Osteogenesis imperfecta (OI) is an inherited disorder of phenotypically heterogeneous affections of the connective tissue. Until now, no definitive treatment for OI has been found. Certain drugs used in osteoporosis are used in these patients such as intravenous bisphosphonates in order to improve bone density. However, in recent years the use of denosumab, an anti-resorptive monoclonal antibody has shown positive results in bone mineral loss. There are no studies that directly compare the use of bisphosphonates and denosumab in OI. The following article presents a case of a 9-year-old patient with diagnosis of OI and a past medical history of epilepsy and cerebral palsy that was treated with bisphosphonates and denosumab.
La osteogénesis imperfecta (OI) es un trastorno hereditario del tejido conectivo fenotípicamente heterogéneo. Hasta ahora no se ha encontrado ningún tratamiento definitivo para la OI. Se aplican ciertos fármacos utilizados en la osteoporosis en estos pacientes como los bisfosfonatos intravenosos para mejorar la densidad ósea. Sin embargo, en los últimos años el uso de denosumab, un anticuerpo monoclonal anti-resorción ha demostrado resultados positivos en la pérdida mineral ósea. No hay estudios que comparen directamente el uso de bisfosfonatos y denosumab en la OI. El artículo presenta un caso de OI infantil en una paciente de 9 años con antecedentes de epilepsia y parálisis cerebral, que fue tratada con bisfosfonatos y denosumab.
Osteogenesis imperfecta (OI) is a congenital disorder. Most cases result from an autosomal dominant defect in the type I collagen encoding gene (COL1A1 or COL1A2), but some may be due to de novo mutations, i.e. mutations that appear for the first time in a family. The most common etiology for this mutation is advanced paternal age.1
OI is characterized by a qualitative and quantitative deficiency in the production of connective tissue, being able to present multiple phenotypic manifestations. The incidence is estimated to be one per 20,000 births, varying according to the underdiagnosis of mild forms.1 This disease is characterized by the presence of fragile bones with a tendency to fracture easily. Patients affected by OI usually present with bone deformity, joint laxity, scoliosis, dentinogenesis alterations, hearing loss, muscle weakness, fatigue, blue sclerae, and short stature.2
The forms of presentation of OI vary considerably from one person to another and, therefore, the various manifestations are not evident in their entirety. There are 11 types of OI based on the severity of the symptoms, according to the Sillence classification.1,3,4
Regarding OI treatment, it is mainly focused on three objectives: to reduce the rate of fractures, to prevent deformities of long bones and to improve mobility and quality of life.
We report the case of a patient with established diagnosis of osteogenesis imperfecta and antecedent of cerebral palsy, who received denosumab as the primary treatment for her condition.
Case reportWe report the case of a 9-year-old female patient with a history of childhood cerebral palsy and epilepsy, who came to the hospital for the first time because of a right femur fracture at 7 months of age while undergoing physical therapy for her cerebral palsy. Two months later during a pediatric control, she suffered a second fracture in the left femur.
The management of the fractures were limb immobilization and rest. At 11 months of age, she suffered a third fracture on her right fibula following a seizure and was diagnosed with osteogenesis imperfecta (OI) type IV based on Sillence's clinical criteria.
