Pneumonia caused by coronavirus, which originated in Wuhan, China, in late 2019, has been spread around the world already becoming a pandemic. Unfortunately, there is not yet a specific vaccine or effective antiviral drug for treating COVID-19. Many of these patients deteriorate rapidly and require intubation and are mechanically ventilated, which is causing the collapse of the health system in many countries due to lack of ventilators and intensive care beds.
In this document we review two simple adjuvant therapies to administer, without side effects, and low cost that could be useful for the treatment of acute severe coronavirus infection associated with acute respiratory syndrome (SARS-CoV-2). Vitamin C, a potent antioxidant, has emerged as a relevant therapy due to its potential benefits when administered intravenous. The potential effect of vitamin C in reducing inflammation in the lungs could play a key role in lung injury caused by coronavirus infection. Another potential effective therapy is ozone: it has been extensively studied and used for many years and its effectiveness has been demonstrated so far in multiples studies. Nevertheless, our goal is not to make an exhaustive review of these therapies but spread the beneficial effects themselves.
Obviously clinical trials are necessaries, but due to the potential benefit of these two therapies we highly recommended to add to the therapeutic arsenal.
La neumonía causada por coronavirus, que se originó en Wuhan, China, a finales de 2019, se ha extendido por todo el mundo convirtiéndose en una pandemia. Desafortunadamente, a día de hoy no existe ninguna vacuna específica para el virus COVID-19, y el tratamiento está siendo de soporte con añadido de antivirales y otros fármacos, sin que hasta la fecha se haya evidenciado un beneficio claro. Muchos de estos pacientes se deterioran rápidamente y requieren ser intubados y ventilados mecánicamente, lo que está provocando el colapso del sistema sanitario en muchos países debido a la falta de ventiladores y de camas de críticos.
En este documento revisamos dos terapias adyuvantes sencillas de aplicar, sin efectos deletéreos y de un coste bajo que podrían ser de utilidad para el tratamiento de la infección por coronavirus agudo severo asociado al síndrome respiratorio agudo (SARS-CoV-2). La vitamina C, un potente antioxidante, se ha convertido en una terapia relevante debido a sus beneficios potenciales cuando se administra por vía intravenosa. El efecto potencial de la vitamina C en la reducción de la inflamación en los pulmones podría desempeñar un papel clave en la lesión pulmonar causada por la infección por coronavirus. Otra posible terapia eficaz es el ozono. Pese a la controversia que siempre le ha acompañado, se ha estudiado y utilizado ampliamente durante muchos años y su eficacia se ha demostrado en múltiples estudios. Sin embargo, nuestro objetivo no es hacer una revisión exhaustiva de dichas terapias sino difundir sus efectos beneficiosos.
Obviamente, los ensayos clínicos son necesarios, pero dado el potencial beneficio de estas terapias, recomendamos incorporarlas al arsenal terapéutico para el tratamiento del SARS-CoV-2.
Coronaviruses are enveloped RNA viruses surrounded by very characteristic spike glycoproteins that form a kind of crown, hence their name. They contain the largest positive-sense single-stranded RNA genome of all known RNA viruses. Once cell entry is achieved, the virion sheds its envelope to begin replication in the cytoplasm of the host cell. After binding to cellular ribosomes the released viral polymerase begins the RNA replication cycle. The newly formed nucleocapsids continue to acquire new envelopes by budding through the membranes of the endoplasmic reticulum of the cell. The virions are then released into the general blood and lymph circulation, ready to infect new cells, other organs, and new hosts. In more than a third of patients, the syndrome progresses to severe disease with respiratory distress and oxygen desaturation that requires ventilatory support approximately 8 days after onset of symptoms. Mortality has been found to vary by transmission group, ranging from 3% to 20%. This suggests that the aetiology of SARS depends on a heterogeneous population of viral quasispecies with varying degrees of virulence.
