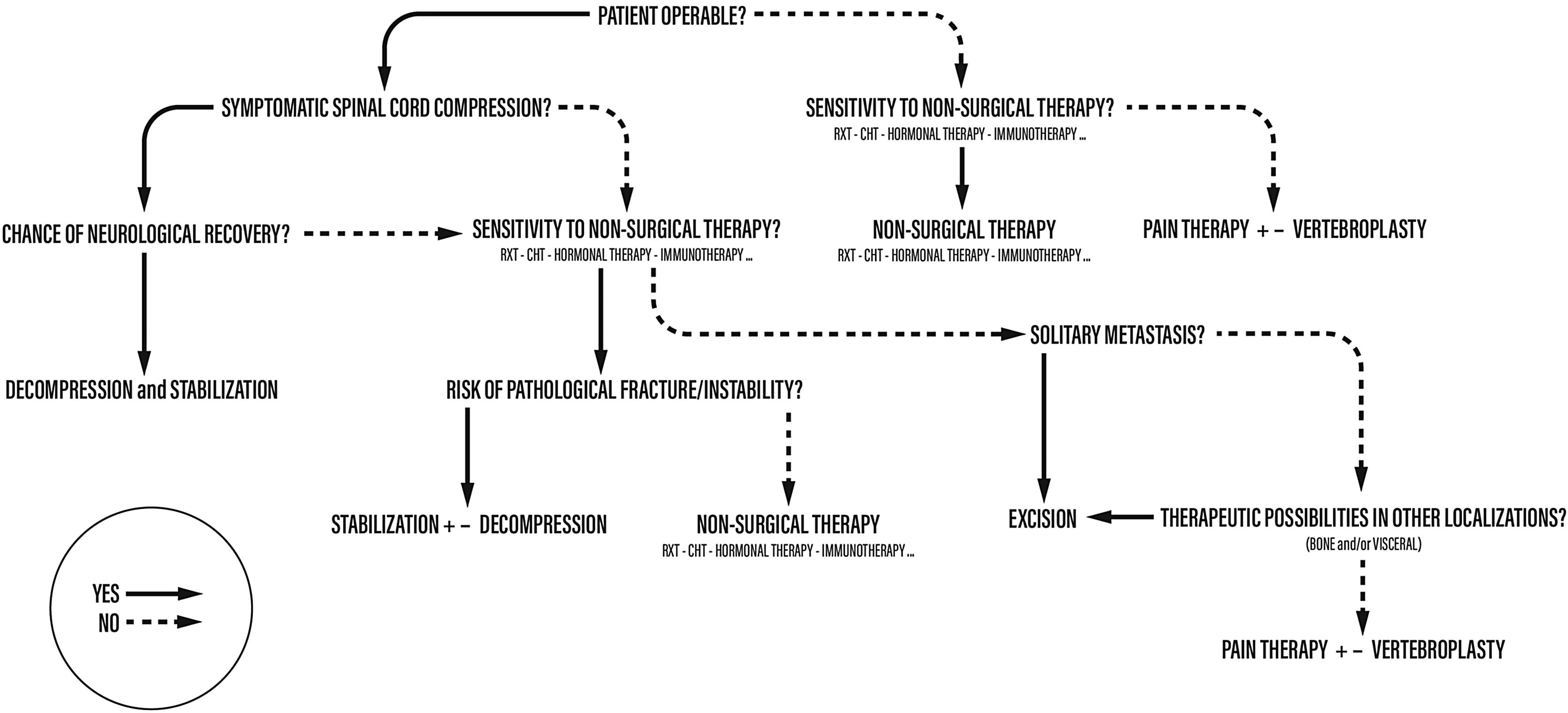
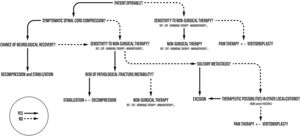
We described an algorithm for the management of spinal metastases in which the importance of single parameters varies depending on when they are contemplated.
Each patient follows his own “personal” sequential process which does not necessarily consider all the parameters each time as some may be irrelevant for the purpose of choosing the type of treatment for that single individual. For instance, a patient in general poor condition with a high “ASA” score is usually not a candidate for surgery, regardless of the primary tumor nature or the number of metastases. For this patient, the most important element would be the sensitivity of the tumor to adjuvant treatment. Similarly, a patient with acute and progressive spinal cord injury would undergo surgical decompression and stabilization without considering a more strenuous intervention.
