To evaluate the clinical and radiological results of a series of patients with a glenoid bone defect treated by reverse total shoulder arthroplasty associated with a bone graft stabilised with a trabecular titanium glenoid component (Axioma SMR Lima®).
Material and methodsRetrospective descriptive study of 16 consecutive patients with an average age of 68.2 years. In 13 cases they were primary arthroplasties and in 3 revision ones. The data included in the study were obtained by reviewing the clinical history. The glenoid defect was classified according to Gupta et al. The pre- and postoperative clinical assessment included the score on the visual analogue pain scale (VAS), the result of the Constant score and the active joint balance. Radiographically, the integration of the bone graft and the fixation of the components were assessed.
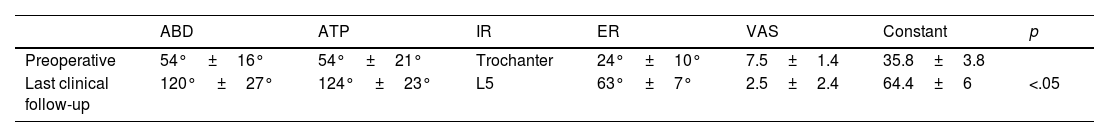
ResultsThe average follow-up was 42.1 months. The average VAS score improved from 7.5 preoperative points to 2.5 points in the last control (p=.006) and on the Constant score from 35.8 pre-surgical points to 64.4 points (p=.001). The average joint balance went from 54° of abduction, 54° of antepulsion, 24° of external rotation and internal rotation to preoperative trochanter to 120° of abduction (p=.001), 124° of antepulsion (p=.001), 63° of external rotation (p=0.001) and internal rotation at L5 in the last clinical control. In all patients, graft integration and the absence of component loosening were observed. The incidence of complications was 6.2%.
DiscussionThe treatment of glenoid defects by reverse total shoulder arthroplasty and a bone graft stabilised by trabecular titanium metaglene presents good clinical and radiological results and a low rate of short-term complications.
Evaluar los resultados clínicos y radiológicos de una serie de pacientes con defecto óseo glenoideo tratados mediante artroplastia total inversa de hombro asociada a injerto óseo estabilizado mediante componente glenoideo de titanio trabecular (Axioma SMR Lima®).
Material y métodosEstudio descriptivo retrospectivo de 16 pacientes consecutivos con una edad media de 68,2 años. En 13 casos se trataba de artroplastias primarias y en 3, de revisión. Los datos incluidos en el estudio se obtuvieron mediante revisión de la historia clínica. El defecto glenoideo se clasificó de acuerdo con Gupta et al. La valoración clínica pre- y postoperatoria incluyó la puntuación en la escala visual analógica de dolor (EVA), el resultado de la escala Constant y el balance articular activo. Radiográficamente se valoró la integración del injerto óseo y la fijación de los componentes.
ResultadosEl seguimiento medio fue de 42,1 meses. La puntuación media en la escala EVA mejoró de 7,5 puntos preoperatorios a 2,5 puntos en el último control (p=0,006) y en la escala de Constant de 35,8 puntos prequirúrgicos a 64,4 puntos (p=0,001). El balance articular activo pasó de 54° de abducción, 54° de antepulsión, 24° de rotación externa y rotación interna a trocánter preoperatorios a 120° abducción (p=0,001), 124° antepulsión (p=0,001), 63° rotación externa (p=0,001) y rotación interna a L5 en el último control clínico. En todos los pacientes se objetivó integración del injerto y la ausencia de aflojamiento de los componentes. La incidencia de complicaciones fue del 6,2%.
DiscusiónEl tratamiento de defectos glenoideos mediante artroplastia inversa e injerto óseo estabilizado mediante metaglena de titanio trabecular presenta buenos resultados clínicos, radiológicos y una baja tasa de complicaciones a corto plazo.
