At the end of 2019, a series of patients affected by lung infection of initially unknown aetiology with clinical presentations very similar to viral pneumonia were registered in China. Sequencing analysis of samples from the lower respiratory tract identified a new type of virus from the family Coronaviridae called SARS-CoV-2 as the causative agent of the outbreak; the agent responsible for the disease that was renamed COVID-19.1 Since then, millions of cases have been identified around the world. The World Health Organization (WHO) initially declared the infection to be a Public Health Emergency of International Concern, and later classed it as a pandemic.2,3
The vast majority of coronaviruses are responsible for mild upper respiratory tract infections in immunocompetent adults, and can cause more serious conditions, such as severe acute respiratory distress syndrome (ARDS) and sepsis in patients with risk factors, namely, cardiovascular disease (10.5%), diabetes (7.3%), chronic respiratory disease (6.3%), high blood pressure (6%) and cancer (5.6%).1,2
Person-to-person transmission occurs mainly by respiratory droplets and by contact with contaminated material through mucosa (oral, ocular and nasal). It can also be transmitted by aerosols in aerosol-generating therapeutic procedures. The average number of secondary cases produced from 1 case has been estimated at between 2 and 3,2,4 while the average incubation period is between 5.2 and 12.5 days, although in some cases it can be as long as 24 days.1
The potential repercussions of the disease have led international, regional and local health organizations to adopt a series of measures to deal with COVID-19 and try to reduce its impact5 not only in society as a whole, but also in healthcare. These measures include changing guidelines and promoting strategies such as telemedicine,6 a service that has been introduced in different specialties, including Pain Treatment Units. Telemedicine is basically applicable in routine, elective, non-urgent medical consultations (clinical evolution, start and follow-up of medical treatments, psychological intervention techniques, etc.).6 In certain cases, however, such as patients with chronic pain refractory to medical treatment who need invasive therapeutic procedures, telemedicine services are ineffective because they exclude direct patient-doctor contact, and with it, the risk of virus transmission.
In this context, intrathecal drug infusion (IDI) pumps and both spinal cord (SCS) and peripheral nerve (PNS) stimulation device are now frequently used as part of the treatment strategy in patients with chronic pain refractory to other therapies.7 SCSs have now been approved by the US Food and Drug Administration (FDA) for the treatment of chronic neuropathic pain of the spine and limbs, post-surgical spine syndrome, and complex regional pain syndrome. However, they have also been used successfully in many other indications, including chronic intractable angina, peripheral arterial disease, peripheral neuropathic pain, and in the treatment of visceral pain.7–9 In IDIs, the intrathecal administration of baclofen is approved by the FDA for the treatment of spasticity, and the intrathecal administration of morphine and ziconotide is approved for the treatment of chronic, refractory, nociceptive, neuropathic or mixed cancer or non-cancer-related pain.7,10
In light of the current situation, we believe it is important to establish guidelines for the management of patients with suspected or serious infection by Coronavirus SARS-CoV-2 scheduled for implantation of electronic systems for the control of chronic pain (IDIs and SCSs). The purpose of these recommendations is to reduce the spread of COVID-19 among both medical personnel and the general population, and they may be updated as the pandemic progresses and new evidence emerges.
Material and methodsWe conducted a systematic search in Pubmed for articles published in English from the last quarter of 2019 to May 2020 using the terms "spinal cord stimulation", "peripheral nerve stimulation”, “intrathecal infusion", "perioperative management”, “perioperative assessment","COVID-19"," SARS-CoV-2” and "chronic pain" both alone and with Boolean operators (AND, OR). All articles considered relevant were reviewed.