Characteristics of severe systemic inflammatory lesionsClinical courseInitial symptoms are usually fever (generally high), chills, headache, myalgia and dry cough, which may evolve to dyspnoea and ultimately respiratory distress. Respiratory deterioration is rapid, and many patients require intubation within the first 48 of onset of respiratory symptoms.
Laboratory studiesIn the early stage of the disease, white blood cell levels in peripheral blood are normal or decreased. Some patients may have abnormal liver function and increased lactate dehydrogenase (LDH), creatine kinase (CK), and myoglobin (Mb) levels. Most patients will have high levels of C reactive protein (CRP) and elevated erythrocyte sedimentation rate (ESR), but normal procalcitonin levels. In severe cases, D dimer levels will be elevated and other clotting indicators may be affected. Elevated levels of inflammatory cytokines, such as IL-2, IL-6, TNFa, can occur during disease progression, together with increased ferritin, an iron storage protein that is a marker of inflammatory response to infection.
Imaging studiesEarly CT scans usually show small patchy areas or a ground glass pattern. A few days later, the lesions increase and become more extensive, with a more established ground glass pattern and/or infiltrating lesions, some with consolidation; pleural effusions are rare. The “white lung” typically found in ARDS rarely occurs in COVID-19.
TreatmentThe combination therapies used so far have been largely unsuccessful. Different combinations, such as hydroxychloroquine sulphate or chloroquine phosphate with azithromycin, appear to show some effectiveness. Interferon is being administered (preferably interferon ?) and various antiviral cocktails are being tested, although efficacy has not yet been proven in clinical trials. The mainstay of therapy, therefore, are supportive measures, because a curative treatment remains elusive.
Two adjuvant therapies, intravenous vitamin C and ozone therapy could play an important role in the therapeutic regimen in these patients.
Intravenous vitamin CIntroductionVitamin C (ascorbic acid or ascorbate), a water-soluble vitamin, is an essential cofactor in numerous enzymatic reactions that mediate a variety of essential biological functions. It is considered a powerful antioxidant with antimicrobial and anti-inflammatory properties. According Nobel prize-winning biochemist Linus Pauling, vitamin C has beneficial effects on cardiovascular health, improves the body's immunity to infection, and can even help treat cancer.1
The seriousness of SARS-CoV-2 infection manifests in lung deterioration. Although the reason for this rapid deterioration remains unclear, its clinical course is similar to macrophage activation syndrome, a secondary form of hemophagocytic lymphohistiocytic syndrome with hypersecretion of proinflammatory cytokines that damage the lungs. This is why intravenous vitamin C, a powerful anti-inflammatory agent, can be effective in COVID-19.2
PharmacokineticsVitamin C acts primarily at the intracellular level, and is transported to cells by specific membrane transporters. Sodium-dependent vitamin C transporters (SVCT) are mainly responsible for the absorption, distribution and retention of vitamin C. Because of the diverse expression and concentration dependency of these transporters, the pharmacokinetics of vitamin C at the physiological level is highly complex, compartmental, and non-linear, but appears to change from zero to first order, showing a constant and dose-independent half-life when administered as an intravenous infusion. After one dose, vitamin C circulates in plasma, is freely filtered by the renal glomerulus, and is reabsorbed at the proximal tubule by the first SVCT (SVCT1). Although SVCT1 regulates vitamin C homeostasis throughout the body, a high affinity, low capacity SVCT (SVCT2) protects metabolically-active cells against oxidative stress, which facilitates vitamin C accumulation where it is needed.3 Dehydroascorbic acid (the oxidized form of vitamin C) is transported by glucose transporters (GLUT), where it is reduced to avoid irreversible decomposition. In situations such as sepsis, vitamin C absorption in cells is reduced due to increased cytokine secretion.
