Spine metastases are a common cause of pain in the oncologic patient which can generate functional limitation, in addition to complications derived from spinal cord compression, radicular compression and fractures. A complex approach to these metastases is required due to the risk of permanent sequelae. With the increase in survival rates due to new emerging treatments, the possibilities of presenting vertebral metastases are increasing, therefore, the management of these lesions should be aimed at pain relief and maintenance of ambulation. Radiotherapy has a fundamental role in the management of these lesions, and technological advances in recent years have made it possible to improve the quality and intentionality of the treatments, going from having a palliative intent to proposing treatments that improve local control. In this article, we describe how the stereotactic body radiotherapy (SBRT) technique, in selected patients, can improve local control and its value in oligometastatic patients and after surgery.
Las metástasis vertebrales son una causa común de dolor en el paciente oncológico lo cual puede generar limitación funcional, además de complicaciones derivadas de una posible compresión medular, radicular y fracturas. Se requiere de un abordaje complejo de estas metástasis por el riesgo de secuelas permanentes. Con el aumento de la supervivencia gracias a los nuevos tratamientos emergentes, las posibilidades de presentar metástasis vertebrales son cada vez mayores, por ende, el manejo de estas lesiones debe ir encaminado al alivio del dolor y el mantenimiento de la deambulación. La radioterapia juega un papel fundamental en el manejo de estas lesiones; disponemos de avances tecnológicos en los últimos años que han permitido mejorar la calidad e intencionalidad de los tratamientos, pasando de ser meramente paliativos a proponer tratamientos que mejoren el control local. En este articulo hacemos una descripción de cómo la técnica de stereotactic body radiotherapy (SBRT) en pacientes seleccionados puede mejorar el control local de forma más duradera, y el valor que tiene en el paciente oligometastásico y tras cirugía.
Spine metastases may present themselves in up to 70% of patients diagnosed with cancer.1
Similarly to bone metastases in any location, vertebral lesions may generate severe pain, functional limitation from the pain and also complications from fractures and radiclar compression or myelopathy from epidural involvement.1,2 It is believed that up to 10% of patients with spine metastases will develop spinal cord compression with the result that treatment approach is much more complicated than bone metastases in other sites, since the risk of permanent incapacitating sequelae is greater.1,2
With advancements in systemic therapy, chemotherapy, immunotherapy, hormone therapy and radiotherapy, patient survival is increasingly greater and this entails a higher possibility of developing metastases over the course of the disease and the need to offer treatments that help to maintain a good quality of life.
The main goal of spine metastases treatment is local control of the disease, pain relief and maintenance of function.3
In general, these metastases have been treated using invasive surgical techniques, with external radiotherapy in palliative doses, or a combination of both.
The treatment of spine metastases requires multi-disciplinary management involving radiation oncologists, spine surgeons, radiologists and medical oncologists.
Historically, external radiotherapy at conventional doses, in the treatment of spine metastases has proven to improve pain control in 60–85% of cases, with a variable durability in keeping with the histology and with better response in breast cancer, prostate cancer and myelomas.4
With the increasing survival of patients with metastatic tumours, the need to achieve improvement in symptoms and function while minimising the risk of adverse effects increases.
Radiotherapy in spine metastasesTraditionally spine metastases have been treated with external radiotherapy at what are considered safe doses and fractionation for spinal cord tolerance. The dose usually used with the purpose of achieving pain relief and local tumour control is divided into 10 fractions of 3Gy, 5 of 4Gy or 1 of 8Gy.
The possibility now exists of administering high, biologically effective doses considered radical, thanks to the ability to adjust the dose and limit the toxic dose at the spinal cord level (Fig. 1).
This technique is known as SBRT (stereotactic body radiotherapy) and is administered between 1 and 5 fractions of >6Gy per fraction.
One of the advantages of SBRT is the improvement of local control over the disease, in addition to an improvement in symptoms. In the spinal column, progression may be associated with neurological morbidity, pain and limited options for adjunct treatments and is therefore an ideal site for treatment with SBRT.
SBRT has been defined as the accurate administration of high-dose, image-guided external radiotherapy, administered in a single fraction or a few fractions, at an extracraneal site, with a dose biologically equivalent to a course of treatment with radical intent administered at standard fractionation (1.8–3.0Gy/fraction).
SBRT has proven to be effective in providing appropriate local control of the disease in combination with surgery or as a single treatment in selected cases. It has therefore become the treatment of choice when complete local ablation of a metastatic tumour is indicated, particularly in patients with oligometastatic cancer.5
When defining whether a patient is suitable for treatment with SBRT, it is important to determine the level of spinal cord involvement, since this affects the possibility or impossibility of being able to use ablative doses that may be prescribed without generating toxicity.
When a patient has spine metastases, we must consider several factors that may help us to decide the most appropriate treatment in each individual situation.
Decision-making will necessarily involve:
- 1.
Patient characteristics: pain, neurological impairment, age, comorbidities, general status, life expectancy and patient preference.
- 2.
Tumour-related factors: histology, molecular profile, systemic disease and therapeutic options.