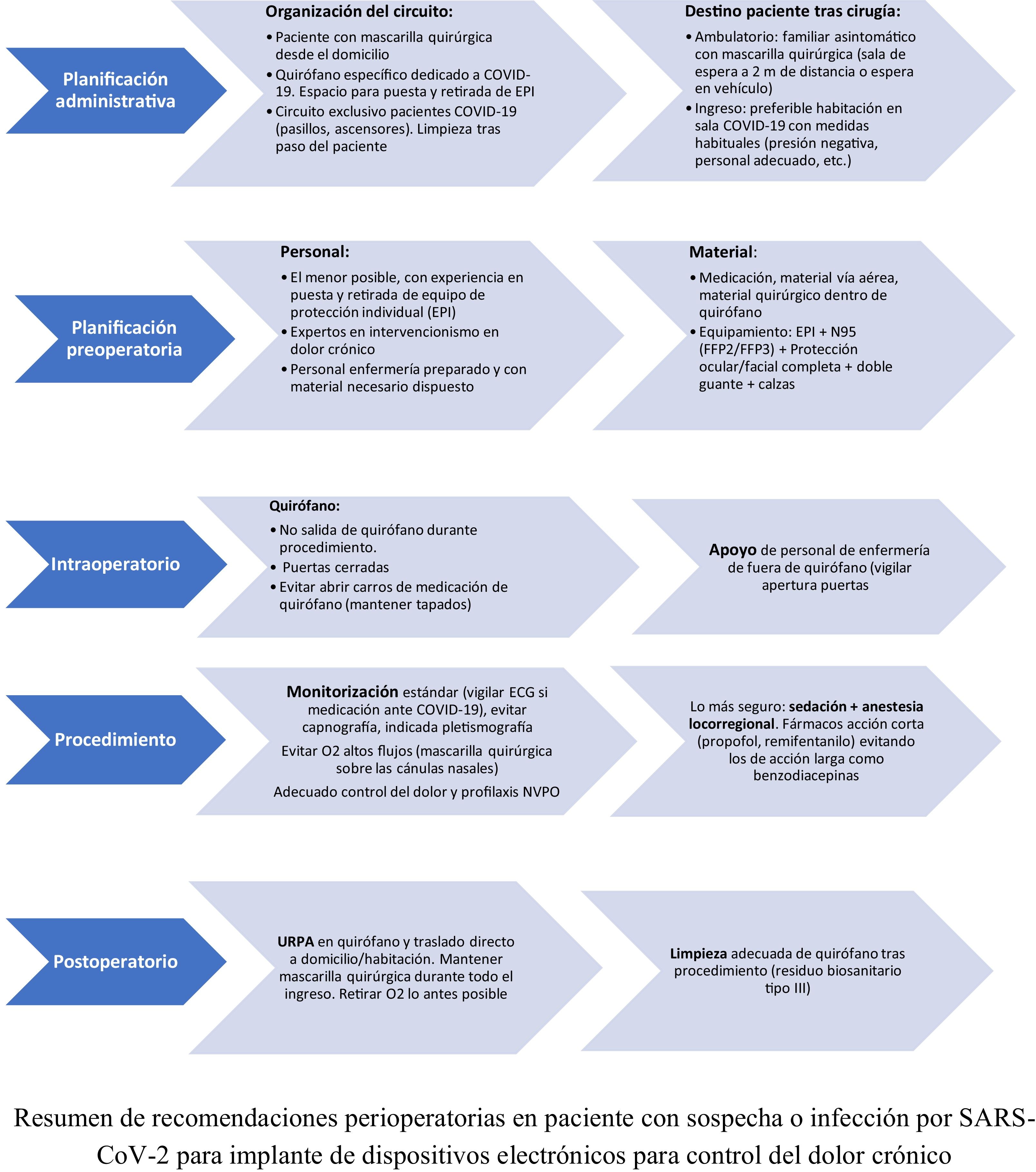
No articles have yet been published on the management and perioperative anaesthetic considerations in patients with suspected or serious infection by SARS-CoV-2 scheduled for implantation of electronic devices for chronic pain control. The recommendations included in these guidelines have been adapted from recommendations established in similar surgical procedures in terms of outpatient care, hospital admission, magnitude, etc. (Fig. 1).
Our overall objective has been to establish perioperative clinical practice guidelines for the management of patients with suspected or confirmed COVID-19 infection in order to reduce the risk of infection for health personnel, other patients, and the community. The recommendations cover the initial evaluation of the patient before the procedure up to the moment they are discharged home.
The indications would also be useful in clinical situations involving infection by microorganisms similar to the SARS-CoV-2 virus.