The number of patients undergoing shoulder arthroplasty has increased significantly in recent years, mainly since the introduction of reverse prostheses.1,2 Surgical reconstruction of the glenoid cavity is the main challenge in arthroplasty in many cases, especially those with bone stock deficit, dysplastic glenoid, or high retroversion.3 Restoration of the joint line is essential to obtain correct deltoid tension to maximise function and decrease the risk of instability. Moreover, it decreases the risk of glenohumeral impingement and the development of long-term notching. This must be achieved without compromising the primary fixation of the glenoid component, as micromovement at the bone-implant interface is associated with premature loosening and the need for implant revision.4
There are different options for reconstructing a glenoid defect. These include eccentric reaming, valid in patients with bone defects or slight version abnormalities, reconstruction with bone graft, glenoid components with metal augments, either concentric or wedge-shaped, and finally, custom components manufactured using three-dimensional printing.1
The aim of the present study was to evaluate the clinical and radiological results of reverse shoulder arthroplasty combined with a bone graft stabilised with a glenoid baseplate with a central trabecular titanium peg in a series of patients with a glenoid bone defect.
Material and methodsDemographic dataWe conducted a retrospective descriptive study of 16 consecutive patients who underwent reverse shoulder arthroplasty combined with glenoid bone graft between June 2015 and September 2018. Consent was obtained from the patients and approval was obtained from our centre's ethics committee (Registration 17/2021) before the start of the study.
Thirteen women (81%) and three men (19%) were included, with a mean age of 68.2 years (range 58–80 years). In terms of aetiology, 13 cases (81%) were primary arthroplasty and 3 (19%) were revision arthroplasty.
The patient data are shown in Table 1.
Demographic data of the patients in the series.
| N | Age | Sex | Aetiology | Glenoid defect | Type of graft | Stabilisation method | Size and length of peg |
|---|---|---|---|---|---|---|---|
| 1 | 59 | H | Revision of reverse prosthesis | C3 | Distal femur allograft | Glenoid baseplate | S (10mm) |
| 2 | 65 | M | Proximal humerus fracture sequelae | C3 | Humeral head autograft | Glenoid baseplate | M (17mm) |