Describimos un algoritmo para el manejo de las metástasis espinales en el que la importancia de los parámetros individuales varía dependiendo del momento en el que se contemplan.
Cada paciente sigue su propio proceso secuencial «personal» que no necesariamente considera todos los parámetros cada vez, ya que algunos pueden ser irrelevantes para el propósito de elegir el tipo de tratamiento para ese individuo. Por ejemplo, un paciente en mal estado general con una puntuación alta de «ASA» generalmente no es candidato para la cirugía, independientemente de la naturaleza del tumor primario o el número de metástasis. Para este paciente, el elemento más importante sería la sensibilidad del tumor al tratamiento adyuvante. Del mismo modo, un paciente con lesión aguda y progresiva de la médula espinal se sometería a descompresión quirúrgica y estabilización sin considerar una intervención más agresiva.
In this manuscript, we described an algorithm for the management of spinal metastases in which the importance of single parameters varies depending on when they are contemplated (Fig. 1).
The world health organization (WHO) estimates an ascending increase of the incidence of cancer with 29.4 million new cases in 2040.1
The most frequent sites of metastases from carcinoma are lung, liver and bone, and the skeletal segment most frequently involved is the vertebral column.
In fact, it is estimated that more than 10% of tumor patients develop symptomatic spine metastases and that 70% of them have evidence of metastatic disease at the time of death.2 This is due to the rich vascularization of the vertebral bodies. There is evidence that blood from many anatomic sites drains directly into the axial skeleton. In a milestone postmortem study, Batson demonstrated that venous blood from the breast and the pelvis flowed not only to the vena cava but also into the venous plexus extending from the pelvis to the epidural and perivertebral veins.3 This may explain, at least in part, the tendency of the breast, prostate and kidney and lung to produce metastases in the axial skeleton.
The life expectancy of cancer patients has increased significantly over the last few decades and this leads to an increase in the population that can potentially develop skeletal metastases.
The presence of vertebral metastases and their treatment affects not only the quality of life but can also affect life expectancy. To date, there is still no universally accepted treatment line, due to the great variety of histotypes of primary tumors and their method of metastasis. However, what is universally accepted is that bone metastasis is just an element of a systemic disease, therefore patients management require a multidisciplinary approach, involving oncologist and a radiotherapist.
The most frequent clinical presentation of a patient affected by vertebral localizations of disease is pain.4 It is usually a pain that is poorly responsive to most analgesic drugs, and it is often underestimated and attributed to problems of a degenerative nature, delaying the diagnosis and the correct diagnostic and therapeutic classification of the patient. The pathological mechanism that determines pain is usually due to one of the following mechanisms, or a combination of several of them:
cortical bone expansion with periosteal stretching and nociceptor activation;
direct compression exerted by the tumor on the spinal cord or nerve roots.
The progressive replacement of healthy tissue by pathological tissue, which not having the same biomechanical properties of healthy bone, can lead to vertebral instability.
The presence of pathological fractures, which typically manifest with acute onset pain, in the absence of apparent trauma.
Another modality of presentation of vertebral metastases, but less frequent than pain, are symptoms of a neurological nature. These can arise from direct compression on the spinal cord or nerve roots, or compression given by bone fragments or bone deformities arising following a pathological fracture.
The first step in the correct management of the patient with vertebral metastases is to make the correct diagnosis.
Vertebral metastases can have three different presentation patterns: osteolytic (lytic, destructive), osteoblastic (blastic, productive), or mixed. Lytic lesions appear destructive, showing loss of both cancellous bone and cortex, and are usually well circumscribed. Blastic lesions, on the other hand, appear hyperdense and are typically expansive and with poorly defined borders. Mixed lesions have both lytic and blastic features.
Lytic lesions can weaken the structure of the vertebra, resulting in impending fracture, while blast lesions can create neurological problems by exerting compressions on the spinal cord and roots. CT scan allows these lesions to be evaluated and classified as lytic, blast or mixed.
The importance of better assessing the bone quality in metastatic lesions is demonstrated in several studies, which show only a 2.3% risk of pathological fracture when <50% cortical bone is involved, but an 80% risk of pathological fracture when >75% of cortical bone is involved.5
However, the imaging in the diagnosis process is enough for a few pathognomonic lesions (e.g., Osteoid osteoma), the laboratory is helpful for a few others (e.g., multiple myeloma), but histological diagnosis is required for the majority.