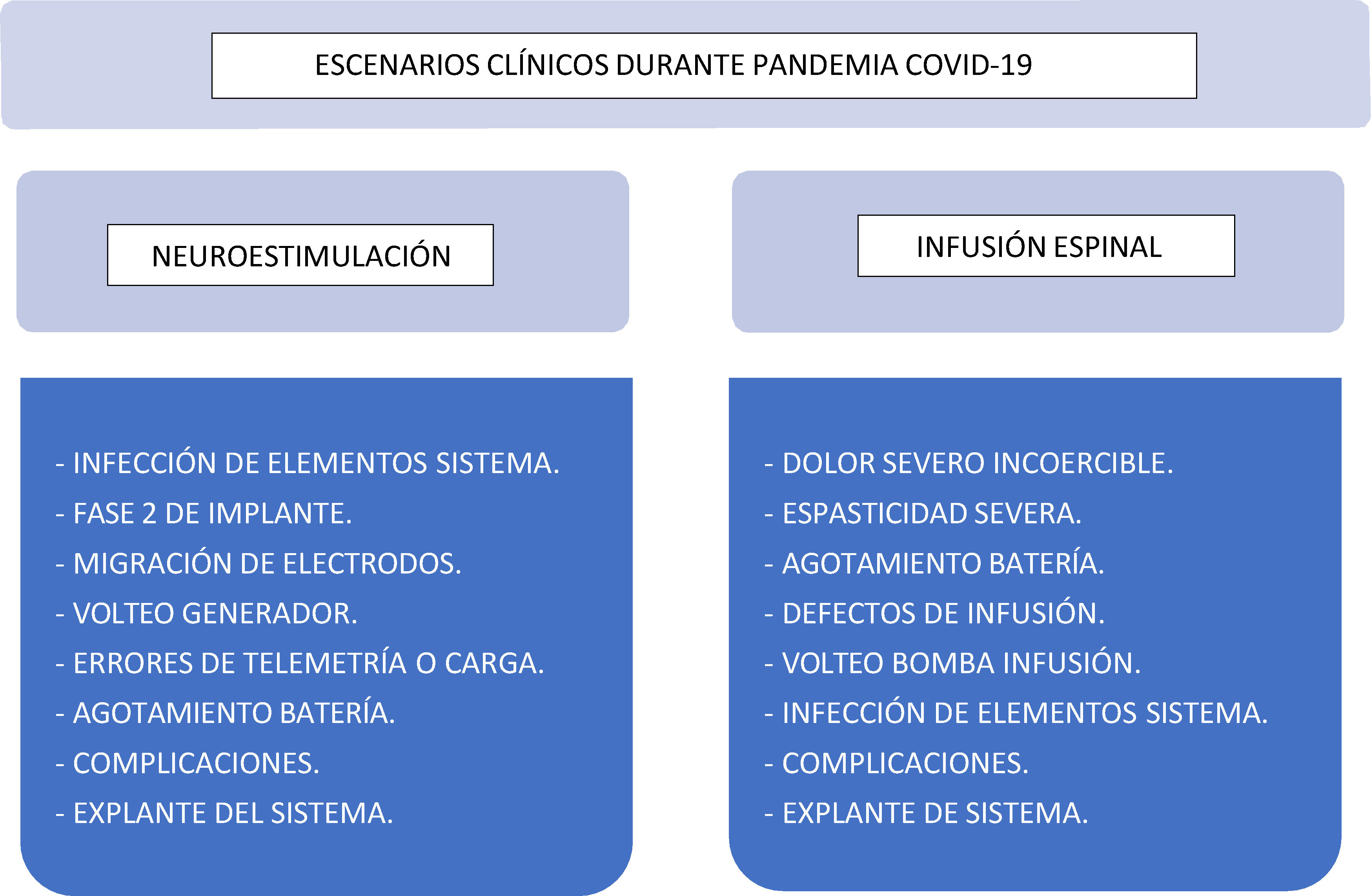
Clinical scenariosIn Pain Treatment Units, perioperative management is required in all patients with suspected or confirmed COVID-19 infection that are not candidates for telemedicine services; that is, patients with pain that is refractory to conventional medical treatment and require urgent implantable therapies or revision of existing implanted systems11,12 (Fig. 2):
- •
Neurostimulation systems: SCS, PNS:
- -
Suspected or confirmed infection of generator or electrodes. Possible need to explant the device.
- -
Phase 2 of implantation of posterior lumbar/cervical cord stimulator, dorsal root ganglion stimulator or subcutaneous stimulator with a temporary external device.
- -
Electrode migration causing neurological deficit or severe pain.
- -
Suspected generator flip-over preventing correct telemetric reading.
- -
Repositioning of the generator due to the inability of the system to perform properly.
- -
End of life generator replacement.