| 3 | 58 | M | Inveterate glenohumeral dislocation | E3A | Humeral head autograft | Glenoid baseplate | XL (29mm) |
| 4 | 66 | M | Inveterate glenohumeral dislocation | E2A | Humeral head autograft | Glenoid baseplate and two cannulated screws | M (17mm) |
| 5 | 60 | M | Proximal humerus fracture sequelae | E3A | Allograft of distal femur | Glenoid baseplate | XL (29mm) |
| 6 | 67 | H | Rotator cuff arthropathy | E3P | Humeral head autograft | Glenoid baseplate | M (17mm) |
| 7 | 76 | M | Revision hemiarthroplasty | C2 | Allograft of distal femur | Glenoid baseplate | S (10mm) |
| 8 | 69 | M | Rotator cuff arthropathy | C2 | Humeral head autograft | Glenoid baseplate | S (10mm) |
| 9 | 78 | M | Proximal humerus and glenoid fracture | E3A | Humeral head autograft | Glenoid baseplate | XL (29mm) |
| 10 | 76 | M | Omarthrosis | E3P | Humeral head autograft | Glenoid baseplate | M (17mm) |
| 11 | 67 | M | Total prosthesis surface revision | C2 | Allograft of distal femur | Glenoid baseplate | S (10mm) |
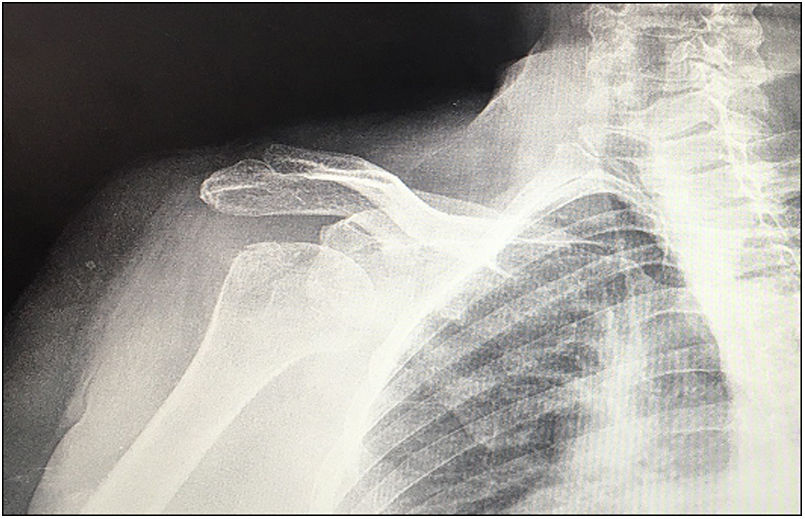
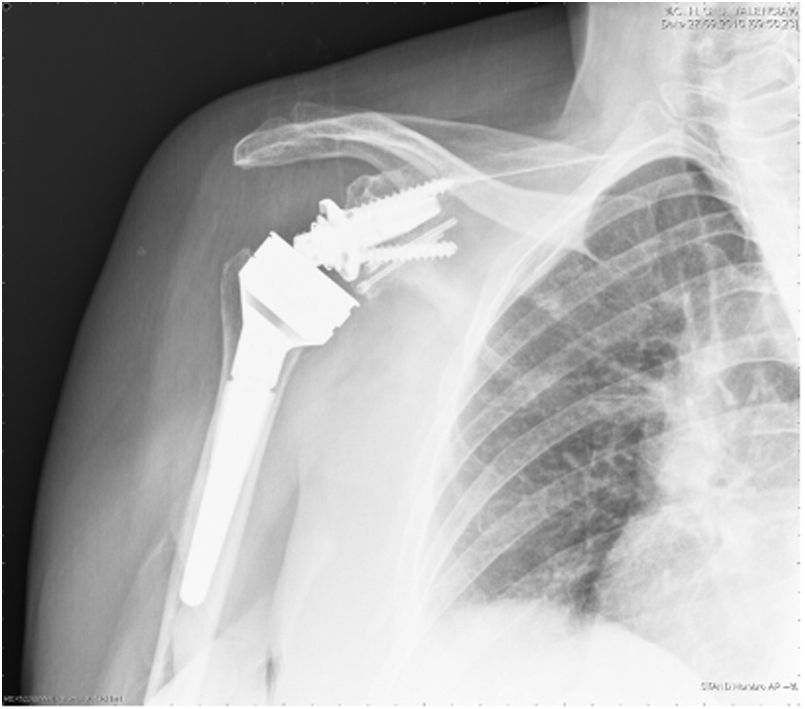
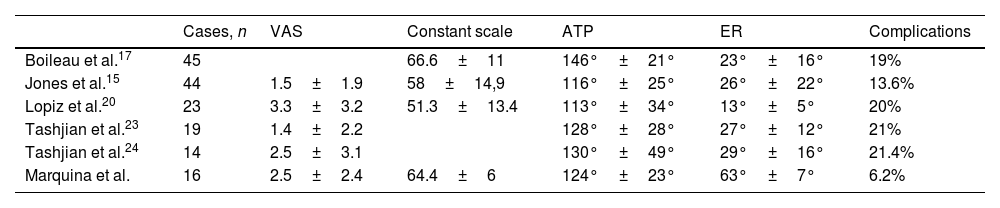
| 12 | 68 | M | Inveterate glenohumeral dislocation (Fig. 1) | E2A | Humeral head autograft | Glenoid baseplate and two cannulated screws | XL (29mm) |
| 13 | 70 | M | Inveterate glenohumeral dislocation | E3A | Humeral head autograft | Glenoid baseplate | XL (29mm) |
| 14 | 62 | H | Omarthrosis | E3P | Humeral head autograft | Glenoid baseplate | XL (29mm) |
| 15 | 70 | M | Rotator cuff arthropathy | E3P | Humeral head autograft | Glenoid baseplate | XL (29mm) |
| 16 | 80 | M | Proximal humerus fracture sequelae | E3A | Humeral head autograft | Glenoid baseplate | XL (29mm) |
The glenoid bone defect was classified according to Seebauer's method.2 Defects are classified as centric (C) and eccentric (E). In turn, centric defects are subclassified into four types: C1 (with a depth<50% of the anteroposterior glenoid diameter), C2 (with a depth>50% and stable vault), C3 (cavitary defect), and C4 (if there is significant destruction of the glenoid and vault). Eccentric defects are subclassified based on the size and primary location of the defect. There are four grades according to the size: E1 (if the defect is small), E2 (defect<30% of bone stock), E3 (defect between 30% and 60% of bone stock), and E4 (defect>60% bone stock). Depending on the location, defects are anterior (A), superior (S), inferior (I), and posterior (P).
