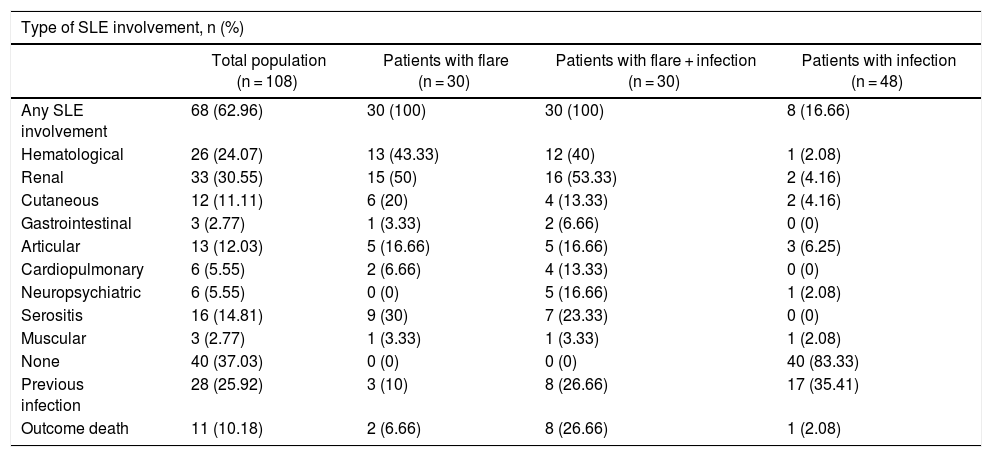
Type of Systemic Lupus Erythematous (SLE) Involvement at the time of admission to the Emergency Department.
| Type of SLE involvement, n (%) | ||||
|---|---|---|---|---|
| Total population (n = 108) | Patients with flare (n = 30) | Patients with flare + infection (n = 30) | Patients with infection (n = 48) | |
| Any SLE involvement | 68 (62.96) | 30 (100) | 30 (100) | 8 (16.66) |
| Hematological | 26 (24.07) | 13 (43.33) | 12 (40) | 1 (2.08) |
| Renal | 33 (30.55) | 15 (50) | 16 (53.33) | 2 (4.16) |
| Cutaneous | 12 (11.11) | 6 (20) | 4 (13.33) | 2 (4.16) |
| Gastrointestinal | 3 (2.77) | 1 (3.33) | 2 (6.66) | 0 (0) |
| Articular | 13 (12.03) | 5 (16.66) | 5 (16.66) | 3 (6.25) |
| Cardiopulmonary | 6 (5.55) | 2 (6.66) | 4 (13.33) | 0 (0) |
| Neuropsychiatric | 6 (5.55) | 0 (0) | 5 (16.66) | 1 (2.08) |