Biological effectsVitamin C is an electron donor, and therefore a reducing agent; hence its antioxidant action. All known physiological and biochemical actions of vitamin C are due to this characteristic. Vitamin C has immunostimulatory effects, antioxidant, anti-inflammatory and antiviral properties, and could also have an antimutagenic effect.4,5 Studies have shown that vitamin C improves neutrophil chemotaxis, phagocytosis, and therefore microbial clearance.6,7 It also promotes T cell and natural killer cell proliferation and modulates their functions.8 Vitamin C is also needed for catecholamine synthesis (formation of adrenaline from dopamine by dopamine beta-hydroxylase)9,10 and adrenal steroidogenesis.11 By recycling tetrahydrobiopterin, a critical cofactor in catecholamine synthesis, vitamin C improve noradrenaline synthesis by increasing expression of tyrosine hydroxylase.12 It is also a cofactor for peptidyl-glycine alpha-amidating monooxygenase, an enzyme that is required for the endogenous synthesis of vasopressin.13 A study in cardiac surgery patients has suggested that pre-operative administration of vitamin C mitigates etomidate-induced adrenal suppression.14 All this has recently sparked considerable interest in the use of vitamin C in the treatment of haemodynamically unstable patients, because the vitamin C-dependent synthesis of the vasopressors norepinephrine and vasopressin may play an important role in supporting cardiovascular function during severe infections and septic shock.15 Nabzdyk and Bittner,16 recently reviewed the use and biological effects of vitamin C in the management of critically ill patients.
Experience in critically ill patientsBurnsVitamin C has traditionally been used in burn patients. Increased capillary leakage is a clinical characteristic of burn injury and is associated with significant fluid and protein extravasation and the generation of free radicals, which have emerged as important mediators of cell injury caused by burns.
Continuous infusion of vitamin C appears to be useful in minimizing the effects of free radical-induced injury and reducing fluid resuscitation requirements in burn patients.17,18 High doses of vitamin C appear to improve microvascular barrier dysfunction without affecting leucocyte activation.19 In a study in dogs with burn injuries, the administration of vitamin C (14mg/kg/h) decreased lipid peroxidation and microvascular protein and the need for IV fluids.20 A randomized, double-blind study in sheep found a significant reduction in net fluid balance and lipid peroxidation in sheep with 40% of total body surface area burns that were resuscitated with fluid therapy along with high doses of ascorbic acid.21 Studies in patients have also been promising. In a randomized and prospective study in patients with more than 30% total body surface area burns, administration of vitamin C (1584mg/kg/day) was well tolerated, reduced IV fluid requirements, and improved lung function, leading to a significant reduction in days on mechanical ventilation.22
SepsisThere has recently been a surge of interest in the use of vitamin C as an adjuvant treatment for sepsis, mainly triggered by the results of the study by Marik et al.,23 who administered a cocktail of vitamin C (1.5g IV every 6h), hydrocortisone (50mg IV every 6h) and thiamine (200mg IV every 12h) to 47 septic patients admitted to the ICU. Mortality in patients receiving this treatment was reduced by more than 30%, irrespective of comorbidities and pre-treatment risk of mortality. Several randomized controlled clinical trials focussed on confirming the beneficial effects of vitamin C and other supplements in critically ill patients with sepsis are currently in progress, including the VICTAS, ACTS, and HYVCTTSSS trials.24–26
Pneumonia and ARDSAcute respiratory distress syndrome (ARDS) is often accompanied by uncontrolled inflammation, oxidative injury, and damage to the alveolocapillary barrier. Unfortunately, there are very few studies in critically ill patients with ARDS who have received IV vitamin C as adjuvant therapy.
