Cysticercosis is a current public health problem related to the poor hygienic–environmental conditions. Developing countries have the highest incidence of this disease, and also countries where there are large amounts of immigrants, who are carriers of Taenia solium.1,2 In Mexico approximately 2% of autopsies performed in patients from public hospitals show evidence of cysticercosis.3 At present, it is estimated that at least 50 millions worldwide are carriers of cysticercosis, half of which are symptomatic, and that approximately 50–80% of them are at risk of die of this disease if they do not receive specialized attention.4
Poor health conditions and a lack of measures for health control are the predisposing factors for this disease. Cysticercosis is acquired by ingesting the T. solium eggs through the intake of contaminated water and food. The activated embryo penetrates the intestinal wall of the host until they reach lymphatic and blood capillaries, which in turn spread them through the circulatory system to several organs and tissues. It is estimated that after 10 weeks, the egg becomes a cysticercus and may survive for several years in the tissues of the intermediary host (human or pig).5 In human beings, cysticerci are mainly located in the central nervous system, producing neurocysticercosis, but it may also be located in a wide variety of tissues, including the muscles, heart, eyes, and skin.5 To the best of our knowledge, only 118 cases of oral cysticercosis have been reported up to date in the international literature.5–10 Treatment of oral cysticercosis consists of surgical excision of the nodule. Histological examination confirms diagnosis. We present a series of 6 patients with this infection and underline the importance of searching for these parasites in other possible affected organs.
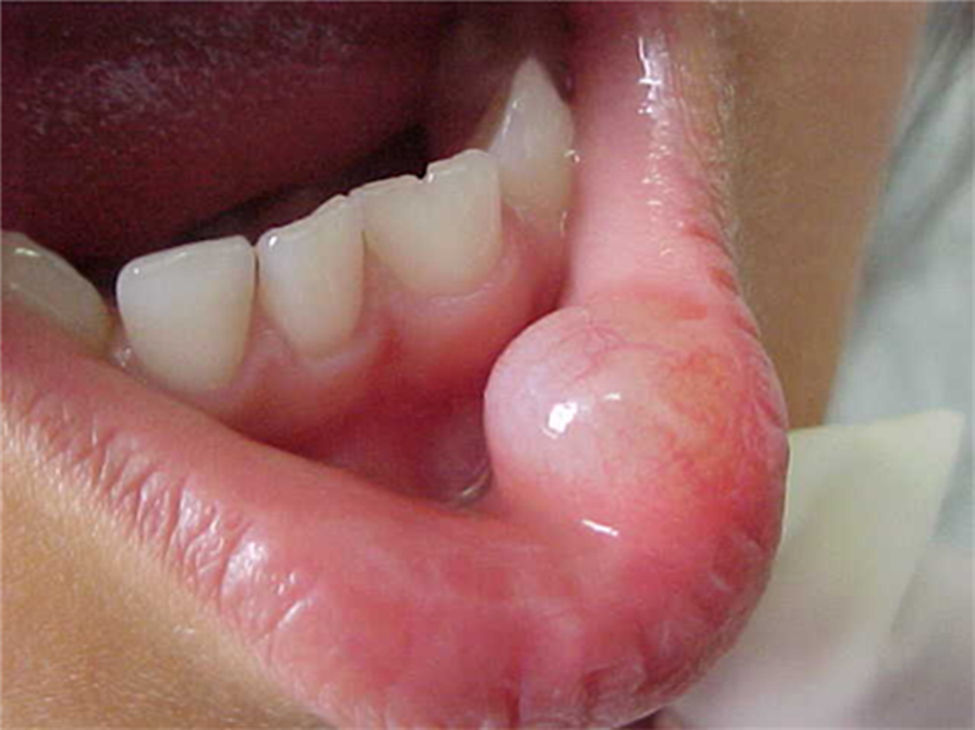
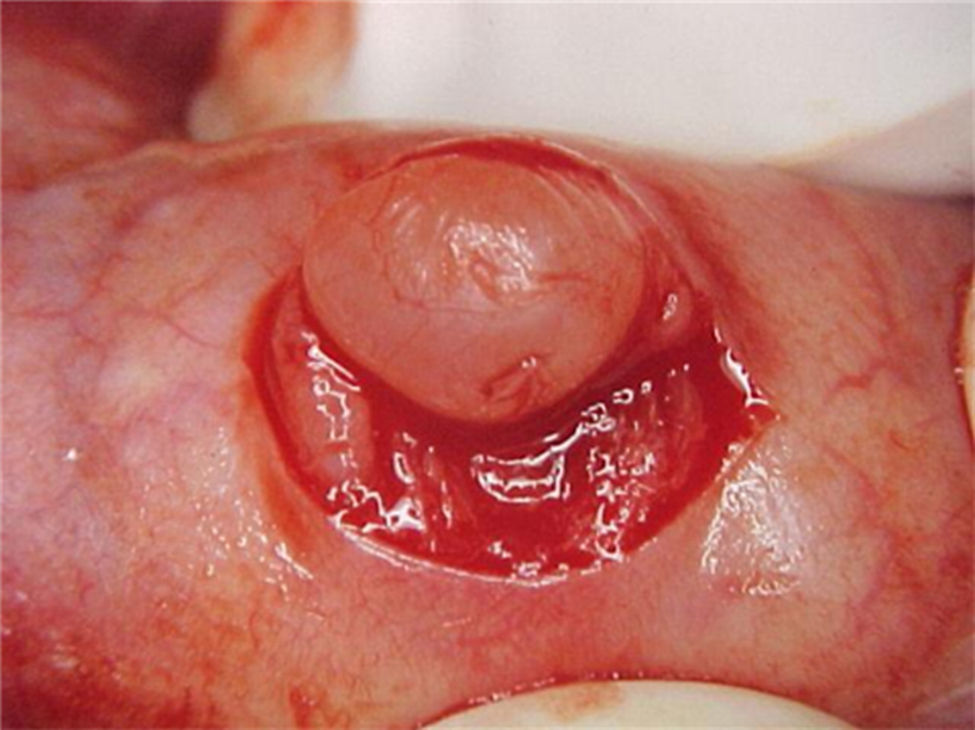
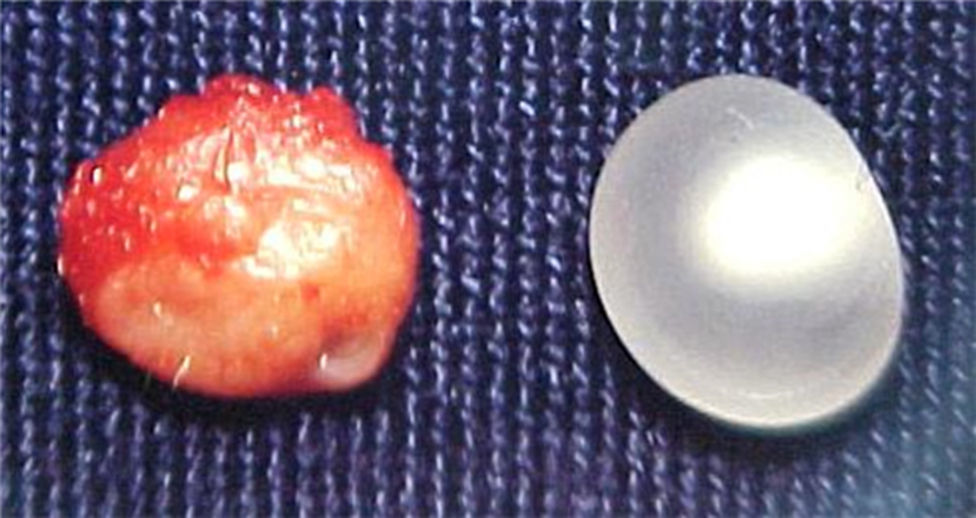
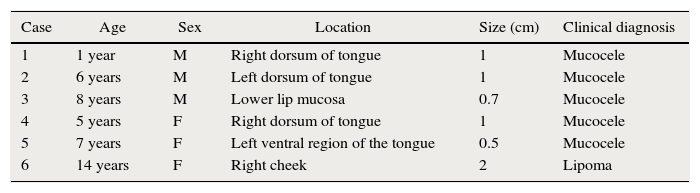
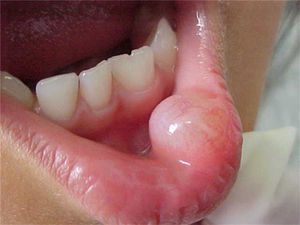
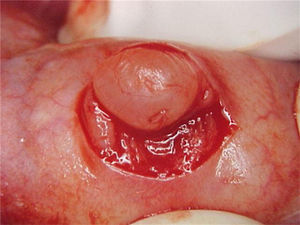
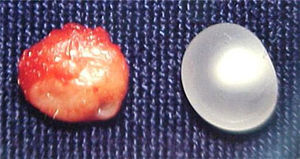
Report of casesSix cases of cysticercosis affecting the oral cavity are presented. They were diagnosed and treated in the Maxillofacial Surgery Department of the Instituto Nacional de Pediatría (Mexico City). The cases presented in this paper were treated from 1999 to 2013. Table 1 shows a summary of the salient clinical features of the affected patients. There were three male and three female whose ages ranged from 1 to 8 and 5 to 14 years, respectively. All the lesions appeared as well defined, mobile and painless submucosal nodules (Fig. 1). Three were located in the dorsum of the tongue, 1 in the ventral surface of the tongue, 1 in the lower lip and 1 within the buccal mucosa. Diameter ranged from 0.5cm to 2cm. All the cases underwent surgical excision (Figs. 2 and 3). The presumptive clinical diagnoses of these lesions were salivary gland mucocele in 5 cases and lipoma in the other case.