| Serositis | 16 (14.81) | 9 (30) | 7 (23.33) | 0 (0) |
| Muscular | 3 (2.77) | 1 (3.33) | 1 (3.33) | 1 (2.08) |
| None | 40 (37.03) | 0 (0) | 0 (0) | 40 (83.33) |
| Previous infection | 28 (25.92) | 3 (10) | 8 (26.66) | 17 (35.41) |
| Outcome death | 11 (10.18) | 2 (6.66) | 8 (26.66) | 1 (2.08) |
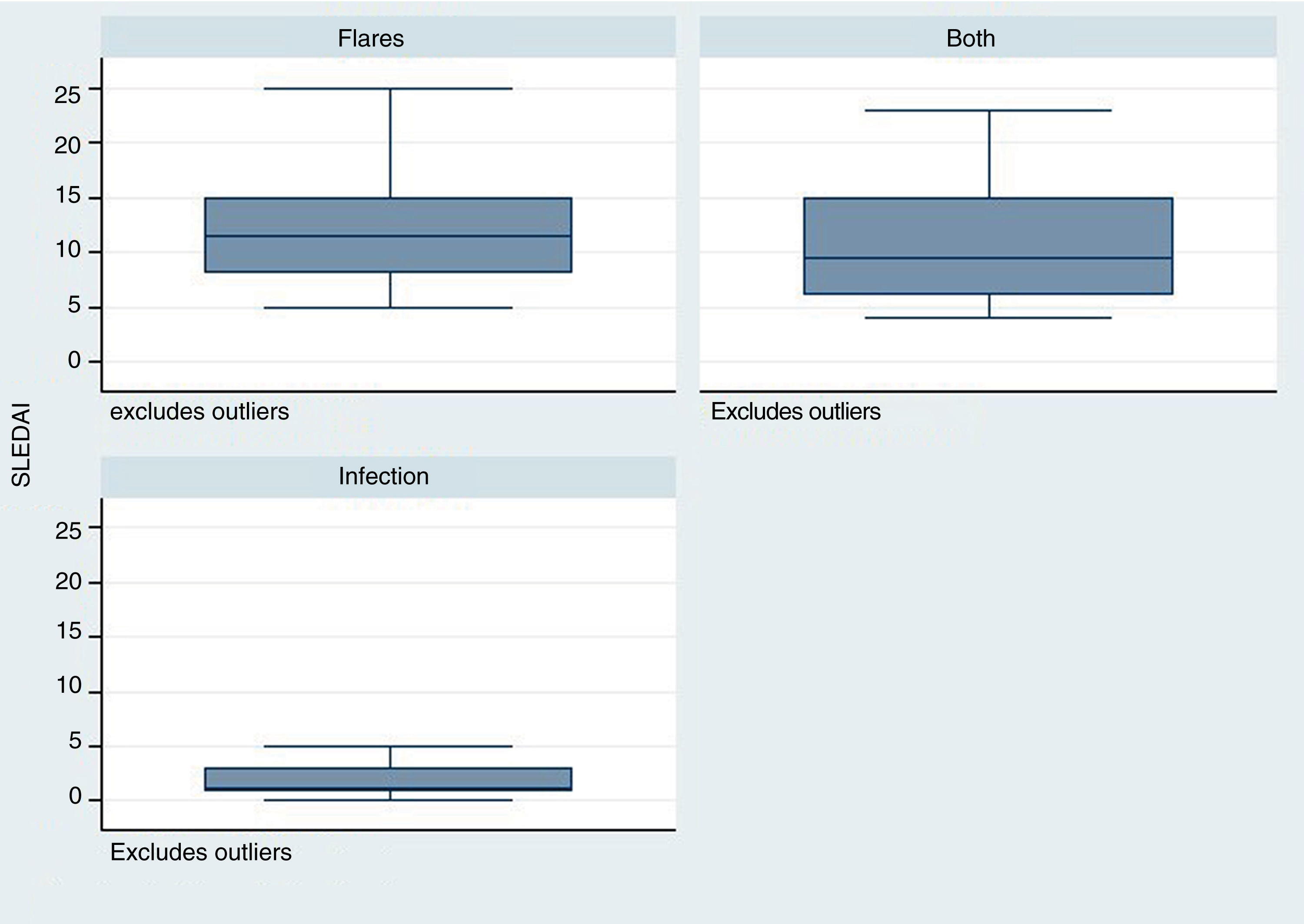
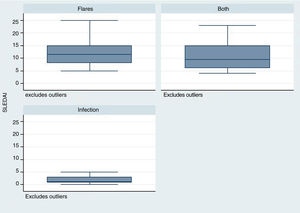
The time elapsed from the time of SLE diagnosis was longer for the infection group, with a mean of 288 weeks (range: 124–480), in contrast with the flare group /24 weeks; R: 0–276) and for the group infection + flare (96 weeks; R: 17–276) (p = 0.001). The SLEDAI score was lower in the infection group – median 1 (R: 1–3), as compared with the other two groups: flare 11 (R: 8–15), infection + flare 9.5 (R: 6–15), as illustrated in Fig. 2 (p = 0.004).