- 3.
Spine-related factors: vertebral site, presence and degree of epidural involvement, previous treatments (radiotherapy or surgery) and spinal stability.5
The circumstances in which SBRT may be useful are: patients with oligometastatic disease, limited or no epidural disease, stable spine, and patients who have previously received radiotherapy selectively after surgery.
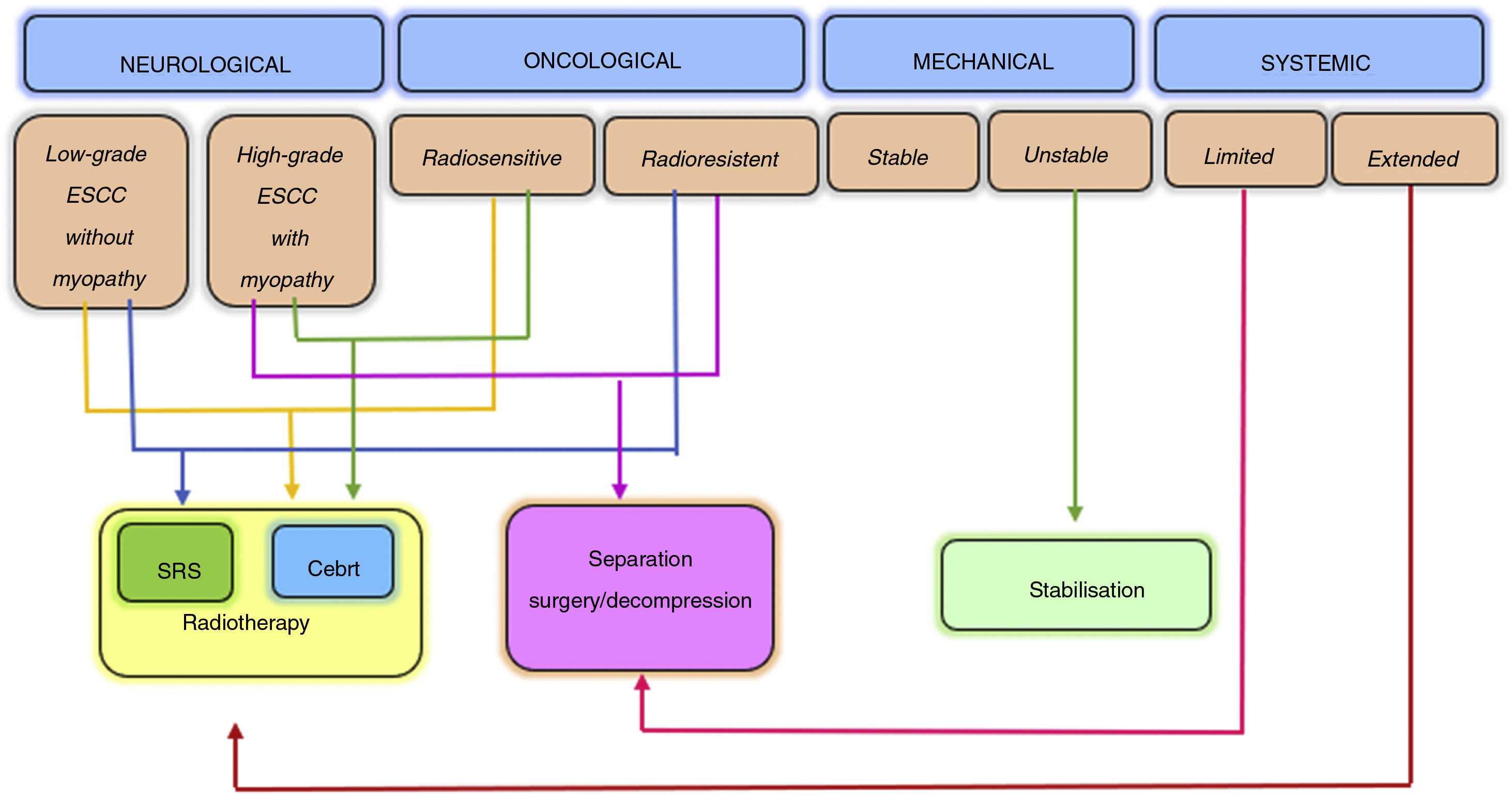
The NOMS decision framework takes into consideration the neurological, oncological, mechanical and systemic characteristics of patients and incorporates the use of radiotherapy, minimally invasive surgery and generally multimodal treatment for spine metastases management. This has been proven to improve local control, pain relief and neurological function recovery or preservation, minimising patient morbidity6 (Fig. 2).