- -
Surgical review of complications: surgical wound dehiscence, haematoma, seroma, suspected infection
- -
- •
Intrathecal Drug Infusion (IDI) devices:
- -
Chronic cancer-related pain refractory to conventional medical treatment (carefully assessing risk/benefit).
- -
Severe and disabling spasticity refractory to oral treatment (carefully assessing risk/benefit).
- -
IDI battery depletion due to severity secondary to baclofen, clonidine, ziconotide, or major opioid withdrawal syndrome.
- -
Infusion malfunctions, under- or over-dosing, which require surgical review of the device (review of the integrity of components, correct connection, presence of granulomas, etc.).
- -
Suspicion of drug infusion pump flip-over, preventing filling.
- -
Suspicion or confirmation of infection. Possible need to explant the device.
- -
Surgical review of complications: Surgical wound dehiscence, haematoma, seroma, suspected infection.
- -
Cancer patients need special considerations during viral pandemics such as that caused by SARS-CoV-2. While cancers vary in natural history, prognosis, and mortality rates, all patients regardless of their cancer type struggle daily with anxiety, fear, and above all refractory pain.13 This is why clinicians must consider special treatment strategies in this patient group, since it is well known that under-treated chronic pain in cancer patients is associated with high mortality rates.14 While organizations such as the American College of Surgeons and the Ambulatory Surgery Center Association (ASCA) recommend delaying the performance of any procedure that can be safely postponed until after the pandemic without significant risk to the patient,15,16 many cancer patients get relief and hope from non-delayed invasive medical treatments such as those provided in Pain Treatment Units. Therefore, given the exceptional nature of the situation, the implantation of definitive IDIs should not be delayed, and pre-implantations tests should either be minimised or obviated.
Recommendations during the procedureFor the purpose of these guidelines, patient management has been divided into three stages: preoperative, intraoperative and postoperative. These recommendations will benefit both the patient and the surgical team involved in their care2,17–20:
- •
Preoperative setting. Pre-procedural considerations.
- •
Staff must be familiar with the physical spaces and the elements available to treat patients with suspected or confirmed COVID-19.
- •
Plan procedures ahead of time in order to maximise barrier precautions and prepare the material needed: Determine the surgical plan and clearly assign each member’s tasks. Prepare anaesthesia drugs and equipment in advance.
- •
Prioritise protective measures for health personnel. Appropriate personal protective equipment (PPE) that protects all team members from inhalation and contact with aerosols and droplets must be available: Protective mask (ideally N95 - FFP2 or preferably FFP3), adjustable goggles or full face mask, splash-proof gown, double gloves, splash-proof cap and boots. Each team member must have previously received training in donning PPE, hand washing, and in removing the PPE to avoid potential mistakes and contamination. Bear in mind that medical professionals are most at risk of contamination when removing the PPE at the end of the procedure, so this must be supervised by a trained personnel.
- •
Make sure that the planned surgical intervention is essential and urgent.
- •
The intervention should, as far as possible, be carried out at a time when staff traffic is at its lowest level.
- •
In order to create and maintain a settings in which patients are least at risk from contracting SARS-CoV-2 from hospital staff, we recommend following the procedures implemented by each hospital’s Preventive Medicine and Occupational Health and Safety services to minimise exposure to the virus.
Preoperative setting. Organization of the pre-surgical circuit:
- •
Outpatient:
- -
Before surgery, establish procedures for actively screening all patients for SARS-CoV-2, based on the hospital’s level of alert, the epidemiological status of the catchment area, and patient- and procedural-related risks. Perform a preliminary evaluation of the patient’s symptoms and epidemiological history approximately 14 days in advance in order to detect symptoms or risk of COVID-19. Advise the patient to observe strict social distancing and to take protective measures during the 2 weeks prior to surgery to reduce the chances of infection. Actively screen patients for SARS-CoV-2 by taking nasal swabs for PCR testing as close as possible to the time of surgery, and at least 72 hours before the intervention. Re-evaluate the patient’s symptoms and epidemiological history 12-24 hours before the intervention. Avoid the systematic use of SARS-CoV-2 serology, chest computed tomography (CT) scans, and specific laboratory tests.
- -
In the final telephone conversation with the patient prior to the procedure explain how the surgery will be organised: arrival, the patient’s escort, and discharge home after surgery.
- -
Both the patient and their escort must wear a surgical mask when they arrive at the surgical suite.