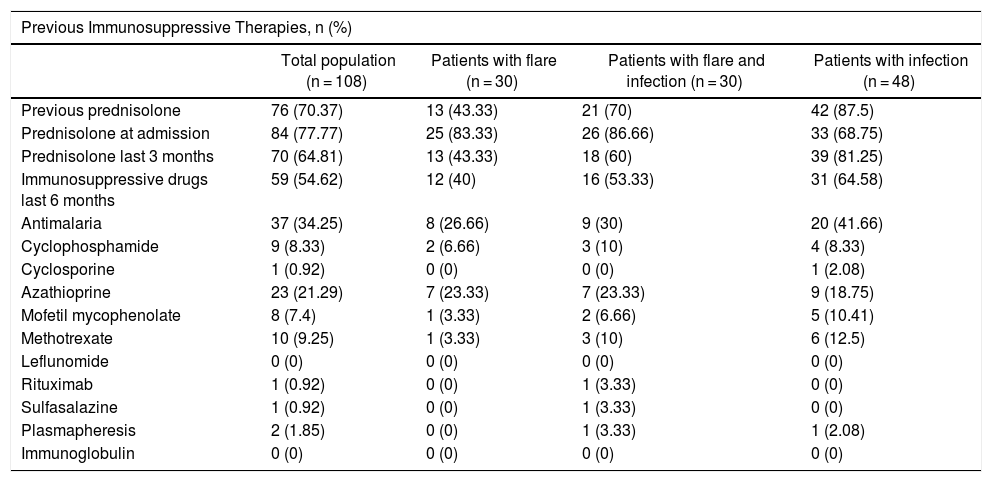
Table 3 shows the treatments received by the patients. Prior prednisolone therapy, particularly over the last 3 months, was more frequent among the group of infected patients (p = 0.001). However, there were no differences in terms of the use of antimalaria agents and other immunosuppressants among the three groups.
Treatments received.
| Previous Immunosuppressive Therapies, n (%) | ||||
|---|---|---|---|---|
| Total population (n = 108) | Patients with flare (n = 30) | Patients with flare and infection (n = 30) | Patients with infection (n = 48) | |
| Previous prednisolone | 76 (70.37) | 13 (43.33) | 21 (70) | 42 (87.5) |
| Prednisolone at admission | 84 (77.77) | 25 (83.33) | 26 (86.66) | 33 (68.75) |
| Prednisolone last 3 months | 70 (64.81) | 13 (43.33) | 18 (60) | 39 (81.25) |
| Immunosuppressive drugs last 6 months | 59 (54.62) | 12 (40) | 16 (53.33) | 31 (64.58) |
| Antimalaria | 37 (34.25) | 8 (26.66) | 9 (30) | 20 (41.66) |
| Cyclophosphamide | 9 (8.33) | 2 (6.66) | 3 (10) | 4 (8.33) |
| Cyclosporine | 1 (0.92) | 0 (0) | 0 (0) | 1 (2.08) |
| Azathioprine | 23 (21.29) | 7 (23.33) | 7 (23.33) | 9 (18.75) |
| Mofetil mycophenolate | 8 (7.4) | 1 (3.33) | 2 (6.66) | 5 (10.41) |
| Methotrexate | 10 (9.25) | 1 (3.33) | 3 (10) | 6 (12.5) |
| Leflunomide | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rituximab | 1 (0.92) | 0 (0) | 1 (3.33) | 0 (0) |
| Sulfasalazine | 1 (0.92) | 0 (0) | 1 (3.33) | 0 (0) |
| Plasmapheresis | 2 (1.85) | 0 (0) | 1 (3.33) | 1 (2.08) |
| Immunoglobulin | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
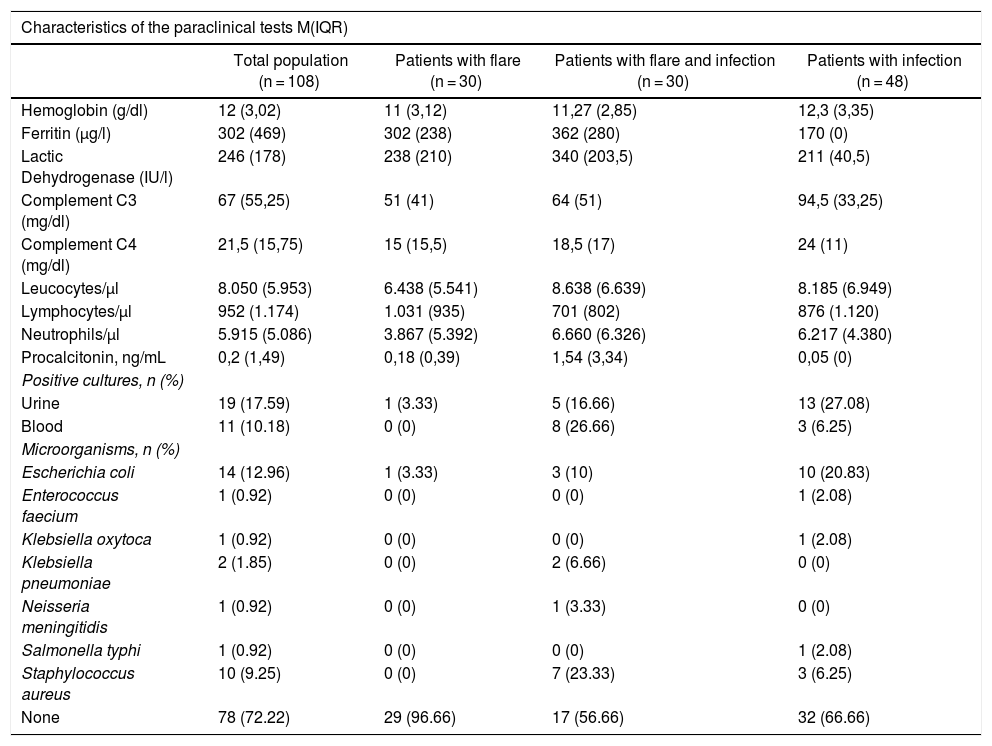
Table 4 shows the results of the diagnostic tests conducted in patients. The median C3 and C4 measurements was higher among the infections group than in the other groups (p = 0.002), and there were no differences in any of the other laboratory tests among the groups.
Characteristics of the Paraclinical Tests Conducted.