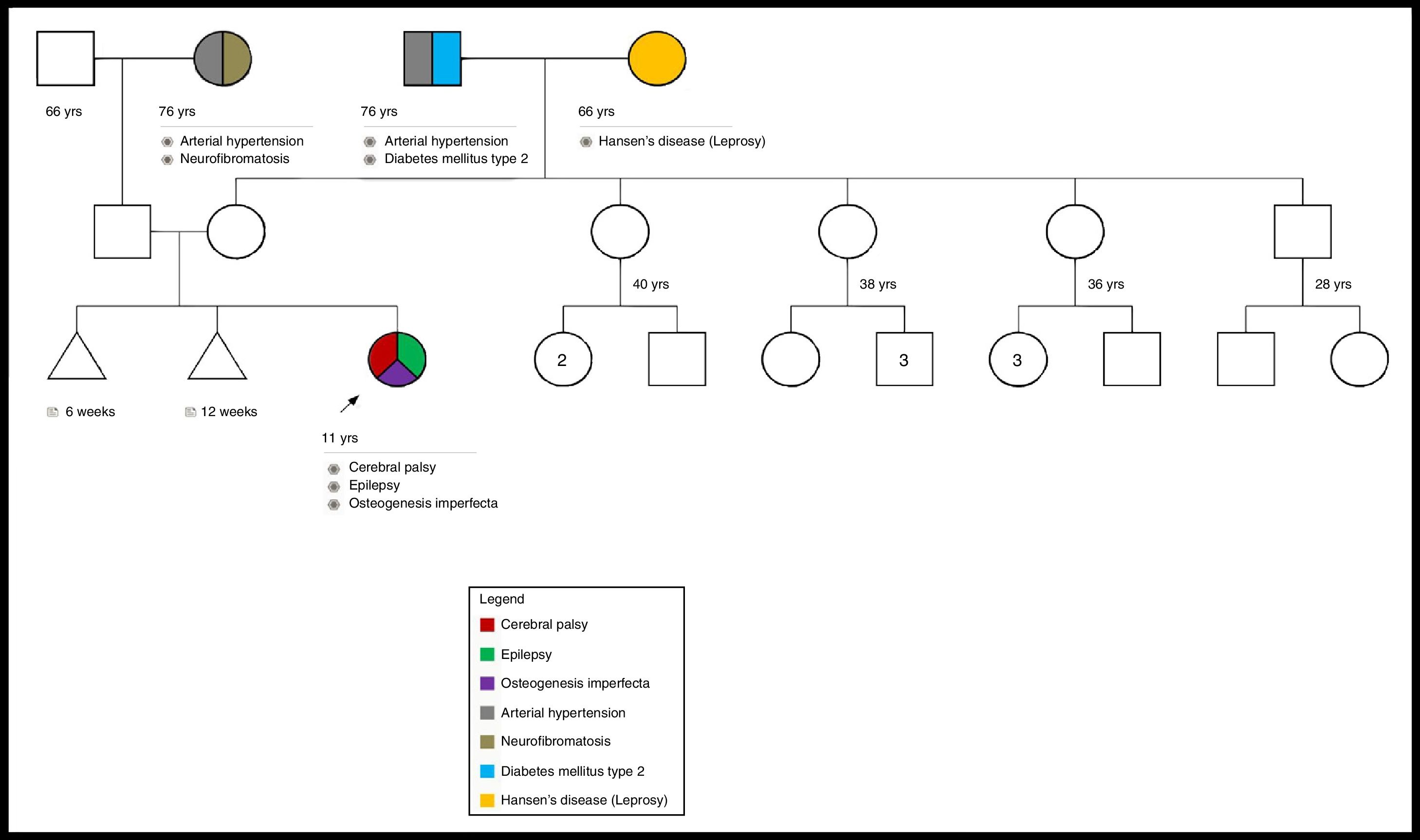
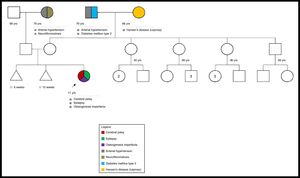
A study was performed to rule out mucopolysaccharidosis, which was negative. Also, a genetic pedigree was made which analyzed three generations and did not reveal a family history of OI (Fig. 1).
On physical examination, the patient presented a low weight and height for her age, her facial features included convergent strabismus, triangular face and wide nasal bridge. Her upper limbs denoted a moderate to severe hypotonia and her lower limbs showed a marked deformity.
An auditory evoked potentials study was performed which concluded that there were bilateral alterations on the auditory pathway.
The management of osteogenesis imperfecta was not considered until 2010, in which intravenous pamindronate was initiated with four administrations every 3 months.
The patient's mother reported that the pain and number of fractures had improved during the period of medication application, but the medication had to be discontinued due to lack of accessibility.
After the suspension of the medication, the patient presented a new left femur fracture that required surgical correction. In 2013, treatment restarts with intravenous zoledronic acid, but due to the difficulty of using the intravenous access that derived from her cerebral palsy, at the end of 2014, the patient was switched to denosumab 60mg/ml subcutaneously, which was easier to administrate, for 3 occasions with an interval of 6 months between each application.
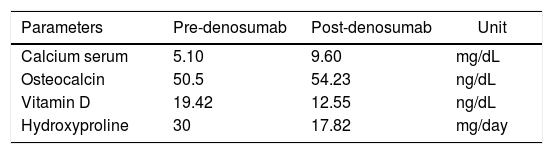
As for markers of bone metabolism, one of bone resorption was identified with decreased levels of hydroxypyridinoline in urine (Table 1).