It is therefore necessary to obtain a tissue specimen, and in the spine, the technique of choice is through a transpedicular CT-guided biopsy. This is the best option because it is the one with the lowest risk of local dissemination of disease and allows, if indicated, surgical excision of the biopsy tract.
Modern treatments of medical therapy (chemo-, hormonal-, immuno-) and radiotherapy have certainly increased the survival of most patients suffering from solid and hematologic tumors, however they are not often able to effectively control pain and functional impairment. Novel tumor biomarkers and tumor epigenetics along with novel hormonal and immunotherapeutic will perhaps positively skew the survival curves even more given better disease control.
The aim of surgery in the management of spine metastases might be one, or an association, of the following:
- -
Neurological function preservation or recovery from a neurological deficit;
- -
Pain relief;
- -
Spinal stability restoration;
- -
Local control of the tumor.
Even though local control of the tumor is a target of the treatment of metastases, it is not always achieved surgically. In fact, the wide variety of histotypes which may deposit in the spine differs in the sensitivity to non-surgical treatments (such as RT, hormonal therapy, and immunotherapy). Moreover, it is intuitive that the longer the expected survival of the patient is, the greater is the possibility that the disease might relapse (with eventual compression of the spinal cord and/or pathological fracture), thus the differential importance of achieving durable local control.
It is important for the surgeon to be aware of the various options available to achieve local control of the various different histotypes, whether surgical or not.
From our prospective the surgical techniques in spine metastasis can be summarized into (1) decompression and stabilization, (2) intralesional excision (curettage or debulking), and (3) en-bloc resection, these latter two followed by reconstructive procedures (with various techniques). All these operations can be performed by either the anterior, posterior, or combined approaches.6
- 1.
Decompression and stabilization: This is the quickest and least aggressive surgical procedure. It can be performed anteriorly or posteriorly through open or minimally invasive approaches or posteriorly. Combined approaches are not commonly used in this setting. A decompressive laminectomy with or without removal of the epidural tumor is combined with posterior stabilization. The authors believe that it is mandatory to stabilize the spinal column at the same time. This procedure is indicated for patients with short-term prognosis who may have neurologic compromise and/or a pathological fracture. Surgeon familiarity and efficiency with these techniques allows this procedure to be performed in an urgent or emergent setting. Anterior decompression and stabilization is more commonly associated with visceral and vascular complications; thus, fewer centers recommend this approach. Preoperative selective arterial embolization can decrease blood loss associated with vascular tumors like renal cell carcinoma and thyroid carcinoma.
- 2.
Intralesional excision (curettage or debulking): The tumor is directly approached either anteriorly, posteriorly, or circumferentially and removed in a piecemeal fashion in order to achieve circumferential decompression of the spinal cord and decrease tumor burden. This procedure is often performed as part of a multidisciplinary approach and is preceded by selective preoperative arterial embolization for select tumors. This operation is indicated for metastases not sensitive to radiotherapy associated with a pathological fracture or spinal cord compression or when a tumor debulking is recommended to enhance oncological treatments.
- 3.
En-bloc resection: This procedure is most commonly performed for patients with primary malignant bone tumors, and it is occasionally recommended for a patient who has a middle- to long-term prognosis and a solitary metastasis from a tumor that is relatively resistant to chemotherapy and radiotherapy. The operation can be performed by a posterior approach alone or a combined anterior and posterior approach. En-bloc resection is associated with a lower local recurrence rate, but the risk-to-benefit ratio is very high due to the morbidity of these long operations (8–16h). En-bloc resection is also considered in highly vascularized tumors as this type of resection may lead to less blood loss than an intralesional excision. In most of the cases, a spine metastasis with such a relevant encroachment of the canal to provoke a cord symptomatic compression is not suitable to en-bloc resection due to the lack of the surgical criteria to perform such kind of procedure. Adjuvant treatment (i.e., RT, hormonal therapy) may decrease the incidence of local recurrence and distant progression of the tumor.7
In choosing the most appropriate treatment, several elements must be taken into consideration: patient's prognosis, the general conditions of the patient, the histotype of the tumor and its sensitivity to adjuvant treatments, the spread of the disease and the neurological conditions.
In the past, many efforts have been made in the attempt to create prognostic scores that could guide the surgical management. With these systems, each parameter is assigned a score and the sum of these scores indicates the most appropriate treatment.