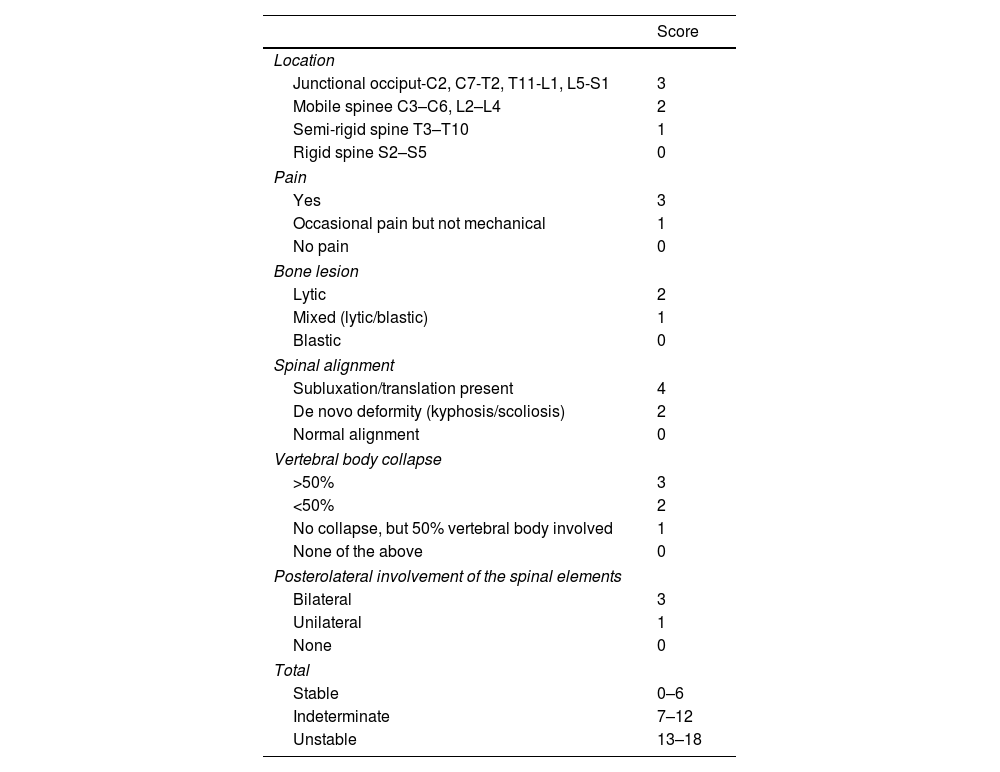
The tools developed by the Spine Oncology Study Group (SOSG) determine spinal stability using the SINS (Spine Instability Neoplastic Score), which includes 6 parameters that predict the need or non need for surgical stabilisation6,7 (Table 1).
SINS (Spine Instability Neoplastic Score) scale.
| Score | |
|---|---|
| Location | |
| Junctional occiput-C2, C7-T2, T11-L1, L5-S1 | 3 |
| Mobile spinee C3–C6, L2–L4 | 2 |
| Semi-rigid spine T3–T10 | 1 |
| Rigid spine S2–S5 | 0 |
| Pain | |
| Yes | 3 |
| Occasional pain but not mechanical | 1 |
| No pain | 0 |
| Bone lesion | |
| Lytic | 2 |
| Mixed (lytic/blastic) | 1 |
| Blastic | 0 |
| Spinal alignment | |
| Subluxation/translation present | 4 |
| De novo deformity (kyphosis/scoliosis) | 2 |
| Normal alignment | 0 |
| Vertebral body collapse | |
| >50% | 3 |
| <50% | 2 |
| No collapse, but 50% vertebral body involved | 1 |
| None of the above | 0 |
| Posterolateral involvement of the spinal elements | |
| Bilateral | 3 |
| Unilateral | 1 |
| None | 0 |
| Total | |
| Stable | 0–6 |
| Indeterminate | 7–12 |
| Unstable | 13–18 |
In general patients with unstable or indeterminate spines (SINS>7 score) should be assessed by a surgical team.6
Another useful tool in decision-making for spine metastases management is the assessment of the degree of spinal cord compression using magnetic resonance.