- -
Only one escort is allowed per patient; the escort must be asymptomatic. Escorts manifesting fever or symptoms will not be allowed entry.
- -
The escort must wait in a room that is large enough to allow a safety distance of at least 2 metres, equipped with hand sanitizers. If this is not possible, the escort will be allowed to wait in their vehicle and be notified by telephone to pick up the patient after the procedure.
- -
- •
Hospital patient:
- -
The patient should be in a negative pressure isolation room that meets established standards (12 air changes/hour, HEPA filter and airlock).
- -
Minimise as far as possible the number of people caring for the patient; ideally, no-one should leave and no new personnel should enter the room during the procedure. Minimise the time spent in the room.
- -
The patient must wear a surgical mask during transfer to the operating room.
- -
- •
General rules in both cases:
- -
On admission or arrival in the surgical suite, add a preoperative report to the patient's medical chart, including their history, allergies, and other routine information.
- -
Do not perform an airway evaluation (give reasons for not doing so).
- -
Either the patient or a family member must sign an informed consent form. Ideally, use a digital document that can be signed electronically.
- -
Perform any additional investigations at the bedside, whenever possible. If transfer is unavoidable, the patient must wear a surgical mask during transfer.
- -
Check preoperative labs, if available, particularly the platelet count, since some patients with SARS-CoV-2 infection have presented thrombocytopaenia, which may contraindicate the intervention or modify the anaesthesia technique (general or regional).
- -
Use transfer routes that are exclusive to SARS-CoV-2 patients, or minimise the personnel present during transfer: Do not take the patient to preoperative waiting areas. Assign a specific operating room to SARS-CoV-2 patients, and place posters on the doors to minimize staff exposure. During transit, make sure that corridors and elevators are clear.
- -
Staff in charge of the transfer and reception in the surgical suite must wear PPE with a FFP2, preferably FFP3, face mask if the distance between the staff and the patient is less than 2 metres.
- -
Check, if possible, that the ventilation is operating at negative pressure and avoid positive pressure. The ideal operating room will have absolute filtration or HEPA filters.
- -
Hand disinfection is essential before and after coming into contact with the patient, as well as when donning and doffing PPE.
- -
Intraoperative management:
- •
There must be enough space in the surgical area to safely don and doff PPE.
- •
Designate the most experienced operator to perform the procedures.
- •
Keep the doors of the operating room closed during the intervention. Only the minimum staff needed, in full PPE, may remain inside. Do not allow students or non-surgical staff to enter the room.
- •
Whenever possible, do not allow device technicians, who occasionally provide support during implantation, to enter the operating room. If their presence is unavoidable, they should be screened for active SARS-CoV-2 infection by means of PCR (nasal swab) as close as possible to surgery, and at least within the preceding 72 hours, and then re-evaluated for symptoms and epidemiological history 12-24 hours before surgery and upon arrival in the surgical suite.
- •
All surgical equipment (masks, video laryngoscopes, tracheal tubes, Guedel cannulas, etc.) and fluids with or without dosing systems must be prepared in advance to avoid the need for opening and handling trolleys. Use disposable material. Similarly, place any drugs that will or might be required on a large tray, and avoid opening and handling drug trolleys as far as possible. Make sure that everything that will or may be required during surgery is in the operating room in order to avoid opening the doors once the patient has entered.
- •
Optionally, place a runner (nurse) outside the COVID operating room to provide any material not available in the room.
- •
Monitoring will depend on the patient's status, the surgery to be performed, and the chosen anaesthetic technique, as in any patient undergoing anaesthesia.
- •
Choose the safest anaesthesia technique for both the patient and surgical team. Airway instrumentation is rarely needed during the implantation of pain control devices, so aerosol-generation is low and risk of airborne transmission is minimal compared to other outpatient procedures (procedure involving the oral cavity, ENT procedures, endoscopies, etc.).
- •
Bear in mind that many of these patients will be receiving hydroxychloroquine and azithromycin to treat the infection. It is essential to monitor for cardiac rhythm disorders (bradyarrhythmias) and QT prolongation. Knowledge of possible drug interactions with other anaesthetic drugs is of vital importance.
- •
We recommend the following protocol based on sedation plus regional anaesthesia:
- -
Avoid preoperative administration of midazolam or long-acting benzodiazepines. If they are unavoidable, use the lowest possible dose, individually titrated. Avoid deep anaesthesia and deep muscle relaxation.