Tokuhashi et al. reported their “Scoring system for preoperative evaluation of a patient's prognosis with metastatic spinal tumor” in 1989.8 It was based on retrospective data collected from 64 patients with spinal metastases and analyzed to develop a comprehensive scoring system identifying six variables on which assess prognostic stratification. A revised version published in 2005 were the item of “Primary site of cancer” were modified. According to this score, three classes of tumor management are calculated: conservative management (score 0–8), palliative surgery (score 9–11) and excisional surgery (score >12) with predicted survival reported at <6, >6 and >12 month respectively.9
Tomita et al. proposed in 2001 a prognostic score base on three factors: the rate of growth of primary tumor, the number of bone metastases and presence/absence pf visceral metastases. The score of the three components are added together to produce an overall score ranging from 2 to 10 from good to poor prognosis respectively. They did not evaluated the performance status because it was considered a reflection of tumor load.10
Many other score have been developed along the years but none of them showed more than 90% consistency between the predicted and the actual survival time to be used in the clinical practice.11
Factors influencing the incidence of complications and survival after surgical treatment of spinal metastases were analyzed and it was observed that preoperative neurological conditions, the nature of the primary tumor and the extent of spinal involvement, are the main determining factors. The systemic spread of the disease or the age of the patient affect the outcome less.12
Therefore, both the type of patient and the type of surgical treatment proposed must be carefully selected.
In 2004 the main author (AG) proposed an algorithm for the management of spinal metastases in which the importance of single parameters varies depending on when they are contemplated13 (Fig. 1).
Each patient follows his own “personal” sequential process which does not necessarily consider all the parameters each time as some may be irrelevant for the purpose of choosing the type of treatment for that single individual. For instance, a patient in general poor condition with a high “ASA” score is usually not a candidate for surgery, regardless of the primary tumor nature or the number of metastases. For this patient, the most important element would be the sensitivity of the tumor to adjuvant treatment. Similarly, a patient with acute and progressive spinal cord injury would undergo surgical decompression and stabilization without considering a more strenuous intervention.
Therefore, patient is not considered only in terms of disease, reducing the choice of treatment to an oversimplified mathematical score, but the patient is studied as a whole: first considering his general conditions, and only subsequently the elements related to the metastatic disease.