| Characteristics of the paraclinical tests M(IQR) | ||||
|---|---|---|---|---|
| Total population (n = 108) | Patients with flare (n = 30) | Patients with flare and infection (n = 30) | Patients with infection (n = 48) | |
| Hemoglobin (g/dl) | 12 (3,02) | 11 (3,12) | 11,27 (2,85) | 12,3 (3,35) |
| Ferritin (μg/l) | 302 (469) | 302 (238) | 362 (280) | 170 (0) |
| Lactic Dehydrogenase (IU/l) | 246 (178) | 238 (210) | 340 (203,5) | 211 (40,5) |
| Complement C3 (mg/dl) | 67 (55,25) | 51 (41) | 64 (51) | 94,5 (33,25) |
| Complement C4 (mg/dl) | 21,5 (15,75) | 15 (15,5) | 18,5 (17) | 24 (11) |
| Leucocytes/μl | 8.050 (5.953) | 6.438 (5.541) | 8.638 (6.639) | 8.185 (6.949) |
| Lymphocytes/μl | 952 (1.174) | 1.031 (935) | 701 (802) | 876 (1.120) |
| Neutrophils/μl | 5.915 (5.086) | 3.867 (5.392) | 6.660 (6.326) | 6.217 (4.380) |
| Procalcitonin, ng/mL | 0,2 (1,49) | 0,18 (0,39) | 1,54 (3,34) | 0,05 (0) |
| Positive cultures, n (%) | ||||
| Urine | 19 (17.59) | 1 (3.33) | 5 (16.66) | 13 (27.08) |
| Blood | 11 (10.18) | 0 (0) | 8 (26.66) | 3 (6.25) |
| Microorganisms, n (%) | ||||
| Escherichia coli | 14 (12.96) | 1 (3.33) | 3 (10) | 10 (20.83) |
| Enterococcus faecium | 1 (0.92) | 0 (0) | 0 (0) | 1 (2.08) |
| Klebsiella oxytoca | 1 (0.92) | 0 (0) | 0 (0) | 1 (2.08) |
| Klebsiella pneumoniae | 2 (1.85) | 0 (0) | 2 (6.66) | 0 (0) |
| Neisseria meningitidis | 1 (0.92) | 0 (0) | 1 (3.33) | 0 (0) |
| Salmonella typhi | 1 (0.92) | 0 (0) | 0 (0) | 1 (2.08) |
| Staphylococcus aureus | 10 (9.25) | 0 (0) | 7 (23.33) | 3 (6.25) |
| None | 78 (72.22) | 29 (96.66) | 17 (56.66) | 32 (66.66) |
Only 12 patients reported the ESR values, and 86 patients reported the CRP values: 24 in the flare group, 27 in the infection + flare group, and 35 in the infection group, with no evidence of any significant differences among the groups. Due to the lack of complete data, the decision was made not to include the ESR and CRP reports in the results table.