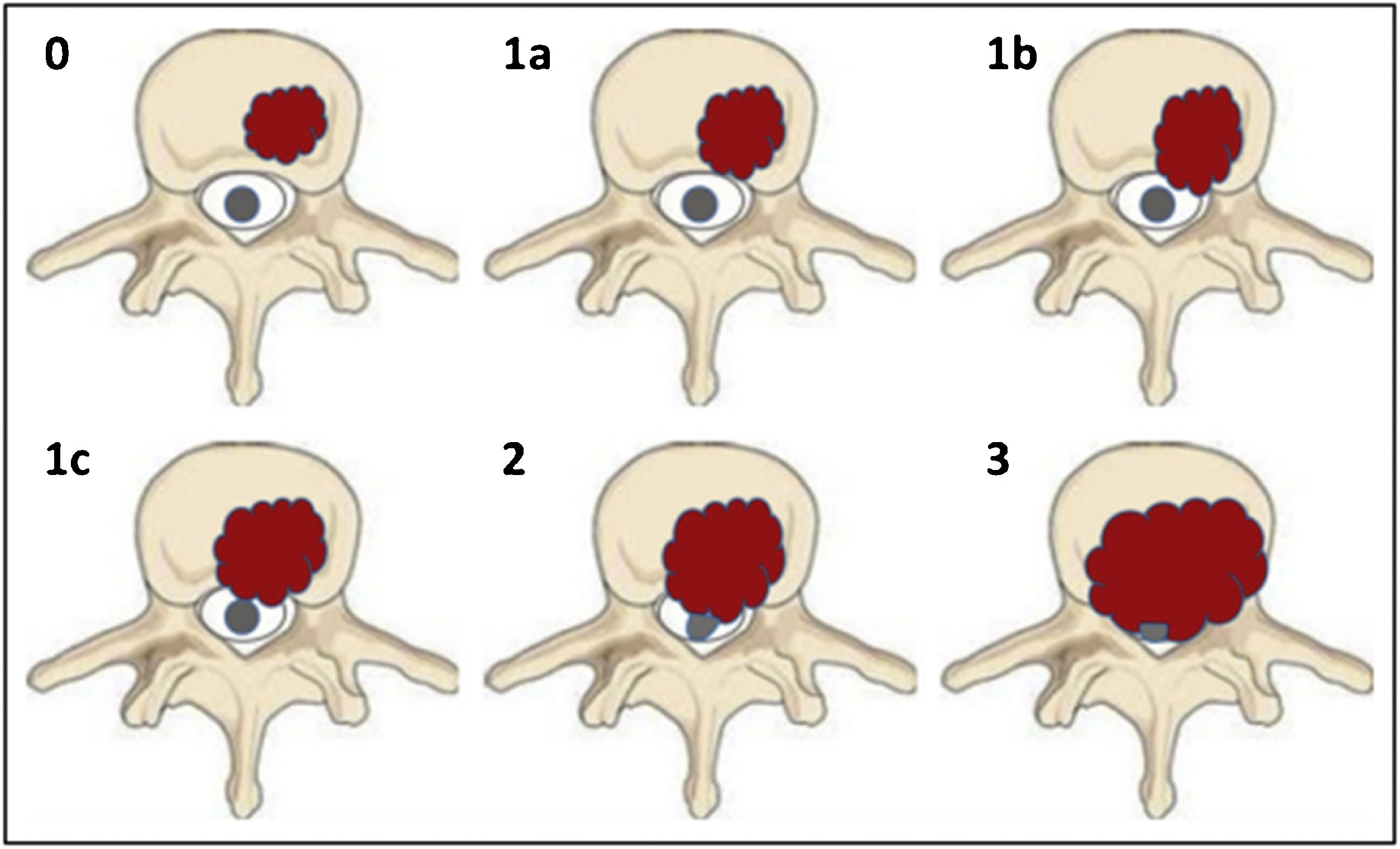
Bilsky et al. classified the ESCC (epidural spinal cord compression) into 4 points8 (Fig. 3):
Grade 0: bone-only tumour.
Grade 1a: tumour with epidural extension, without thecal sac displacement.
Grade 1b: tumour with epidural extension and thecal sac displacement, but without contact with the spinal cord.
Grade 1c: tumour with epidural extension and spinal cord abutment but no displacement.
Grade 2: tumour with spinal cord displacement or compression, without circumferential extension, with cerebrospinal fluid visible around the cord.
Grade 3: tumour with circumferential extension and/or which causes spinal cord compression with no cerebrospinal fluid visible around the cord.8
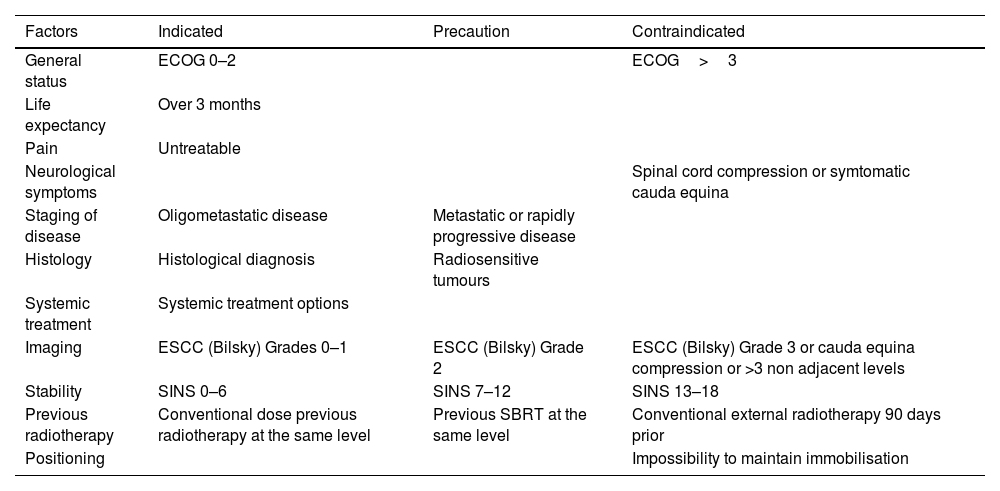
Taking into account the tools previously described, the indications for assessing treatment with SBRT are summarised in Table 2.
