Cancer is in Spain the second cause of death in women (22%) and the first in men (31%). In this chapter, we describe the most frequent types of spinal metastases, their most frequent locations within the spine, as well as their clinical behaviour. We also analyse the neurological conditions most frequently associated with spinal metastases: root compression, spinal cord compression, cauda equina, and spinal cord involvement.
El cáncer es en España la segunda causa de muerte en mujeres (22%) y la primera en varones (31%). En este capítulo describimos los tipos más frecuentes de metástasis raquídeas, sus localizaciones más habituales dentro de la columna vertebral, así como su comportamiento clínico. Analizamos también los cuadros neurológicos más comúnmente asociados a las metástasis de columna: compresión radicular, compresión medular, cauda equina y afectación medular.
In Spain, cancer is the second cause of death in women (22%) and the first in men (31%). Lung, breast, prostate and colon cancer are the most frequent. In most cases, death occurs as a result of metastasis and complications, rather than from the primary tumour.
The most frequent sites of metastasis are the liver, lung and bone, in that order.1 Seventy to 80% of malignant tumours will give rise to bone metastases; of these, 70% will be in the spine.2 Within the spine, the most frequent locations are thoracic (60–80%), lumbar (15–30%) and cervical (<10%).3 It is estimated that almost half of patients with spinal metastases will have metastases at multiple levels of the spine.4 Spinal metastases can be located intradurally (intramedullary or extramedullary) or extradurally. Approximately 95% are extradural.5
In 2013, we conducted a review of 279 cases of metastases treated at our centre between 2006 and 2010. The mean age was 65 years, with 60% of the cases being male. At the time of diagnosis, 55.6% were living a normal life (Karnofsky>80), 33.7% required assistance (Karnofsky 50–70) and 10.7% were bedridden (Karnofsky 10–40). Extrathecal bone lesions were present in 80.4% of cases and internal organ involvement was present in 58.7%. The most frequent primaries were: lung 26.1% (n=73), breast 21.8% (n=61) and prostate 10.7% (n=30). Spinal cord injury was present in 16% (30 incomplete and 17 complete). 10.2% started with pathological fracture, with 82.8% presenting pain as the initial symptom. Thirty patients (10.7%) underwent surgery.
PhysiopathologyMetastatic spreadCancer cells can spread to the spine through several mechanisms: via the arterial system, Batson's venous plexus, cerebrospinal fluid (CSF) and by direct anatomical continuity from a neoplasm near the spine. The most relevant form of dissemination to the bone is through the circulatory system, particularly the venous system.6 There is communication between the veins of the breast and Batson's plexus in the thoracic region and, therefore, breast cancers often metastasise to the thoracic vertebrae. The lungs drain their blood through the pulmonary veins to the left side of the heart, which can spread cancer cells throughout the body.7 The prostate drains through the pelvic plexus into the lumbar region, so prostate cancers metastasise to the lumbosacral vertebrae.7 Colon cancer metastasises first to the liver and lungs via the portal system and the cava, respectively.7 In most cases, the posterior vertebral body is the initial site of involvement, spreading later to the pedicles.