Animal studies have shown that vitamin C increases resistance to infection caused by coronavirus, and also modifies susceptibility to infection.27 Nathens et al.28 gave 594 critically ill surgical patients 1g of ascorbic acid every 8h combined with oral vitamin E for 28 days, and observed a significantly lower incidence of acute lung injury and multi-organ failure. In a clinical study published by Sawyer et al.,29 a 50% reduction in mortality was observed after high IV doses of ascorbic acid and other antioxidants (tocopherol, N-acetylcysteine, and selenium) were given to patients with established ARDS. Bharara et al.30 obtained good outcomes and no side effects after administering 50mg/kg every 6h for 96h to treat recurrent ARDS. Fowler et al.31 described the successful outcome in a 20-year old woman with viral ARDS (rhinovirus and enterovirus D68) who received IV vitamin C. In another study in patients with severe pneumonia, significantly lower in-hospital mortality was observed in the group treated with vitamin C.32 Clinical trials carried out at the Virginia Commonwealth University have showed that high plasma levels of vitamin C act “pleiotropically” to attenuate systemic inflammation and correct sepsis-induced coagulation abnormalities while attenuating vascular injury (CITRIS-ALI clinical trial; identifier NCT02106975). These critically ill patients often present low levels of antioxidants, and in this context vitamin C is likely to have a positive effect.
ARDS secondary to COVID-19 is physiologically different from typical ARDS since extravascular lung water (EVLW) levels are normal or only marginally increased; by definition, therefore, this is not typical ARDS. Lung compliance in these patients is also adequate, although they present severe hypoxia due to intrapulmonary shunt. This suggests the presence of a microvascular and/or macrovascular disease, hence the significant D-dimer elevation, or some other process that has not yet been clarified. Furthermore, pulmonary embolism appears to be common in these patients, and autopsy series have frequently found microthrombi in the pulmonary circulation.
Intravenous vitamin C is already being used in China against COVID-19. A phase-2 clinical trial in which patients receive 24g of IV vitamin C daily for 7 days has been registered at Zhongnan Hospital at Wuhan University (identifier: NCT04264533) to confirm the effectiveness of this treatment. The group of experts in the clinical treatment of COVID-19 in Shanghai has recommended including high daily doses of vitamin C in the treatment of critically ill patients with SARS-CoV-2 infection, since continuous use also seems to achieve a significant improvement in the oxygenation index.33 Some hospitals have already added IV vitamin C and oral zinc (220mg) to the azithromycin and hydroxychloroquine treatment regimen, even in patients with non-severe involvement. The North Shore University Hospital ICU in New York is also administering 3g vitamin C every 6h and Marik 12g daily.
Administration protocolHigh doses of vitamin C are needed to treat the pneumonia and hyperinflammation caused by COVID-19. Several protocols involving different doses and frequency of administration have recently emerged. The controversy surrounding the pro-oxidant effect of high-dose vitamin C has not been demonstrated in vivo and the dose required to produce this effect remains unclear. The cytokine storm generates reactive oxygen species that can be effectively treated with 30–60g of vitamin C, while relatively high levels of vitamin C can improve the chemotaxis of white blood cells (neutrophils, macrophages, lymphocytes, B cells, NK cells).
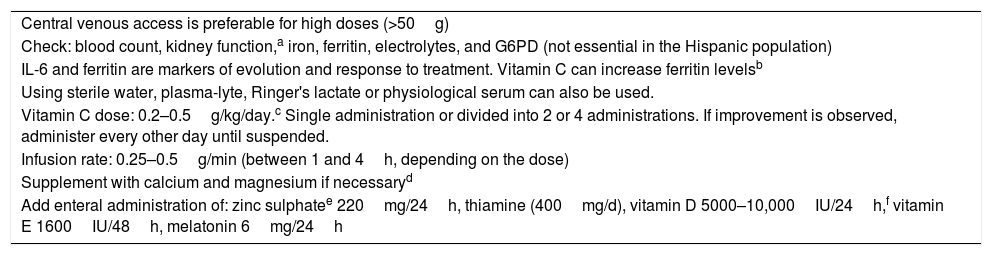
Table 1 shows our administration protocol. This protocol is flexible and must be adapted to the clinical status of the patient and to the adjuvant therapies administered. For example, if the patient is receiving ozone, the dose and frequency of vitamin C must be adjusted because it can be antagonistic; wait for 3h after ozone administration before administering vitamin C.