The proposed treatment algorithm starts from the diagnosis of spinal metastases. Subsequently the first step is the anesthesiological evaluation, during which the operability of the patient must be established: if the patient were inoperable (high ASA), non-surgical options would be considered. The next step takes into consideration the histotype and its sensitivity to adjuvant therapies: if the tumor does not respond to any form of treatment, the only option for the patient would become pain therapy.
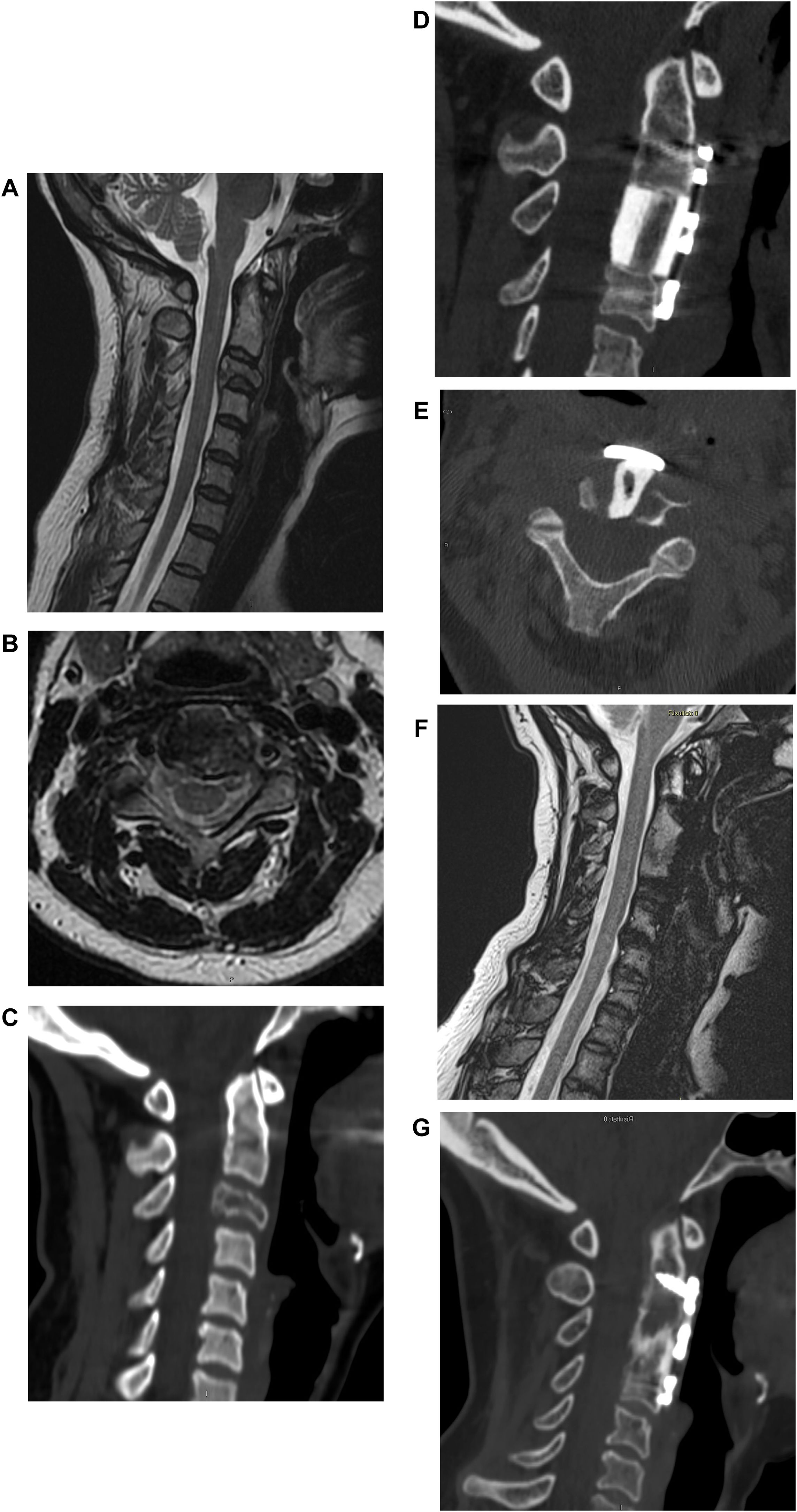
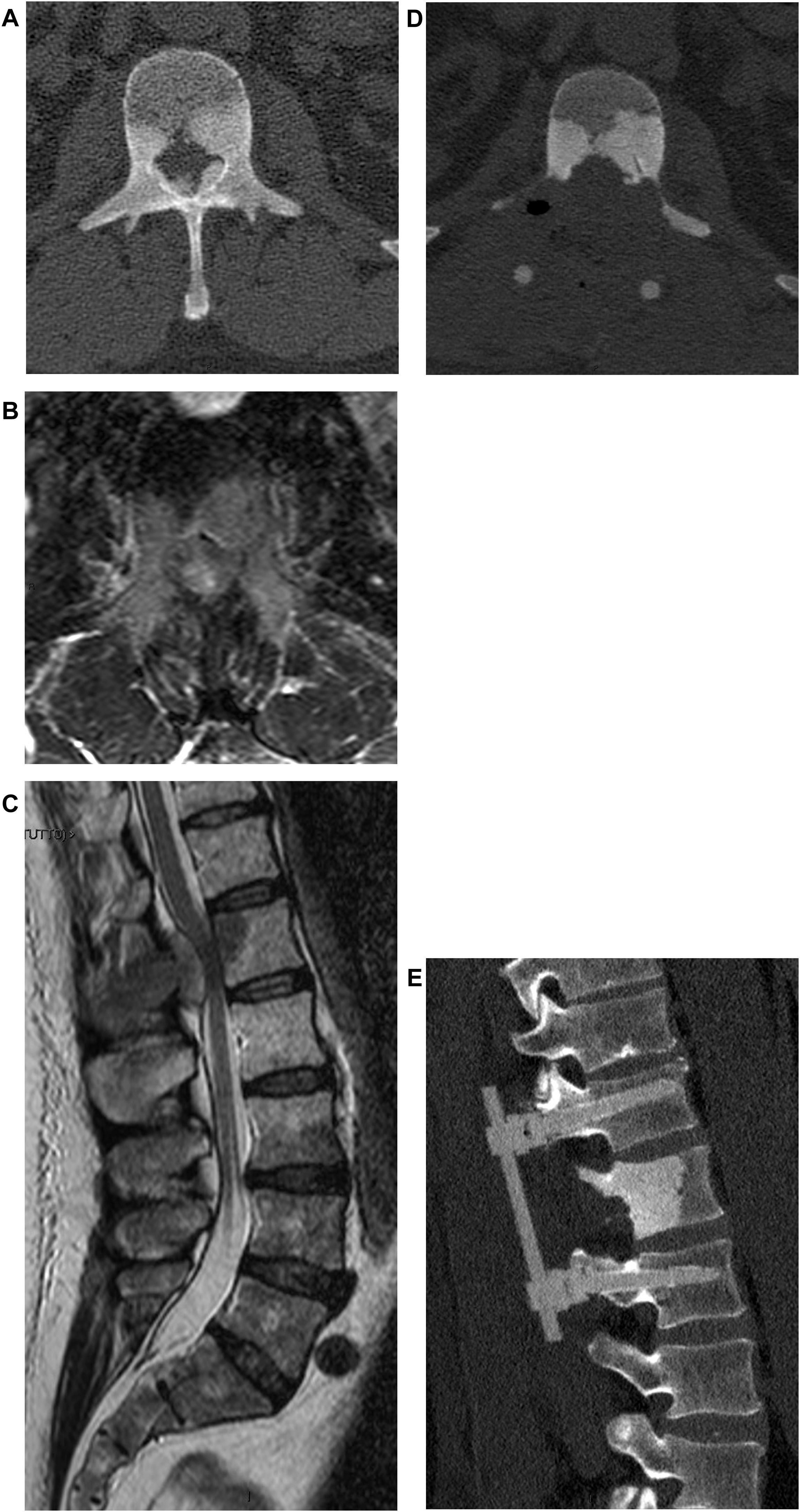
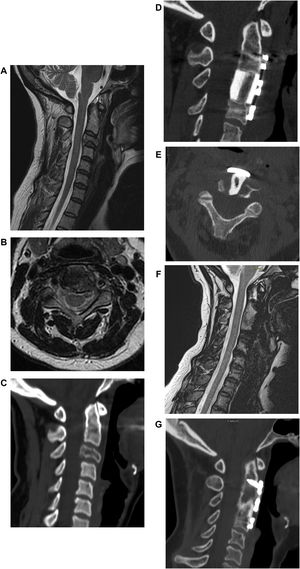
If the patient is judged operable, the extent of spinal cord compression and the severity of the neurological damage are assessed. If a neurological deficit is present, the possibility of recovery is evaluated based on the time since the onset of symptoms (Figs. 2 and 3).
Male, 58 years old. L1 prostate cancer spinal metastasis with epidural spinal cord compression type 2 according to Bilsky classification with impairment of neurological function to the lower limbs. Decompression, debulking and stabilization with carbon fiber reinforced PEEK instrumentation by posterior approach.
If a neurological recovery is excluded at this stage, the sensitivity to adjuvant treatments should be re-evaluated. On the other hand, if the patient has an acute and progressive spinal cord injury, emergency surgery must be performed.