Cellular mechanismsAt cellular and molecular level, the development of a bone metastasis is a complex process. Neoplastic cells must first spread to the primary site at the expense of pre-existing cells and the stroma of the organ of origin and surrounding tissues, then detach from it by reduction of cell–cell adhesion molecules, then reach the blood vessels and penetrate them through the lamina and endothelium, and then migrate with the bloodstream. All these processes depend on the increased or decreased expression of different proteins, mainly integrins, cadherins, immunoglobulins and selectins.8 The bone marrow, especially of the axial skeleton, has the conditions of microcirculation which slows down blood flow, available space, receptor expression, neoangiogenesis capacity, ideal for the establishment of these neoplastic cells, etc.9
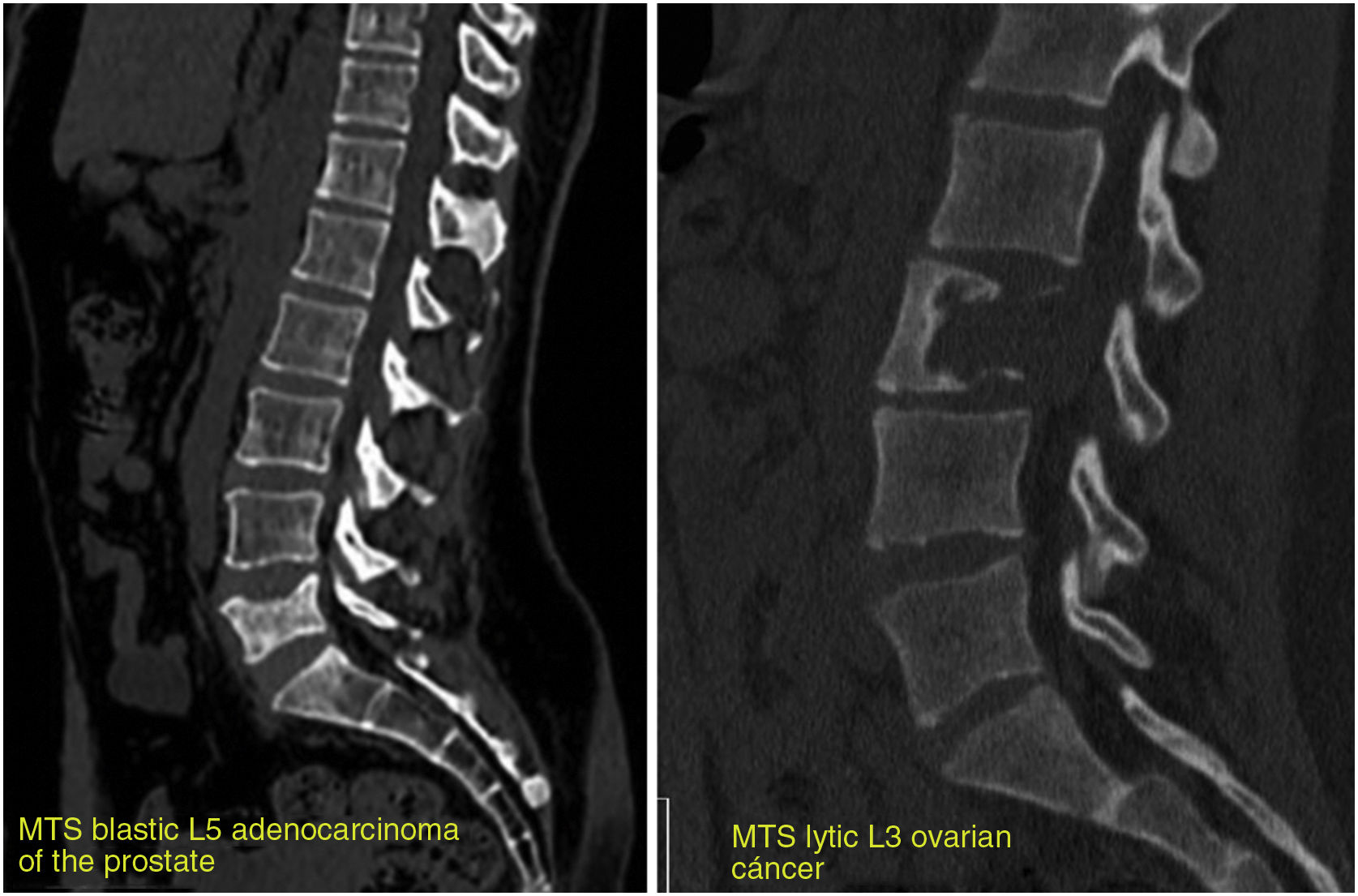
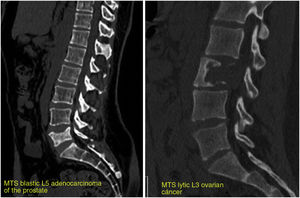
In normal individuals, there is a continuous and balanced process of bone resorption by osteoclasts and bone formation by osteoblasts. In cancer cells, this balance is lost, creating lytic, blastic or mixed lesions in the bone.10 When metastatic cells invade the bone, they produce growth factors that directly stimulate osteoclastic and/or osteoblastic activity, altering the balance of bone formation/destruction. Therefore, osteolytic lesions are caused by an excess of osteoclastic activity accompanied by reduced osteoblastic activity, and not by the direct effects of metastatic tumour cells on bone.11 Osteoblastic lesions are an expression of increased bone formation by osteoblasts around tumour cells, accompanied by decreased bone formation. As an example, in breast cancer, osteolytic lesions predominate, although at least a quarter of lesions are thought to be osteoblastic.12 In prostate cancer, most tumours are osteoblastic in nature. However, a lesion may contain both osteoblasts and osteoclasts. These lesions are visualised differently on radiological studies: osteoclastic lesions appear lytic (dark appearance due to bone destruction), and osteoblastic lesions appear sclerotic (light appearance due to bone tissue formation); when both components are present, the lesion shows a mixed pattern (Fig. 1).