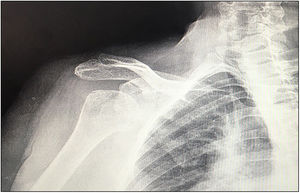
In our study we found 3 cases that were type C2 (18.7%), 2 cases C3 (12.5%), 2 cases E2A (12.5%), 5 cases E3A (31.2%), and 4 cases E3P (25%) (Fig. 1).
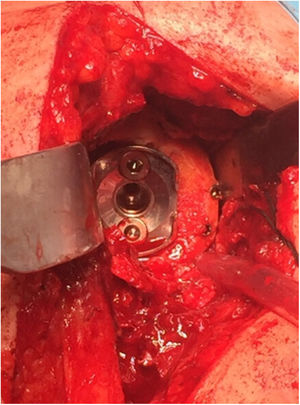
Surgical procedureThe surgery was performed using a deltopectoral approach with the patient in a beach-chair position. Preoperative antibiotic prophylaxis was given in all cases with 2g intravenous cefazolin. We used the SMR reverse total shoulder arthroplasty (Lima Corporate, San Daniele del Friuli, Italy). The glenoid component of this prosthesis has two central peg options: the first is made of hydroxyapatite-coated porous titanium alloy and is intended for primary arthroplasty; the second, used in all cases in this series, is made of trabecular titanium and has different lengths, ranging from 10.5 to 29.6mm (Fig. 2). The aim of the latter is to maximise osseointegration while allowing a bone graft of variable thickness. It is therefore indicated in patients with poor bone quality, revision arthroplasty, or in cases with glenoid defect.
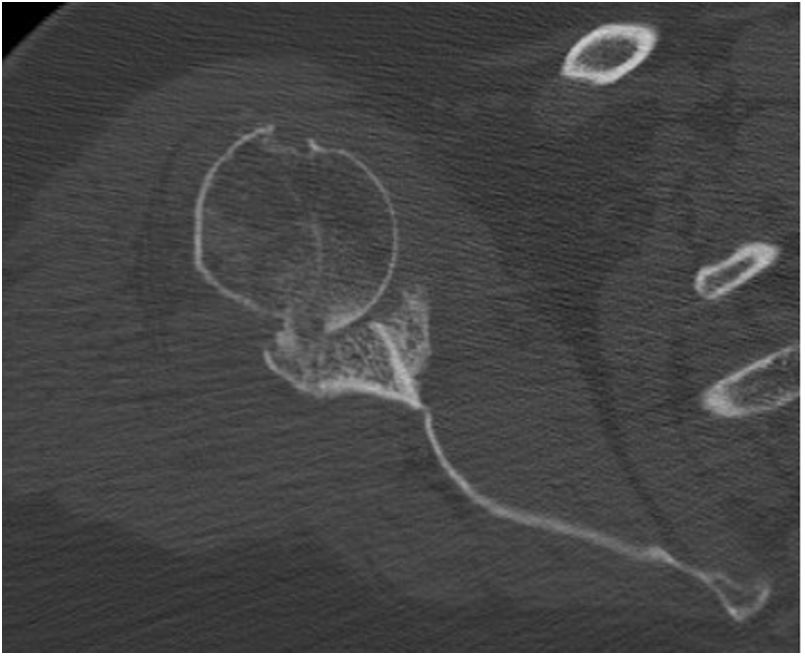
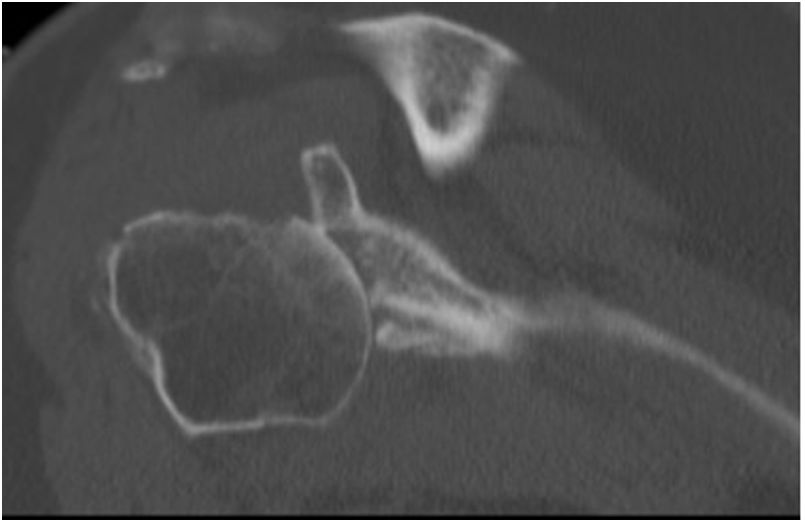
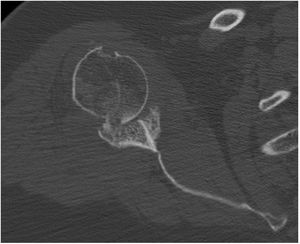
Preoperative planning was performed using computed tomography (CT), determining the optimal position and angulation of the central peg to obtain maximum contact with the host bone. CT was also used to determine the thickness of the graft required and the need, or otherwise, to correct the glenoid version by carving a wedge-shaped graft measured over the least eroded ridge of the residual glenoid (Figs. 3 and 4).
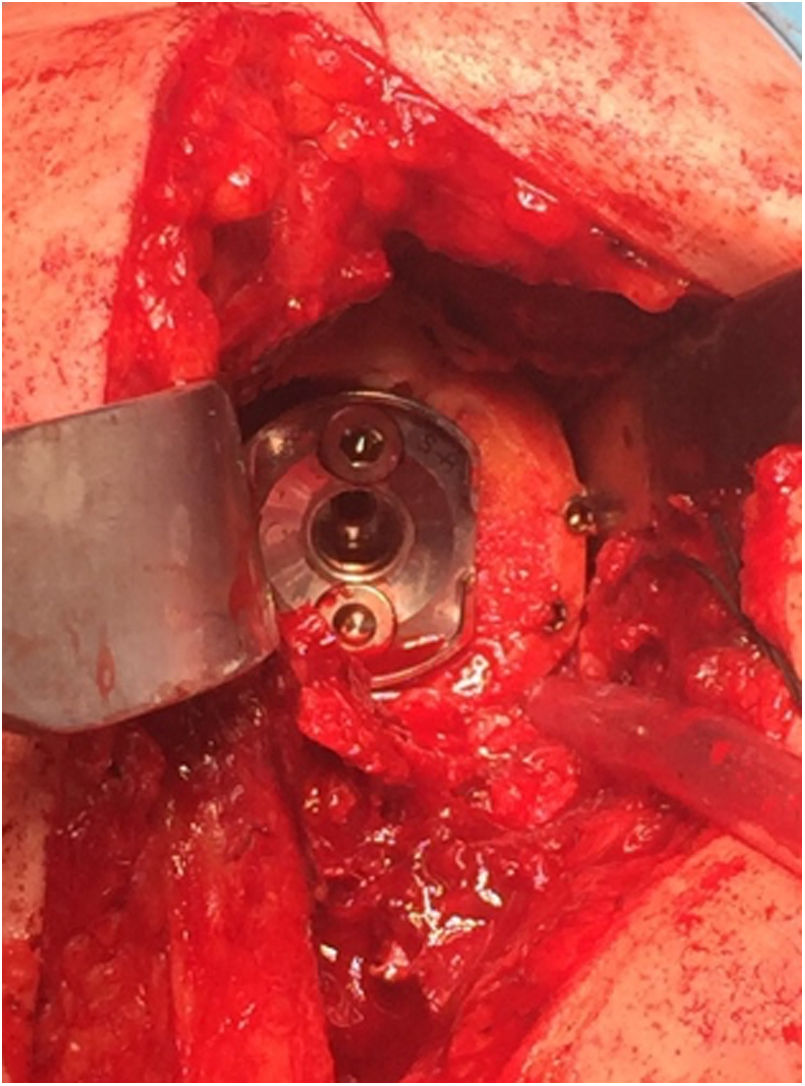