Clinical findings in the present series of 6 cases of oral cysticercosis.
| Case | Age | Sex | Location | Size (cm) | Clinical diagnosis |
|---|---|---|---|---|---|
| 1 | 1 year | M | Right dorsum of tongue | 1 | Mucocele |
| 2 | 6 years | M | Left dorsum of tongue | 1 | Mucocele |
| 3 | 8 years | M | Lower lip mucosa | 0.7 | Mucocele |
| 4 | 5 years | F | Right dorsum of tongue | 1 | Mucocele |
| 5 | 7 years | F | Left ventral region of the tongue | 0.5 | Mucocele |
| 6 | 14 years | F | Right cheek | 2 | Lipoma |
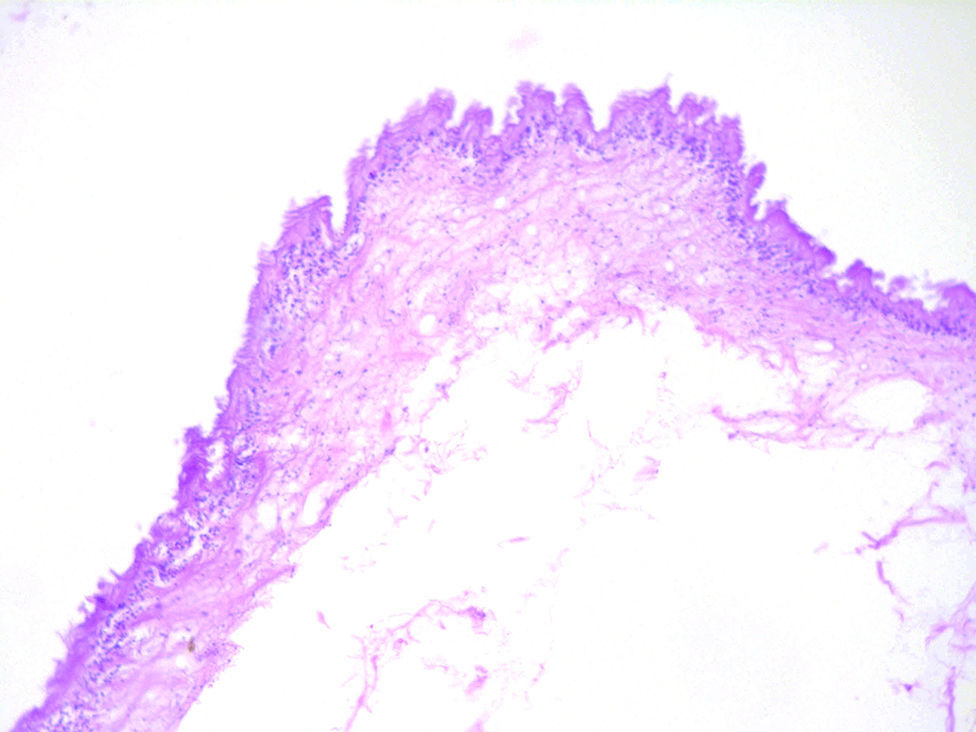
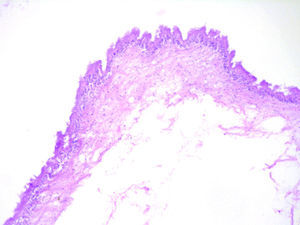
Histologically, viable cysticerci were detected in two cases. These showed an external dense fibrous capsule that surrounded a delicate, double-layered membrane composed by an outer acellular eosinophilic layer and an inner, sparsely cellular lining. The outer part of the capsule contained a mild to moderate inflammatory cell infiltrate, in which lymphocytes, plasma cells and histiocytes predominated. On the inner aspect the inflammatory infiltrate exhibited aggregates of neutrophils and eosinophils, which were more abundant in the four cases of degenerated cysticerci. The cyst in all cases contained the larval form of T. solium, whose cephalic extremity (scolex) had a structure similar to that of the adult cestode, with distinct suckers and a crown of hookers (rostellum). Caudal to the scolex is the duct-like invagination segment consisting of a free duct with a digitiform coating lined by anhistic membrane. The four cases with degenerative changes exhibited colloid degeneration of their structure and abundant granular mineralization (Fig. 4).