Protocol for IV Vitamin C administration in COVID-19 infection.
| Central venous access is preferable for high doses (>50g) |
| Check: blood count, kidney function,a iron, ferritin, electrolytes, and G6PD (not essential in the Hispanic population) |
| IL-6 and ferritin are markers of evolution and response to treatment. Vitamin C can increase ferritin levelsb |
| Using sterile water, plasma-lyte, Ringer's lactate or physiological serum can also be used. |
| Vitamin C dose: 0.2–0.5g/kg/day.c Single administration or divided into 2 or 4 administrations. If improvement is observed, administer every other day until suspended. |
| Infusion rate: 0.25–0.5g/min (between 1 and 4h, depending on the dose) |
| Supplement with calcium and magnesium if necessaryd |
| Add enteral administration of: zinc sulphatee 220mg/24h, thiamine (400mg/d), vitamin D 5000–10,000IU/24h,f vitamin E 1600IU/48h, melatonin 6mg/24h |
Protect the administration system from light, as it is photosensitive and easily oxidized.
Ferritin is a good marker of therapeutic response and prognosis; however, vitamin C can increase ferritin levels and confuse interpretation, so if IL-6 is not available, dose adjustment must be based on other parameters.
ConclusionVitamin C can be effective in the treatment of SARS-CoV-2 due to its antioxidant effect, its antiviral properties, its capacity to boost the immune system, and its anti-inflammatory properties. Vitamin C can also help remove the alveolar fluid that accumulates during ARDS by preventing neutrophil activation and accumulation and by reducing damage to the alveolar epithelium. Intravenous infusion of up to100g is safe, provided the precautions described above are taken.
Ozone therapyIntroductionDespite sufficient scientific evidence to support the clinical use of ozone, a gas made up of three oxygen atoms (O3), ozone therapy has not yet been fully accepted. Ozone generators produce the gas from pure oxygen by passing it through a high voltage gradient (5–13mV) according to the formula:
This yields a gas mixture consisting of at least 95% oxygen and no more than 5% ozone; for example, a concentration of 50 µg contains 97.5% oxygen and 2.5% ozone. A medical ozone generator produces ozone concentrations ranging from 1 to 100µg/ml, but concentrations of 15–70µg/ml are used for medical purposes. Ozone therapy is simple to administer, extremely effective, well tolerated, and has virtually no side effects.
Russia, Cuba and Turkey have recognized the benefit of ozone in their legislation. In Spain, specific regulations are in force in some autonomous communities, and also in other countries such as Italy.
The effectiveness of ozone against pathogens is well recognized: ozone appears to be the best agent available for sterilizing water.34 Due to its biological properties, ozone therapy could be administered to complement standard COVID-19 treatment regimens.
PharmacokineticsOzone is a gas that dissolves in pure water in accordance with Henry's law, relative to temperature, pressure, and ozone concentration. Unlike oxygen, ozone reacts as soon as it dissolves in any biological fluid. In the body, it has a half-life of milliseconds due to its high affinity for double covalent bonds, preferably carbon-carbon, found in the polyunsaturated fatty acids that travel in albumin molecules. By this means it creates longer half-life metabolites called ozonides – reactive oxygen species and lipid oxidation products, including peroxides, hydroperoxides, and aldehydes. These molecules act as messengers of the biochemical and immunomodulatory effects of ozone, which are the key to ozone therapy. Ozone reacts immediately with biomolecules that have this double bond, and produces rapid oxidation that results in secondary molecules, which are the basis of its therapeutic action. The therapeutic efficacy of ozone is therefore due to the moderate and controlled oxidative stress produced by its reactions with various biological components.35
Biological effectsViruses can be susceptible to ozone, albeit to a variable degree. Evidence has shown that viruses with a lipid envelope, including coronaviruses, are most sensitive to ozone.