In 12 of the 13 patients (75% of the total series) undergoing primary surgery, the patient's own humeral head was autografted. In the remaining 4 cases (25%) – 3 cases of revision surgery and one case of proximal humerus fracture sequelae, in which most of the humeral head was necrotic – allograft bone from the femoral condyle was used. In all cases, the graft was carved attempting to obtain at least 3mm of cortical bone around the entire circumference of the graft to increase its structural strength. After glenoid reaming to remove the cartilaginous remnant, the graft was temporarily stabilised on the host bone using K-wires and the central hole of the peg was then drilled. In 2 patients operated for inveterate glenohumeral dislocation with a severe anterior eccentric defect requiring an L-shaped graft, after placing a central broach the wires were replaced by two 3.0mm cannulated screws (Fig. 5). The graft was finally fixed by impacting the glenoid component supplemented by two screws, superior and inferior, 6.5mm in diameter. The mean duration of the surgical procedure was 96min (range 65–140min).
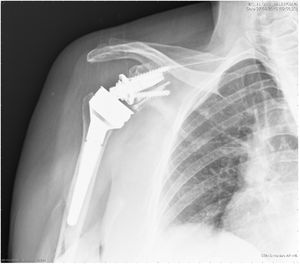
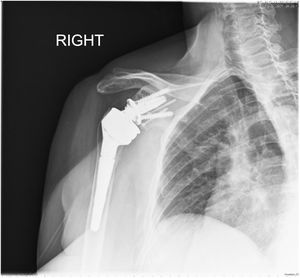
Postoperatively all patients were immobilised using a conventional sling, and passive mobilisation of the joint by a trained family member was started up to 90 degrees antepulsion on the first day after surgery. Active mobility was delayed until the sixth postoperative week. Clinical and radiological follow-up took place in the centre's own outpatient clinic at 1 month, 3 months, 6 months, 12 months, and then annually (Figs. 6 and 7).