Treatment indications with SBRT.
| Factors | Indicated | Precaution | Contraindicated |
|---|---|---|---|
| General status | ECOG 0–2 | ECOG>3 | |
| Life expectancy | Over 3 months | ||
| Pain | Untreatable | ||
| Neurological symptoms | Spinal cord compression or symtomatic cauda equina | ||
| Staging of disease | Oligometastatic disease | Metastatic or rapidly progressive disease | |
| Histology | Histological diagnosis | Radiosensitive tumours | |
| Systemic treatment | Systemic treatment options | ||
| Imaging | ESCC (Bilsky) Grades 0–1 | ESCC (Bilsky) Grade 2 | ESCC (Bilsky) Grade 3 or cauda equina compression or >3 non adjacent levels |
| Stability | SINS 0–6 | SINS 7–12 | SINS 13–18 |
| Previous radiotherapy | Conventional dose previous radiotherapy at the same level | Previous SBRT at the same level | Conventional external radiotherapy 90 days prior |
| Positioning | Impossibility to maintain immobilisation |
ECOG: Eastern Cooperative Oncology Group; ESCC: epidural spinal cord compression; SBRT: stereotactic body radiotherapy; SINS: Spinal Instability Neoplasic Score.
One consideration to take into account is that patients with a Bilsky Compression Grades 2–3 at a single level and with a life expectancy of at least 3 months, could be managed with surgical decompression and subsequent SBRT as long as there is at least 2mm of separation with the spinal cord and the instruments used in the surgery do not interfere with the images to plan radiotherapy treatment.9
SBRT planningFor accurate performance of a vertebral SBRT, an adequate immobilisation of the patient is required that allows interfraction reproducibility, minimising uncertainty and possible toxicities. In general, rigid but comfortable immobilisation systems are used, such as vacuum mattresses. If the location is in the cervical spine, thermoplastic masks are used.
For the outlining of the different structures, especially the spinal cord, and the contouring of the target volume, it is imperative to perform and merge magnetic resonance images with T1–T2 sequences that include at least one vertebral body above and below the area to be treated.
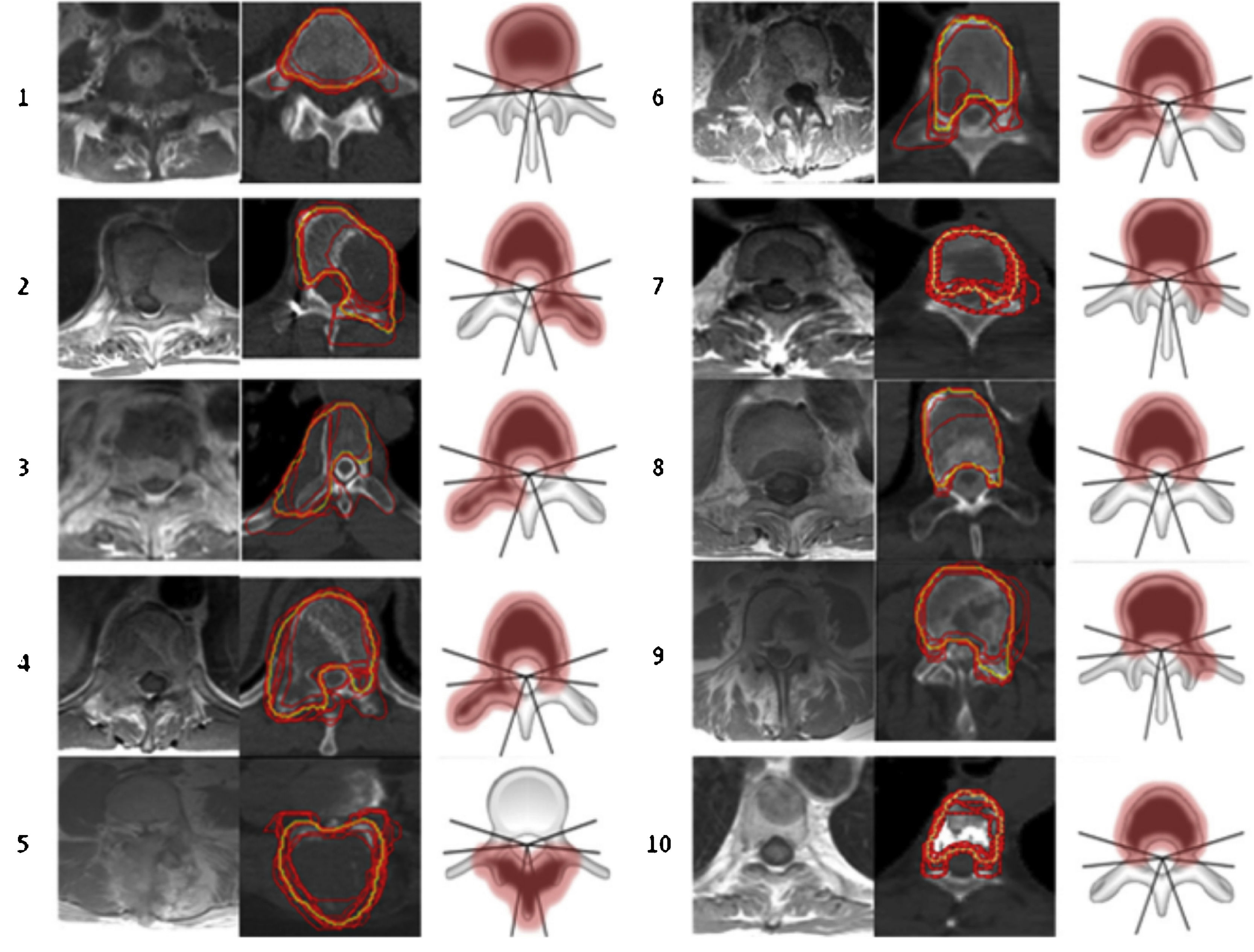
For outlining SBRT treatment volumes the “International Spine Radiosurgery Consortium” (Fig. 4) guidelines are used.10
According to this international consensus, 3 types of volume are outlined: GTV (Gross Tumour Volume) which includes the tumour lesion visible both in the CT and in the fusion with the resonance image. The CTV (Clinical Target Volume) covers areas of potential microscopic extension and a PTV (Planning Target Volume) is the margin of the CTV that is usually less than 3mm, depending on the location of the lesion and the proximity to the spinal cord. Additionally, to avoid uncertainties in both positioning and intrafraction movement, a safety volume of 2–3mm is contoured around the spinal cord (Fig. 5).