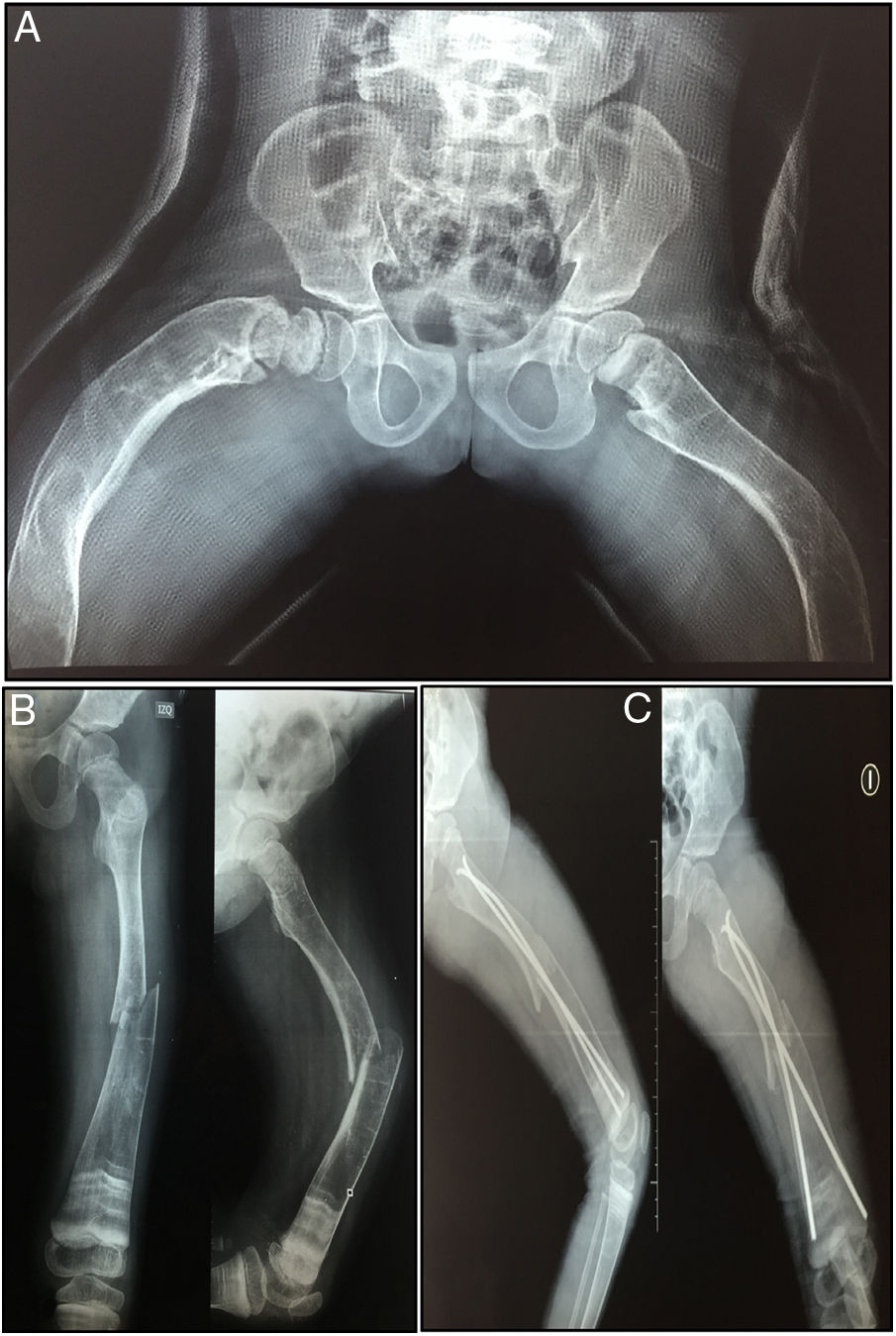
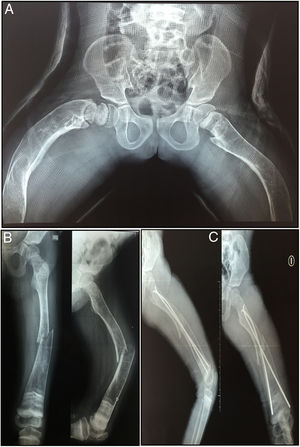
The patient has not presented fractures since the last application of the medication. Until the decision to use denosumab, the patient had presented a total of 40 fractures distributed between clavicles, hips and lower limbs (Fig. 2).
DiscussionWe present the case of a 9-year-old patient diagnosed with OI. This disease belongs to a heterogeneous group of genetic disorders that affect the connective tissue.
The incidence worldwide has been set to a range from 1:10,000 to 1:20,000.1,5 Up to 2000, 120 cases were reported in Ecuador, according to data from the Ecuadorian Federation of Osteogenesis Imperfecta.