The information obtained in the preoperative consultation when the patient was included on the waiting list, and from the last follow-up present in our centre's electronic history was used to assess and compare the clinical and radiological results. Both the functional assessment and the radiological findings were performed jointly by two senior surgeons with expertise in shoulder surgery who were not involved in the surgical procedure. For clinical assessment we used Constant's shoulder scale,5 the visual analogue pain scale (VAS) from zero (no pain) to 10 (maximum pain),6 and we measured active range of motion using a goniometer. For the radiological assessment, graft integration was studied using anteroposterior, axillary, and scapular projections,7–10 fixation of the glenoid component according to the method proposed by Ha et al.11 and Gustas-French et al.,12 and that of the humeral component according to Martínez Díaz et al.13 The graft was considered fully integrated when there was radiographic evidence of union, no complete radiolucent lines at the interfaces between the baseplate, bone graft and/or glenoid, and no evidence of component loosening at the last clinical follow-up.10 The Sirveaux classification14 was used to classify development of scapular notching. We also recorded the incidence of intraoperative and postoperative complications.
Statistical analysisWe used SPSS 22 and XLSTAT statistical software for MAC OS for processing the data. The descriptive analysis of categorical variables is expressed as absolute and relative frequencies; quantitative variables are described with mean and standard deviation (SD). Both quantitative and qualitative variables were tested for normality using the Kolmogorov–Smirnov test. Since there was no normal distribution of the sample, for the analysis of the variables included in our study we applied the non-parametric Wilcoxon signed-rank test. The significance level was set at 5% in all the statistical analyses.