DiscussionOral lesions due to cysticercosis are very rare, in spite of the high prevalence of neurocysticercosis worldwide. In the largest Latin-American study of oral cysticercosis reported by Delgado et al.,5 the most frequently affected site was the tongue, followed by the buccal mucosa, lower lip, and the upper lip. In that study there were 16 cases retrieved from Peru, Guatemala and Mexico; of these, there were only two pediatric patients, which coincides with the rest of the literature with respect to the fact that the number of pediatric patients is lower as compared to adults affected by this disease. According to our review of the English and Spanish literature, only 31 (25%) out of 124 cases reported to date that presented complete information about age, gender and specific location (including the present series) have occurred in this age group.5–25 Of these, 14 were males and 17 females. Age ranged from 1 to 12 years, with most cases (19) located on the tongue.
The differential diagnosis of oral cysticercosis depends on the location of the lesion. Nodules located on lips and cheeks may be misdiagnosed as mucocele, fibroma, lipoma, or a salivary gland adenoma, while the nodules located within the tongue may simulate fibroma, granular cell myoblastoma or a submucous mesenchymal tumor.5,6,10,12,13,14,17 In most cases pediatric oral cysticercosis has been misdiagnosed clinically as mucocele, as it happened in 5 out of the 6 cases included in the present series, with the exception of a case which showed a 2cm lesion within the cheek, which was clinically diagnosed as lipoma. Mucocele rarely exceeds 1.5cm in larger diameter, and the clinical presentation depends on its depth and the degree of keratinization of the covering mucosa. All these characteristics are similar to those described in our patients; however, cysticercosis has a firmer consistency as compared to mucoceles and the lipomas; thus, this feature must suggest the possibility of a cysticercus. The importance of its recognition lies in that sometimes lesions clinically diagnosed as mucoceles or other apparently benign lesions are surgically excised without histopathological confirmation of the diagnosis. Although cysticercosis has to be confirmed by histopathological examination, some authors suggest that the use of other diagnostic tools such as fine needle aspiration may be also useful. This method has demonstrated the identification of some parts of the parasite in a high percentage of the cases.11,20,26,27,28,29 Laboratory findings in patients with cysticercosis reveal that the Enzyme-Linked Immuno-Sorbent Assay (ELISA) is positive to Cysticercus cellulosae.5
Treatment of oral cysticerci is based on symptoms and depends on the affected anatomical zone. Praziquantel and Albendazole are potent anthelmintic drugs used in the treatment of cysticercosis, replacing Niclosamide, which was the drug of choice for the treatment of the disease for a long period. Drugs should be used especially in cases where surgical treatment is risky or impossible, as in neurocysticercosis. Treatment may be unnecessary in asymptomatic individuals, but most cases are treated when the lesion is clearly evident, interferes with function or if it is traumatized. In the oral tissues, treatment of choice is surgical excision of the lesion as well as to perform a detailed study in each case in order to exclude the presence of the tapeworm in other sites.5,8,20 The six pediatric patients we presented with cysticercosis in oral cavity were asymptomatic, with well defined lesions, mobile, painless and firm in consistency. Such lesions were completely removed without complications, because the surgical site was easily accessible.
This infection could cause neurocysticercosis, which in turn predisposes to cancer, gliomas, astrocytomas and oligodendroglyomas.30–32
ConclusionsWe can state that it is important to consider the diagnosis of cysticercosis in oral solitaire nodular lesions presenting in patients living in endemic areas, since we know that Mexico has a great deal of cases with this disease in other regions of the body. This paper emphasizes the role of the dentist in the detection of a disease that can have more serious involvement, as well as the importance of routine histological examination of every lesion excised from the oral cavity.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Source of fundingNone declared.
Conflict of interestThe authors declare no conflict of interest.