RANKL and OPG balanceThe final effectors of bone remodelling are: the receptor activator of nuclear factor κβ ligand (RANKL), its natural receptor (RANK) and osteoprotegerin (OPG), all belonging to the tumour necrosis factor (TNF) family. RANKL is a protein expressed by osteoblasts and their precursors under the control of hormones, cytokines and proresorptive growth factors. The binding of RANKL to its natural receptor on the cell surface of osteoclasts and their precursors, RANK, increases osteolysis by different mechanisms: it stimulates the fusion of the pre-osteoclasts, increases their adhesion to the bone, stimulates their function and increases their survival by preventing apoptosis.13 OPG is a protein synthesised by osteoblasts and stromal cells. It acts by preventing the binding of RANKL to its natural receptor RANK. In this way, OPG blocks all the actions of RANKL, having the final effect of inhibiting osteolysis.14 Due to the antagonistic effects of the RANKL and OPG proteins, bone remodelling depends on the balance between the two, and this is regulated by multiple factors.
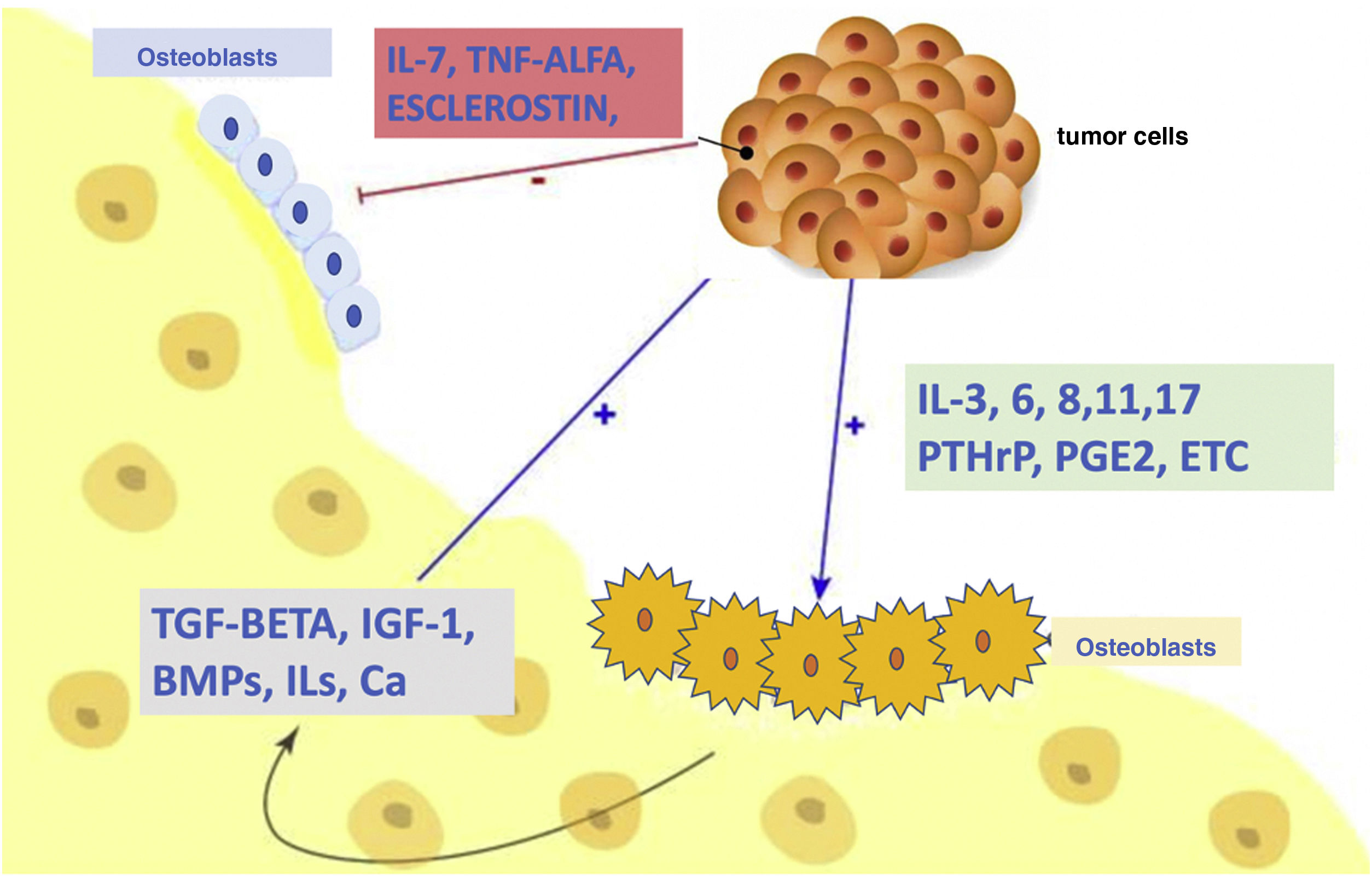
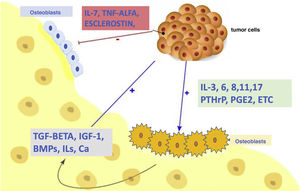
As mentioned above, normal bone remodelling maintains a proper balance between the action of osteoclasts (bone resorbing cells) and osteoblasts (bone-forming cells). Skeletal malignancies, including bone metastases, disrupt the OPG–RANKL–RANK signal transduction pathway and promote increased osteoclast formation, which accelerates bone resorption and induces bone loss. This osteolysis, in turn, leads to the release of bone-derived growth factors, contributing to a “vicious cycle” in which interactions between tumour cells and osteoclasts lead not only to increased osteoclastogenesis and osteolytic activity, but also to aggressive tumour cell growth and behaviour.10 Therefore, osteolytic complications associated with bone metastases are caused by tumour-induced alterations in the OPG–RANKL–RANK system (Fig. 2).