ResultsThe mean follow-up was 42.1 months (range 27–60 months), with no loss to follow-up. Mean postoperative length of stay was 2.5 days (range 2–4 days).
The preoperative and last follow-up results of the VAS scale, Constant test, and active range of motion are shown in Table 2.
Mean pre-and postoperative VAS, Constant and active range of motion scores (p<.05).
| ABD | ATP | IR | ER | VAS | Constant | p | |
|---|---|---|---|---|---|---|---|
| Preoperative | 54°±16° | 54°±21° | Trochanter | 24°±10° | 7.5±1.4 | 35.8±3.8 | |
| Last clinical follow-up | 120°±27° | 124°±23° | L5 | 63°±7° | 2.5±2.4 | 64.4±6 | <.05 |
ABD: abduction; ATP: antepulsion; ER: external rotation; IR: internal rotation.
From the radiological assessment, bone graft integration was observed in all patients. Furthermore, no images compatible with loosening of the glenoid or humeral component were observed in any case. Three of the patients (18.8%) developed Sirveaux grade I scapular notching visible on radiological control 3 years after the surgery in two cases, and in the remaining case after 4 years.
Only one of the 16 patients (6.2%) had early postoperative complications: an ulnar nerve neuropraxia that completely resolved 6 months after the surgery.
DiscussionThe presence of a bony defect or severe abnormality of the glenoid version compromises the results of shoulder arthroplasty and increases the difficulty of the surgical procedure. In a study by Gupta et al.2 of 94 patients with glenoid defects of varying degrees treated by reverse shoulder arthroplasty, combined with bone graft in patients with more severe defects, it was concluded that the latter had the worst clinical outcomes and greater likelihood of early failure of the arthroplasty.
There are currently different options for the treatment of glenoid defects. These include eccentric glenoid reaming, autograft or allograft bone, and glenoid components with metal augments (wedges), or custom-made by 3D printing.1
Eccentric reaming of the glenoid cavity is often chosen for minor bone defects. The purpose of this procedure is to regularise the articular surface, thus allowing uniform contact with the prosthetic component. There is no clear limit to the amount of glenoid erosion that can be safely corrected by this method. However, excessive reaming would lead to medialisation of the glenohumeral joint line and thus decrease soft tissue tension and increase the risk of prosthetic instability and scapular notching.3,15–17 Therefore, most authors agree that abnormalities of the glenoid version greater than 15 degrees should not be treated with reaming alone, and other options should be considered, such as bone grafting or glenoid baseplate with metal augments.3
Several authors have reported good results using glenoid baseplates with posterior wedge-shaped metal augments to treat severe glenoid defects.18 Theoretically, compared to bone graft, they require less surgical time and avoid complications such as graft resorption and lack of graft incorporation. However, they have disadvantages, such as the absence of bone stock restoration, complicating future revisions, increased stress at the host-bone interface, increasing the risk of long-term loosening, and the higher cost of the implant.19
This paper presents the results obtained in a series of 16 patients with various conditions associated with glenoid bone defect using reverse arthroplasty and bone grafting. The main advantages of grafting over eccentric reaming are the restoration of bone stock and the preservation or restitution of the joint line, avoiding excessive medialisation of the centre of rotation. The indications for bone grafting could therefore be grouped into asymmetric bone defects not suitable for reaming and cavitary or segmental defects of sufficient size to prevent stable primary fixation of the glenoid component.3
Theoretical disadvantages of bone grafting include increased difficulty of the surgical technique and the lack of incorporation or resorption of the bone graft on the glenoid surface, which could lead to premature loosening of the components.3 In this regard, in our series we obtained a 100% graft integration rate. Malhas et al.1 present similar results in a study of 45 patients with glenoid defect (23 primary and 22 revision surgeries) treated by reverse arthroplasty and stabilised bone grafting, as in our series, with trabecular titanium glenoid baseplate, reporting 93% integration with a minimum follow-up of 2 years. Boileau et al.,17 using a glenoid component with a smaller diameter central peg than that used in our series and coated with hydroxyapatite, obtained complete incorporation of the graft in 98% of a series of 42 patients treated by reverse arthroplasty and bone autograft of the humeral head.