Finally, only 27.77% of all patients had some type of microbiological isolates, of which the most relevant one was urine (17.59%); Escherichia coli was the predominant isolate (12.96%), and it was more prevalent among the infection group.
The number of days with symptoms was less for the infection + flare group (p = 0.002) but there were no statistically significant differences in the number of days with fever, in the temperature, or in the heart rate at admission. In the flare + infection group, all patients had some lupus-associated involvement, as compared with 17% (8/48) of the patients in the infection group (p = 0.0001). With regards to the presence of myalgia, it was more frequent in the flare group (p = 0.02), as was also the case with respect to hematological involvement (p = 0.001). The production of sputum was less frequent in the flare group (p = 0.03). There were no differences in terms of other clinical findings, such as compromised renal function, vomiting, diarrhea, serositis, or joint, muscular or neuropsychiatric involvement.
The power with 108 subjects in terms of the exploratory analyses is 55.36%.
DiscussionThis is one of the first regional trials describing the clinical and paraclinical characteristics of patients with a diagnosis of SLE that presented with fever, and analyzing patent populations only with flares, with active infection, or both. Our population is similar to that in the trial by Torres-Ruiz et al.,10 published in 2017, as well as to the population of other Latin American authors, where the average age of the patients was 33 years old, and the percentage of females ranged between 87 and 89%,11 which is also consistent with larger population trials in which the people mostly affected by the disease and relapses are young women.
The time elapsed from the time of diagnosis of SLE in most cases is short. However, the patients with longer time since diagnosis presented a higher frequency of infection as compared to the other groups, which is consistent with the literature that claims that a longer duration of the disease is an independent risk factor for the development of infections. Jeong et al.12 conducted a retrospective, case control trial with 110 patients, with a view to identifying risk factors for infection in patients with SLE; the risk was increased when the duration of the disease exceeded 8 years, particularly for pulmonary and soft tissue-related community acquired infections. This is associated with increased exposure to immunosuppressive therapy, as well as to a chronic and greater compromise of the innate and adaptative immune system.3
Similarly, the SLEDAI score was lower in the infection group, as compared against the other two groups. This scale rates the disease activity and allows to discriminate based on disease severity.13 It should be highlighted that the group with the highest score in this scale was the group with flares. Various epidemiological studies have reported a relationship between higher disease activity, particularly with severe activity (SLEDAI > 12), and a higher rate of infectious complications. This fact was mentioned by Rúa-Figueroa et al.14 in a multicenter cohort trial published in 2019, where 114 episodes of bacteremia were analyzed, in order to determine its incidence in patients with SLE, and the incidence was more frequent among patients with flares (66% of the cases presented severe activity according to SLEDAI).
With regards to the characteristics of the symptoms at admission, no significant differences were found with regards to temperature, heart rate, or the rest of the clinical variables. Multiple trials have been conducted aimed at establishing whether the presence of fever or other clinical characteristics may help to differentiate a reactivation of the disease, from an infectious process.7,15 Even since the seventies, studies have been conducted in patients with SLE and fever; these studies found that 60% corresponded to lupus flares, 23% were due to infections and 12% miscellaneous.16 Beça et al.17 published in 2015 a retrospective cohort for the development and validation of an algorithm to predict risk in this setting. They found that fever is associated with infection in 48% of the cases, and with flares in 45%, and considered that the use of three additional variables — days with fever, anti-dsDNA and CRP— could be helpful when used in combination, to differentiate flares from infection in patients with SLE.
Only 17% of the cases in the infection group presented some type of compromise due to SLE, as compared to the lupus flare group, or as compared to the group with both, where surprisingly the involvement was of 100%. This is associated with a strong coexistence between severity of the disease activity and higher risk of infectious complications, evidencing the 100% involvement of the group with both.14 Moreover, patients with flares experienced more symptoms such as myalgia, and greater hematological involvement than those with infection. However, there were no significant differences in terms of the other types of involvements due to SLE. The literature suggests that there is no single finding, or multiple clinical findings able to predict with certainty, whether it is infection of flare, and such difference if difficult to establish, even in combination with paraclinical parameters.18,19
Moreover, another big question mark that arises when analyzing the risk factors associated with infections in patients with lupus is the effect on the immune system, secondary to the various treatment regimens. The results of this study showed that prior prednisolone treatment, particularly over the last 3 months, was more frequent in the group of infected patients, as compared against other groups. However, no significant differences were identified with the use of other immunosuppressive medications.