The coronavirus envelope is rich in cysteine, and viral activity depends on the conservation of these residues. Cysteine contains a thiol or sulfhydryl group (–SH); many viruses, including coronaviruses, require these reduced sulfhydryl groups for cell entry and fusion.36 Sulfhydryl groups are susceptible to oxidation, and therefore to the oxidizing effect of ozone. Peroxides created by ozone administration oxidize cysteines37,38 and show long-term antiviral effects that can further reduce viral load. Once their capsid is removed, virions cannot survive or replicate, and the creation of dysfunctional viruses due to the action of ozone offers unique therapeutic possibilities.
Ozone also has an immunomodulating action through the activation of various cytoplasmic transcription factors by second messengers, specifically: (1) hypoxia-inducible factor 1-alpha (HIF-1-alpha), (2) nuclear factor Kappa B (NF-?B), and (3) nuclear factor erythroid-2-related factor 2 (Nrf2). These factors release proteins that set in motion all the beneficial mechanisms associated with ozone. Some are activated or modulated before others, which is why ozone therapy is a cumulative dose treatment.
A particularly important effect of ozone therapy is the improvement in tissue oxygenation, since it increases oxyhaemoglobin by increasing 2–3 DPG levels, which in turn stimulates glycolysis. This increases energy levels, in the form of increased ATP, which allows erythrocytes to maintain and improve oxygen release to more hypoxic tissues. COVID-19 patients are usually hypoxic, so the oxygenating effect of ozone will be very beneficial. Some studies suggest that ozone may enhance the phagocytic activity of neutrophils, and the hydrogen peroxide released by monocytes and lymphocytes is known to be involved in intracellular signalling and capable of activating a tyrosine kinase that phosphorylates NF-?B and leads to the synthesis of different proteins.39,40 NF-?B plays a key role in regulating the immune response to infection and the inflammatory response.41,42 The ability of ozone to induce the release and modulation of interferons and of some anti-inflammatory cytokines (IL-4, IL-6, IL-10, TNFß) is also of major importance.43
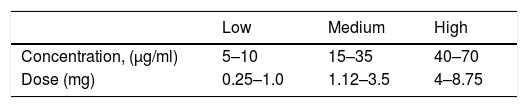
Major autohemotherapyMajor autohemotherapy (MAH) consists of drawing venous blood, usually between 50 and 225ml, and mixing it with oxygen-ozone at concentrations of 15–70µg/ml. The ozone-enriched blood is then reinfused into the body through an IV route.35
Concentrations and dose in ozone therapy are expressed as follows:
- •
Concentration of ozone: µg/mL.
- •
Dose: concentration per volume of ozone (µg/mL×mL=µg de ozono)
The total dose, therefore, is calculated by simply multiplying the ozone concentration by the volume of gas. For example, if 50ml of blood is ozonated with 150ml of gas (1:1 ratio) with an ozone concentration of 30µg/ml, the total equivalent dose will be 4.5mg of ozone.
Ozone concentrations for systemic use range from 10 to 70µg/ml. Concentrations above 80µg/ml should be avoided given the risk of haemolysis, decreased 2.3 DPG levels, and the resulting inability to activate immunocompetent cells.
The side effects of ozone therapy are minimal; the World Federation of Ozone therapy (WFOT) estimates the incidence of complications to be 0.0007%. Given its beneficial effects, adjuvant medication may need to be adjusted, for example, antidiabetic or antihypertensive medication may need to be down-dosed.
Many studies have been published on ozone therapy and its anti-viral action, but a comprehensive review of the literature is beyond the scope of this article. Cespedes et al.44 treated patients with chronic hepatitis B for 1 year with HAM, and observed negativization of the surface antigen, positivity of antibodies against the surface antigen, significant decrease in viral load to undetectable values, and normal transaminase values, which resulted in functional recovery associated with a favourable immune response. They also treated HIV-AIDS patients for 2 years, and obtained a significant decrease in viral load to undetectable values and an increase in CD4 and CD8.45. Robert Jay et al.46 cured 5 Ebola patients after treating them with ozone for 10 days. The Ebola virus, like COVID-19, also induces a cytokine storm that is neutralized by the immunomodulatory action of ozone.