Evidence in favour of SBRTMajor retrospective studies have demonstrated the efficacy of spinal SBRT with lasting local control, progression-free survival and palliation rates in approximately 70–95% of patients who received treatment for the first time, in repeated radiation or as adjuvant treatment after surgical resection.10
Different treatment regimes with SBRT exist, which include 18–24Gy in a single fraction, 24Gy in 2 fractions, 24–30Gy in 3 fractions, 30Gy in 4 fractions and 30–40Gy in 5 fractions. The dose is determined by location, tumour size, risk of spinal fracture (this may reach 39% in a single session),11 the proximity of the spinal cord to the volume to be treated, and the experience or preferences of each hospital in guaranteeing interfraction treatment reproducibility.
“Pain flare” has been described as an SBRT-associated effect. This consisted in a transitory increase in pain, during and on finalising treatment. Its incidence is 14–68% of patients and it can be controlled by administrating dexametasone as preventative treatment. Spinal compression fractures may appear in 11–39% of cases. In retrospective analysis lytic lesions have been identified as risk factors, compromise of >50% of the vertebral body, having a baseline fracture and a poorly aligned spine.11 In general, an intermediate/high SINS entails greater risk and these patients may benefit from minimally invasive surgical techniques to stabilise the spine prior to SBRT treatment.11
SBRT-associated myelopathy is a rare complication that may present in .4% of cases, and is conditioned by the maximum dose the spinal cord receives, in accordance with the treatment scheme used.12
Spinal cord tolerance of 1, 3 or 5 fractions is well described in the literature. The safe doses established for the spinal cord in a treatment with SBRT are 12.4Gy, 20.3Gy and 25.3Gy respectively, with a risk of myelopathy under 1%.12
For re-irradiations there is also information that allows us to make therapeutic decisions avoiding spinal cord damage related to SBRT. However, several factors must be taken into account, the initial dose received and the fractionation, the volume to be treated in the second treatment, the maximum dose accumulated in the spinal cord and the time elapsed since the first treatment. With this it is possible to define if reirradiation should be performed with conventional fractionation or if it is possible to do it with SBRT.
A randomised phase II–III study (SC24) has recently been published, conducted in Australia and Canada, in which the use of SBRT (2 sessions of 12Gy) is compared with external radiotherapy at conventional palliative doses (20Gy in 5 fractions), for pain control in spine metastases.13 This study included patients with painful vertebral metastases confirmed by MRI, with a maximum of 3 consecutive affected vertebrae, and without spinal instability or spinal cord compression. At 3 months of treatment, 35% of patients treated with SBRT had a complete response to pain, compared to 14% of patients treated with conventional radiotherapy. As adverse effects, Grade 3 pain occurred in 4% of patients and Grade 1 vertebral fracture in 11% treated with SBRT, compared to 5% and 17% of patients treated with conventional radiotherapy, without any deaths. Therefore, these results indicate that the use of SBRT has better results in a palliative setting, without producing relevant adverse effects.13
In a systematic review carried out in 2020, it was concluded that vertebral SBRT achieves local control rates of 90% one year after treatment in de novo vertebral metastases, higher than 80% in the context of adjuvant radiotherapy after surgery and higher than 65% in cases of reirradiation. The most common adverse effect was vertebral fractures in 10–15% of patients.14