Most studies have concluded that there is a relationship between the use of certain immunosuppressive drugs and a higher rate of infections, particularly with prednisolone doses above 20 mg/day.18–20 A prospective trial with 92 patients with SLE reported that in those patients receiving maintenance doses or higher doses (> 20 mg/day) fever was usually associated to infection, and even with the development of sepsis.21 The relationship with the risk of infection by opportunistic germs has also been assessed, showing that the use of prednisolone during the first 3 months after making the diagnosis, at medium doses (between 15-30 mg/day) and at high doses (30–60 mg/day) vs. low doses, reported a hazard ratio (HR) of 1.72 (95% CI: 1.02–2.91) and of 1.96 (95% CI: 1.17–3.28), respectively, representing a higher risk. However, a limitation of the trial is the fact that these patients did not have an adequate control of the disease.22 Some studies are controversial with regards to the risk of infection and the use of other immunosuppressive medications, such as cyclophosphamide.14
Among the laboratory tests conducted, higher complement levels were found in the infection group as compared to the other groups, but there were no significant differences between the other groups. Several authors have conducted studies comparing some acute phase reactants, such as CRP, ESR, and other markers to determine their value when trying to establish whether it is infection or flare.23 Most have suggested CRP as a helpful marker to make this differentiation; however, a higher cut point should be considered (usually >10 mg/dl), since lower values have been observed in patients with flares.18,24 Other acute phase reactants, such as ferritin or ESR, apparently fail to contribute to establish such difference.25,26
Procalcitonin measurements have been controversial in this setting.27,28 Some studies, including a meta-analysis published by Liu et al.29 in 2017, included 8 studies with 205 patients with SLE flare and 198 patients with SLE and infection, but failed to establish any significant differences (standardized mean difference of ―0.45 (95% CI: ―0.96 to 0.06). In contrast, Serio et al.30 conducted a systematic review including 12 articles until 2013, observing higher procalcitonin levels in patients with bacterial infections (values >0.5 μg/l). Others report a sensitivity of 75% and a specificity of 90% with the use of procalcitonin in patients with autoimmune diseases.4,31 The real value of procalcitonin is still being debated and, in view of the findings, most authors recommend a combination of multiple laboratory parameters together with the clinical findings, in order to achieve a more objective differentiation. Further studies with new markers are currently underway in order to optimize this differentiation. Calcium binding proteins (S100A8/A9) have been mentioned, as well as the delta neutrophil index, the neutrophil/lymphocyte index, and the platelet/lymphocyte index, which have been associated with SLE flares, and probably to a higher prediction of bacterial diseases in these patients32; however, further studies are needed with larger populations, in order to confirm this premise.
One of the weaknesses of this study is the selection and information bias, keeping in mind that the analysis involved patients in one third-level of care hospital, its retrospective nature, the fact that it was limited to one single healthcare center, and the impossibility to differentiate the type of infection by organ. Additionally, this is a descriptive trail and hence the comparisons are merely exploratory. The comparisons of variables where significant differences were found, should be validated with other type of studies.
ConclusionThe differentiation between infection or flare in patients with SLE is currently a diagnostic challenge. Notwithstanding the fact that multiple studies have been conducted to determine different scales, or clinical and paraclinical markers with diagnostic predictive value, there is no clear recommendation yet about their usefulness and application.
In accordance with the analysis herein, there is a need to study the discriminative power between infection and flare in patients with SLE, of the variables, the use of steroids over the last 3 months, the SLEDAI score, and the time elapsed since the time of the SLE diagnosis.
FinancingNo funding was received by the authors.
Conflict of interestsThe authors have no conflict of interests to disclose.
Please cite this article as: Beltrán A, Mora C, Bastidas AR, Aragón Guzmán DM. Caracterización de pacientes con lupus y fiebre: actividad, infección o ambas. Rev Colomb Reumatol. 2020;27:95–102.