Four cases (25%) in our series used distal femoral allograft and 12 (75%) used proximal humeral autograft, with no significant differences in the degree of graft incorporation or incidence of complications between the two cohorts. Similar results were reported by Lopiz et al.20 in a study of 23 patients with glenoid bone defect treated by reverse prosthesis combined with bone grafting, with 100% consolidation of autografts versus 92% of allografts (mainly proximal tibia). In contrast, Jones et al.,15 in a series of 44 patients treated with reverse arthroplasty and bone graft (29 humeral autograft, one iliac crest autograft, and 14 femoral head allografts), found a total graft incorporation rate of 58.6% with autograft, and 41.7% with allograft. However, this difference in the degree of graft integration did not translate into differences in clinical outcomes between the two groups of patients. The anatomical origin of the allograft varies according to the series consulted, with good results in most cases.21 However, some authors, such as Ozgur et al.,22 advise against the use of allografts from femoral diaphysis, and found that 87% of these failed despite the addition of bone morphogenetic protein (BMP-2) in all cases.
The functional results obtained in our series are consistent with those presented by other authors, as can be seen in Table 3.
Comparative table of functional results from our and other authors’ case series.
| Cases, n | VAS | Constant scale | ATP | ER | Complications | |
|---|---|---|---|---|---|---|
| Boileau et al.17 | 45 | 66.6±11 | 146°±21° | 23°±16° | 19% | |
| Jones et al.15 | 44 | 1.5±1.9 | 58±14,9 | 116°±25° | 26°±22° | 13.6% |
| Lopiz et al.20 | 23 | 3.3±3.2 | 51.3±13.4 | 113°±34° | 13°±5° | 20% |
| Tashjian et al.23 | 19 | 1.4±2.2 | 128°±28° | 27°±12° | 21% | |
| Tashjian et al.24 | 14 | 2.5±3.1 | 130°±49° | 29°±16° | 21.4% | |
| Marquina et al. | 16 | 2.5±2.4 | 64.4±6 | 124°±23° | 63°±7° | 6.2% |
ATP: antepulsion; ER: external rotation.
Our complication rate was low, with only one case out of 16 (6.2%) suffering ulnar nerve neuropraxia. Lopiz et al.20 describe a complication rate of 20% in 23 patients treated with reverse arthroplasty and grafting with a mean follow-up similar to that of the present study (38 months). Other authors report complication rates of between 12% and 31% in patients treated with reverse arthroplasty and grafting for glenoid defect, the most frequent being instability, infections, and aseptic loosening.1,2,15,24,25
Study limitationsThe main limitations of our study lie in its retrospective design, the limited number of patients and limited clinical follow-up, which means we can only provide short-term results from our series of patients. Another limitation, common to other studies mentioned,20 is the assessment of graft incorporation into the host glenoid by plain X-ray, as this involves greater interobserver variability than assessment by CT. However, Paul et al.,10 in a systematic review including 11 studies with 393 patients undergoing reverse shoulder arthroplasty combined with autograft or glenoid bone allograft, found no statistically significant difference in the rate of bone graft union when CT was used as an adjunct to plain X-rays (94% glenoid graft integration rate). In addition, the heterogeneity of aetiologies for the indication for surgery, as well as whether the surgery was primary or revision of a previous prosthesis, may be a factor influencing the patients’ functional outcomes.
ConclusionsThe use of bone graft combined with trabecular titanium glenoid components is a valid option in the treatment of patients with glenoid defects by reverse shoulder arthroplasty. Although it is a complex procedure, it has a high rate of graft incorporation and low short-term complication rate.
Level of evidenceLevel of evidence IV.
Conflict of interestsThe authors have no conflict of interests to declare.
FundingThe authors declare that they have received no funding for the conduct of the present research, the preparation of the article, or its publication.