A randomised phase 2/3 trial (RTOG0631) assessed pain control at 3 months, with a 16Gy treatment regimen in one fraction compared to 8Gy in one fraction, and although the phase 2 study found that the fraction A single dose of 16Gy/1 was safe, preliminary phase 3 results reported at ASTRO 2019 (American Society for Radiation Oncology) did not show any improvement in pain control at 3 or 6 months after treatment. Although pain control at 3 months is considered to be an important endpoint, the aforementioned data sets indicate that SBRT is more likely to provide local and pain control over time.15
In 2018, the results of a phase II study were published, randomizing 55 patients with de novo vertebral metastases to receive 30Gy in 10 fractions vs. 24Gy in one fraction of SBRT. Results showed 3-month complete pain response rates of 43.5% with SBRT vs. 17.4% with conventional fractionation, being even higher at 6 months (52.6 vs. 10%) without G3 toxicity in the SBRT group, nor cases of myelopathy in any of the groups.16
Postoperative SBRTThe value of surgery in spine metastases has potential advantages, since it can provide decompression of neural structures, spine stability, and is useful for providing diagnosis with neurological symptomatic relief.17
Different surgical approaches are used to treat spine metastases, and the main objective is to resect the largest possible tumour volume. However, surgery alone cannot completely eliminate the disease, usually requiring complementary treatment with radiotherapy.17
A recent concept for the management of spine metastases is “separation surgery”, the concept of which is to restore the anatomical distance between the tumour and the spinal cord, through microsurgical techniques that allow decompression of the medullary canal, and achieve a distance between the tumour and the bone marrow of at least 2–3mm, which allows the administration of high doses of radiation, minimising possible bone marrow toxicity.17
Several studies have demonstrated the value of radiotherapy after surgery in patients with spinal cord compression. More recently, the North American AOSpine multicenter study confirmed that, in patients with symptomatic spinal cord compression, surgical intervention provided immediate and sustained improvement in postoperative ambulatory status, quality of life, and pain score.18
Surgery is also indicated for mechanical instability, as the pain caused by instability is not effectively alleviated by radiation therapy alone.
A systematic review published in 2021 found a one-year local control rate of 70–95%.18 Some of these studies describe predictive factors of response, such as residual epidural disease after surgery, the grade of compression (Bilsky) at diagnosis, the dose of SBRT (18–26Gy in a single session vs. 18–40 in 3–5 fractions), certain histologies such as sarcomas and high tumour volume, pain control at 3–6 months, the Karnofsky index and the possibility of receiving systemic treatment. The results show a low toxicity profile, with a risk of vertebral compression fracture of 5.6%.18
Indications for postoperative SBRT are:
- •
Patients with oligometastatic disease and/or with long survival.
- •
Patients with large tumour volume with radio-resistant histologies such as sarcomas, renal and colon carcinoma, and melanoma.
- •
Patients with prior external radiotherapy, susceptible to resection.
The doses of SBRT can be variable, but it has been seen that higher doses achieve better local control: 18–26Gy in 1–2 fractions or 27Gy in 3 fractions achieve more local control of the disease with respect to 24–30Gy in 5 fractions. Local control rates are 84–95% in different series.5
The material used in surgery is important for treatment planning and volume demarcation: materials with carbon fibber reduce artefact and reduce uncertainty in dosimetry.18
The recommended times to administer the treatment should not exceed 4 weeks after surgery. One week is recommended for minimally invasive procedures and 2 weeks after open surgery.18
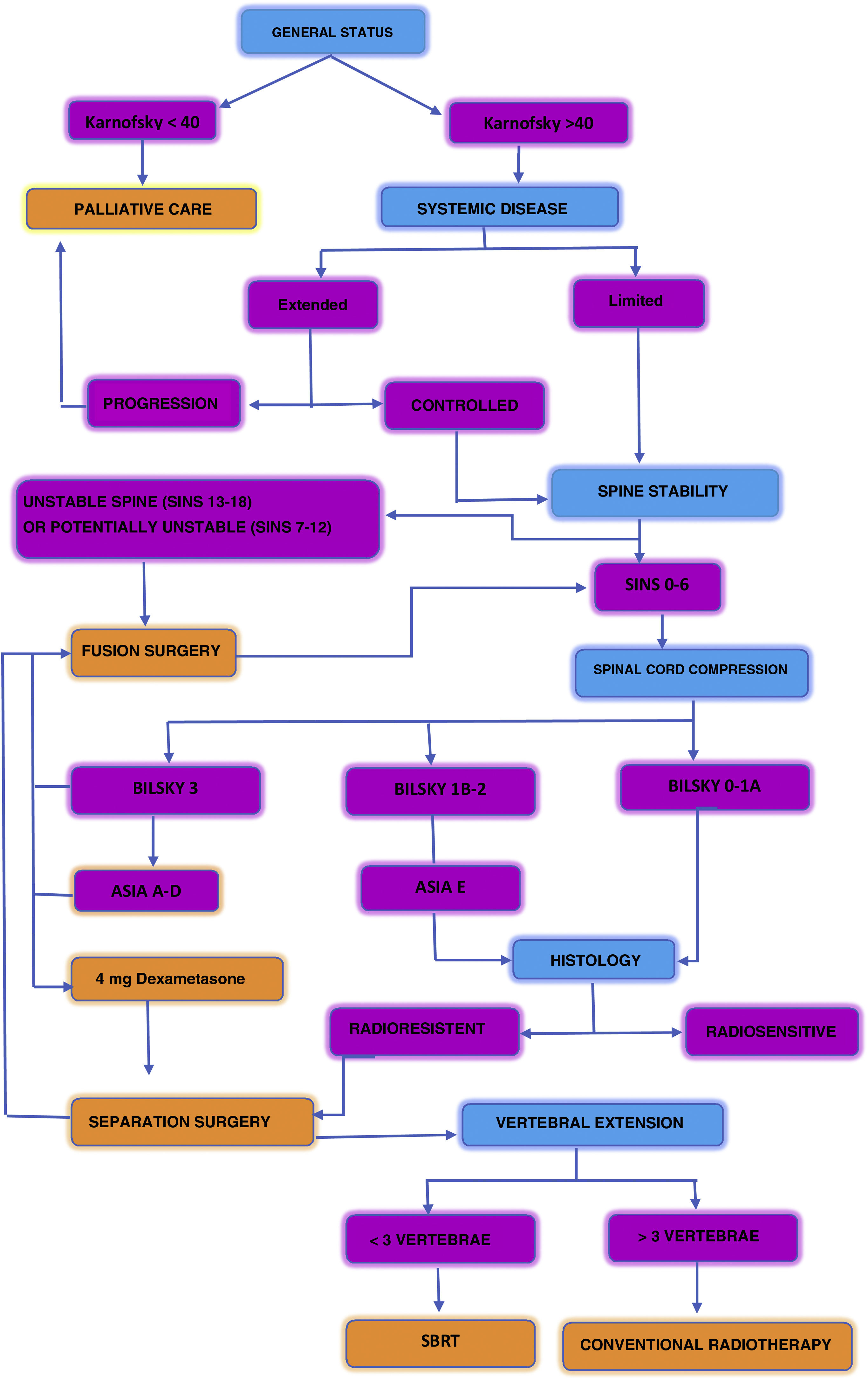
Fig. 6 describes a decision algorithm proposed in the management of patients with spinal metastases.17