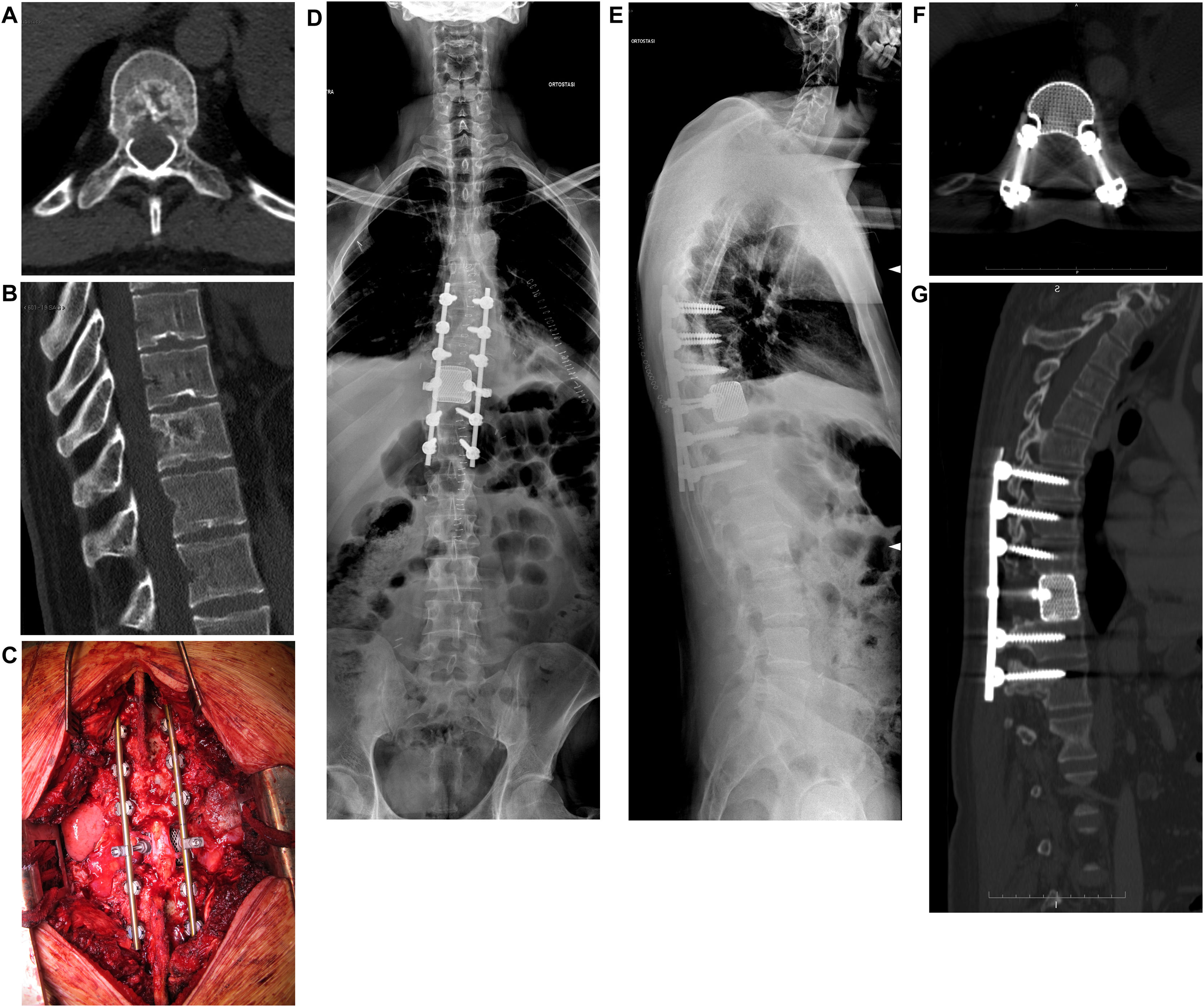
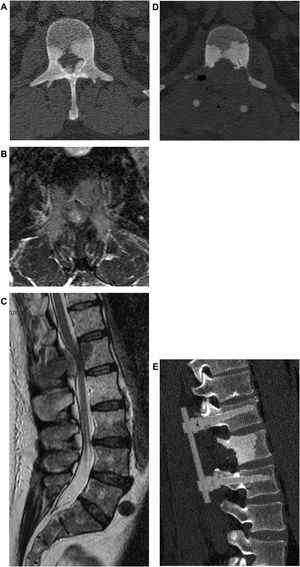
If there is no neurologic deficit or the damage is stable, sensitivity to adjuvant treatments is evaluated. If the tumor is not responsive and an isolated metastasis is present, resection of the lesion may be chosen (Fig. 4). Surgical decompression and stabilization are indicated instead if the metastases are multiple and are treatable. If they are not treatable, only pain therapy will be administered.
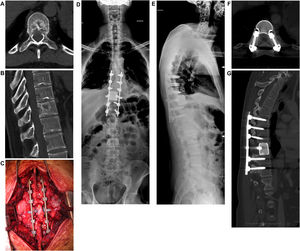
When there is no deficit or the lesion is recoverable, and the tumor is responsive to some form of adjuvant treatment, the pathologic fracture (actual or impending) is evaluated. In fact, this parameter is crucial in guiding the choice toward surgical decompression and stabilization treatment, or adjuvant treatment alone. Tumor resection can be performed en-bloc with a wide margin or by intralesional debulking. In general, en-bloc excision is suggested by the authors in case of isolated metastases of histotypes unresponsive to radio- and chemo-therapy treatments.14
Level of evidenceLevel of evidence iv.
Conflict of interestsThe authors declare they have no conflict of interest.