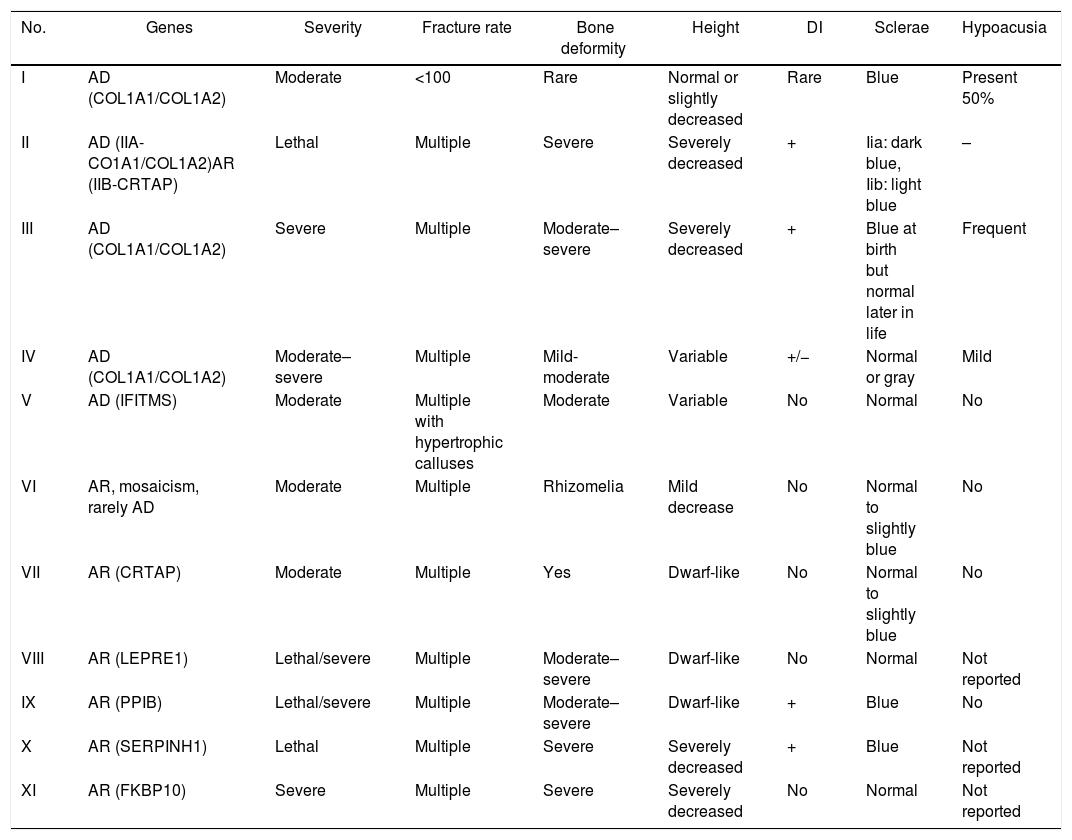
The classification for OI was established by Sillence et al. (1979) based on phenotypic and genotypic characteristics. According to Sillence's classification, the patient presents clinical characteristics of type IV OI (Table 2). As for the etiology, three possible theories are considered: direct paternal inheritance, de novo mutation and mosaicism.
Osteogenesis imperfecta classification.
| No. | Genes | Severity | Fracture rate | Bone deformity | Height | DI | Sclerae | Hypoacusia |
|---|---|---|---|---|---|---|---|---|
| I | AD (COL1A1/COL1A2) | Moderate | <100 | Rare | Normal or slightly decreased | Rare | Blue | Present 50% |
| II | AD (IIA-CO1A1/COL1A2)AR (IIB-CRTAP) | Lethal | Multiple | Severe | Severely decreased | + | Iia: dark blue, Iib: light blue | – |
| III | AD (COL1A1/COL1A2) | Severe | Multiple | Moderate–severe | Severely decreased | + | Blue at birth but normal later in life | Frequent |
| IV | AD (COL1A1/COL1A2) | Moderate–severe | Multiple | Mild-moderate | Variable | +/− | Normal or gray | Mild |
| V | AD (IFITMS) | Moderate | Multiple with hypertrophic calluses | Moderate | Variable | No | Normal | No |
| VI | AR, mosaicism, rarely AD | Moderate | Multiple | Rhizomelia | Mild decrease | No | Normal to slightly blue | No |
| VII | AR (CRTAP) | Moderate | Multiple | Yes | Dwarf-like | No | Normal to slightly blue | No |
| VIII | AR (LEPRE1) | Lethal/severe | Multiple | Moderate–severe | Dwarf-like | No | Normal | Not reported |
| IX | AR (PPIB) | Lethal/severe | Multiple | Moderate–severe | Dwarf-like | + | Blue | No |
| X | AR (SERPINH1) | Lethal | Multiple | Severe | Severely decreased | + | Blue | Not reported |
| XI | AR (FKBP10) | Severe | Multiple | Severe | Severely decreased | No | Normal | Not reported |
AD: autosomal dominant; AR: autosomal recessive; DI: dentinogenesis imperfecta.