Algorithm of decisions proposed in the management of patients with spine matastases.
The main advantage of proton therapy over photon-based radiotherapy is a better dose distribution, with high conformality and dose gradient, while decreasing the integral dose to healthy tissues, thanks to the physical properties of protons.19
Generally speaking, proton therapy is recommended for reducing possible side effects relating to radiotherapy, in tumours with a good prognosis or for does scaling aimed at increasing local control. In chordoma and spinal chondrosarcoma, proton therapy is considered standard in the postoperative setting or for unresectable disease. Clinical trials have shown that proton therapy allows the treatment dose to be increased without complications, despite the proximity to organs at risk such as the bone marrow.20–22
Due to its physical properties and the clinical experience gained in the treatment of spinal sarcomas, proton therapy could also be of benefit in the treatment of spine metastases.
There are no clinical trials concerning the treatment of spine metastases, other than in a palliative setting. Given the current context of limited access to proton therapy, spine metastases are not considered within the current indications. However, despite the lack of clinical data, proton therapy could be considered in selected patients with oligometastatic disease with a good prognosis, or in patients who have received previous radiotherapy treatments close to the primary tumour.
In comparison to the photon-based technique, protons are used in a hypofractionated scheme. In a comparative planning study between SBRT with IMRT (Intensity Modulated Radiation Therapy) vs. particle therapy (Carbon Ion Therapy and Protons), Rief et al. demonstrated a benefit of particle therapy in spinal cord preservation, with no significant difference in volume coverage. For other organs such as the lung or intestine, the dose was also reduced, but the advantage was smaller, bearing in mind that the dose administered with IMRT was already within the tolerance of normal tissues.23
In postoperative treatment cases, caution should be exercised in the case of fixation materials. The presence of metallic material near the tumour may affect planning accuracy due to artefacts in CT planning and dosimetry.24
Because of these uncertainties, preoperative proton radiotherapy is preferred whenever possible. If surgery is performed first, the use of common metallic artefact reduction algorithms, the use of dual-energy CT to improve image quality, facilitate contouring, and reduce dose calculation uncertainties is recommended. The integration of implant composition into the dose calculation is also required. Ballistic trajectories that avoid the passage of the radiation beam through the metallic material are preferred.25 Non-metallic implants such as carbon fibre reinforced polyetheretheretherketone are a promising material to overcome these difficulties.26
ConclusionsTreatment of spine metastases is complex and requires a multi-disciplinary approach with consideration of different factors to achieve the best clinical outcome for the patient.
SBRT is an effective and safe treatment for patients with spine metastases, with the ability to administer ablative doses that do not affect healthy organs, within a short treatment period. The main challenge is the patient selection criteria, since not all patients are SBRT candidates. The benefit in oligometastic patients, with localised tumours and a stable spine has been proven, both for pain symptom control and local disease control.
Spine surgeons play an essential role in many of these patients, since through minimally invasive surgical techniques combined treatment may be administered which improve the oncological outcome of these patients.
Level of evidenceLevel of evidence i.
Conflict of interestsThe authors have no conflict of interests to declare.