Experience in critically ill patientsUnfortunately, despite the beneficial effects achieved with ozone therapy, its application in critical units is very limited. In patients receiving MAH, oxygenation of vital organs and areas of ischaemia was improved, and respiratory, cardiac and renal functions were supported. If the patient's metabolic status does not deteriorate excessively after 3–4 days of MAH, increased antioxidant enzyme synthesis and haemoxygenase-1 induction can reduce the oxidative stress caused simultaneously by infection, inflammation, tissue necrosis, and dysmetabolism. Bocci and Brito47 reported the case of a patient who developed ARDS in the postoperative period of aortic dissection and was treated with ECMO. Improvement was observed after initiation of MAH with a starting dose of 40µg/ml and successive concentrations of 25µg/ml on the following 2 days. Doctors in Italy have started treating COVID-19 patients with MAH. The studies have not yet been published, but remarkable benefits have been reported. We have also started MAH in our hospital; the patient sample is still small, but clinical and analytical results after just 2 treatment sessions are spectacular. We hope to publish our results soon.
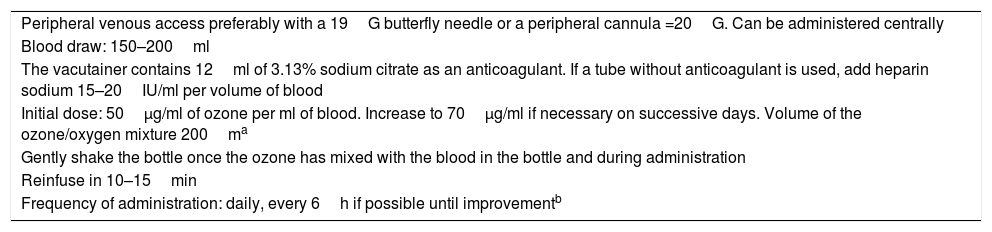
Administration protocolThe protocol described here (Table 2) is flexible, since it depends on the patient's clinical status and evolution. Patients that have received ozone therapy have improved both clinically and analytically since the first treatment session. Ozone therapy is safe, and the frequency of treatment can be increased as needed.
Protocol for administration of major autohaemotherapy in COVID-19 infection.
| Peripheral venous access preferably with a 19G butterfly needle or a peripheral cannula =20G. Can be administered centrally |
| Blood draw: 150–200ml |
| The vacutainer contains 12ml of 3.13% sodium citrate as an anticoagulant. If a tube without anticoagulant is used, add heparin sodium 15–20IU/ml per volume of blood |
| Initial dose: 50µg/ml of ozone per ml of blood. Increase to 70µg/ml if necessary on successive days. Volume of the ozone/oxygen mixture 200ma |
| Gently shake the bottle once the ozone has mixed with the blood in the bottle and during administration |
| Reinfuse in 10–15min |
| Frequency of administration: daily, every 6h if possible until improvementb |
If vitamin C is included, administer 3h after ozone therapy.
Ozone has biological properties that suggest it may have a possible role in SARS-CoV-2 therapy. The abundant cysteine in the characteristic spike on the viral surface of coronaviruses can be easily damaged by ozone. Abundant cysteine residues in viral membrane proteins mediate virus binding and entry into the host cell. This cysteine appears to be functionally important for the production and maintenance of the virus. MAH has proved to be an effective and safe therapy. Ozone is capable of damaging these cysteine residues, and is also the best known anti-inflammatory immunomodulator; therefore, given the characteristics of COVID-19, we believe ozone can be an effective therapy.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Hernández A, Papadakos PJ, Torres A, González DA, Vives M, Ferrando C, et al. Dos terapias conocidas podrían ser efectivas como adyuvantes en el paciente crítico infectado por COVID-19. Rev Esp Anestesiol Reanim. 2020;67:245–252.