- -
Use regional techniques to perform the intervention or to control postoperative pain. This will reduce or eliminate the need for opioids and therefore avoid postoperative nausea and vomiting, respiratory depression or cognitive deterioration. The possibility of preoperative administration of transversus abdominis plane block for the implantation of electronic devices for chronic pain control has been described.21
- -
It is essential to administer postoperative nausea and vomiting prophylaxis to reduce the risk of aerosol generation during coughing and vomiting.
- -
Manage postoperative pain with non-opioid drugs, such as paracetamol or NSAIDs.
- -
Use short-acting intravenous sedatives (propofol, remifentanil), titrated individually.
- -
When administering intraoperative oxygen therapy in an awake patient, it is preferable to use low-flow nasal cannulas covered with a surgical face mask. Avoid potential aerosol-generating systems, such as high-flow oxygen, Venturi masks or non-invasive ventilation. Hui et al. showed that the dispersion of the particles exhaled by the patient increased in direct relation to the progressive increase in the oxygen flow (distances of 0.2, 0.22, 0.3 and 0.4 metres during the administration of 4.6, 8 and 10 l/min, respectively).22
- -
Monitoring can be performed under direct clinical vision, impedance plethysmography, and capnography.
- -
If devices such as point-of-care ultrasound are used, place a plastic cover over the ultrasound unit and cable to minimize contamination. Non-essential parts of the ultrasound trolley can be covered to minimize exposure to droplets.
- -
- •
If airway instrumentation is required, follow the protocol established by Montero et al.2
Postoperative management:
- •
Post-anaesthesia care will take place in the operating room (avoid transferring the patient to another unit). Patients should remain in recovery as long as needed to guarantee their safety during transfer to the ward, their stay in the ward, and their discharge at home, if applicable. Transfer patients requiring greater postoperative surveillance to an airborne infection isolation room set aside exclusively for COVID patients, equipped with adequate monitoring and staffed with personnel with PPE, as stipulated.
- •
Patients must always wear a surgical mask during the postoperative period and discharge.
- •
Avoid aerosols, high-flow nasal cannulas or non-invasive ventilation as far as possible, and withdraw supplemental oxygen therapy as soon as possible.
- •
Any patient discharged home must receive a list of instructions and advice on self-care. The same instructions must be given over the phone to their primary caregiver.
- •
If the patient is to be transferred to a ward after the intervention, notify the ward staff and await confirmation that they are prepared to receive the patient.
- •
Coordinate with the hospital’s security service to expedite the transfer (use of elevators, avoid risky corridors, etc.).
- •
Inter-transfer monitoring is at the discretion of the anaesthesiologist, using the protective, cleaning and disposal measures established in the corresponding protocols.
- •
During transfer, the patient’s face and/or low-flow nasal cannulas or other oxygen delivery system, if used, must be covered with a surgical mask.
- •
The same recommendations for transferring patients to the operating room must be used during postoperative transfer.
- •
After the patient has left the operating room, leave as much time as possible before the next patient is received (to eliminate airborne contaminants).
- •
After the procedure, the operating room, work areas, and potentially contaminated surfaces must be cleaned and waste (type III biosanitary waste) eliminated as per the hospital’s standard procedure, paying particular attention to the recommendations of the hospital’s Preventive Medicine Service.
Pain Treatment Units may need to organise the perioperative management of patients with suspected or confirmed COVID-19 infection, many of them with pathologies that are refractory to conventional medical treatment and urgently require a review of their existing implantable pain management device, or the implantation of a new device.
It is essential to establish specific perioperative clinical guidelines for patients with chronic pain requiring this type of invasive procedure in order to reduce the risk of infection for health personnel, other patients, and the community at large.
These indications are also applicable in situations involving infection by microorganisms similar to the SARS-CoV-2 virus, and in the case of the latter will be updated as the pandemic evolves and new evidence emerges.
FundingThis manuscript has not received any type of funding.
Conflict of interestsThe authors declare that they have no conflict of interest.
Please cite this article as: Asensio-Samper JM, Rubio-Haro R, Hernández-Cádiz MJ, De Andrés J. Manejo perioperatorio de pacientes con sospecha o infección grave por coronavirus SARS-CoV-2 programados para implante de dispositivos electrónicos para el control de dolor crónico. Rev Esp Anestesiol Reanim. 2020. https://doi.org/10.1016/j.redar.2020.06.010