We consider this to be a de novo mutation because the genealogical study did not show evidence of familial inheritance or mosaicism. The patient could not be subjected to a bone mineral density study because of her cerebral palsy.
FDA-approved treatment regimens for OI include drugs used for other conditions such as osteopenia and osteoporosis such as bisphosphonates, which have shown a positive effect on increasing the quality of life of people with OI. Physical therapy and rehabilitation play an important role in maintaining the mobility of these patients.6 Also, in some cases, orthopedic surgeries may be necessary.
In 2010, the FDA approved denosumab (a monoclonal antibody), for the treatment of osteoporosis in postmenopausal women at high risk of fracture and in 2011 by the European Commission.
Denosumab is a human monoclonal antibody (IgG2) that targets and binds with high affinity and specificity to RANKL, preventing activation of the RANK receptor on the surface of osteoclast precursors and osteoblasts. By impeding the interaction of RANKL/RANK, the formation, function, and survival of osteoclasts is inhibited and this leads to decreased bone resorption in the trabecular and cortical bone.7 Recently, this drug has been used in the treatment of OI giving long-term positive results.7–9
Intravenous bisphosphonates were first suggested as a treatment to improve bone fragility in children with severe OI 25 years ago. Their use has demonstrated improvements in pain, longitudinal growth, muscle strength, vertebral and long bone mass, and a decrease in the rate of fracture.10,11
After initiation of treatment with bisphosphonates, the number of fractures and their frequency decreased. These results are consistent with the study by Astrom et al. which demonstrated that bone mineral density improves with long-term intravenous bisphosphonates and is more beneficial if physiotherapy is added.6
Following the recovery of a severe, surgically corrected, left femur fracture, it was decided to initiate treatment with subcutaneous denosumab due to the difficulty of intravenous administration of bisphosphonates, as this drug has the same antiresortive properties as bisphosphonates and has a simpler administration route considering the preexisting conditions of the patient (cerebral palsy, epilepsy).
Semler et al. studied the use of denosumab in patients with OI type VI showing favorable results in a period of 2 years. During this process, deoxypyridinoline (also called pyrilinks-D) was measured to verify its efficacy.9
Deoxypyridinoline or pyrilinks-D is one of two pyridinium cross-links that provide structural rigidity to type I collagen found in bone. It is excreted and measured unmetabolized in the urine and is a specific marker of bone resorption and osteoclastic activity.12
The results in the study carried out by Semler et al. showed a decrease in bone resorption with decreasing levels of deoxypyridinoline in urine9 which was also evident in our case after the first dose of denosumab.
The interval between the denosumab injections was chosen based on experience from adults with osteoporosis. However the results on studies performed by Semler and Hoyer et al. indicate that a 6-month interval might be too long for patients with OI. An 8–10-week interval seems more appropriate to ensure a constant suppression of bone resorption by osteoclasts.9
In addition to its efficacy in normalizing bone resorption in patients with OI, denosumab potentially offers another advantage compare to the bisphosphonates. The humanized antibody degrades within 3–4 months after injection and hence does not remain the body. Bisphosphonates are stored in the skeleton for years, a fact that has led to a debate about their long-term safety use for children.7,9
According to Hoyer-Kuhn et al., treatment with denosumab should be continued till the end of growth, similar to the currently use regime for biphosphonates. Bone turn over is especially high during childhood and adolescents, and a strong effect of an antiresortive treatment can be expected, this might also decrease the risk of rebound effect after the end of therapy.8
So far the patient has received three doses of denosumab with intervals of 6 months between each dose with satisfactory results, improving her quality of life significantly.
ConclusionOI is a rare congenital disorder whose treatment is focused on improving quality of life. Bisphosphonate therapy remains a suitable pharmacological option to improve bone density in these patients but studies with denosumab show that this drug is also effective and thus, it should be considered as an alternative to bisphosphonates as first-line therapy for osteogenesis imperfecta.
Conflict of interestThe authors declare no conflict of interest.