Osteoblastic metastasesOsteoblastic metastases are mainly found in prostate cancer, but also in small cell lung cancer, Hodgkin's lymphoma and medulloblastoma. In prostate cancer, increased bone-forming activity results in interwoven bone, which is characterised by an osteosclerotic appearance distinct from the typical lamellar structure seen in normal bone. A histopathological analysis of these bone metastases usually shows a large number of osteoblasts adjacent to the prostate cancer cells.15 In contrast, osteoblasts are almost always absent in normal bone or in bone metastases from other cancers (such as breast, lung and kidney), which mostly contain osteoclasts.16 An increased serum OPG level has been established in patients with advanced prostate cancer.17
Clinical manifestations of bone metastasesIntroductionBone metastases have a very significant impact on the quality of life and survival of patients who suffer from it, with the result that assessment of the patient's general functional status is of great importance. There are several scales used to assess the patient's general condition: the Karnofsky Scale (KPS), the Edmonton Symptom Rating Scale, the Katz Index of Independence in Activities of Daily Living (Katz ADL), etc. The Karnofsky Scale (KPS) is the most widely used; it was defined by Bruchenal and Karnofsky in 1949. The functional status of a patient is assessed on a scale from 0 to 100 points, ranging from total well-being (100%) to death (0%), decreasing by 10 points at each level. According to the results, patients are divided into three groups: group A (100–80%) can perform daily activities independently; group B (70–50%) can perform daily activities with assistance; and group C (<40%) requires continuous assistance and is progressively approaching death18 (Table 1).
Karnofsky Scale (KPS) from 0 to 100 points, classifying functional status into groups A, B and C.
| Summary Karnofsky Scale |
|---|
| Group A 100–80% can perform daily activities independently |
| Group B 70–50% can perform daily activities with help |
| Group C <40% requires continuous assistance and progressively approaches death |
Metastases can cause bone pain, fractures, neurological deficits and symptoms associated with systemic disease (asthenia, anaemia, weight loss, hypercalcaemia, etc.). In some, a palpable mass may also be found, especially in the case of large sacral metastases.
PainPain is the most common manifestation in patients with spinal metastases. It is estimated that approximately 80–95% of patients will complain of pain.17 It will be the initial symptom of spinal metastasis in 10% of patients. Patients with spinal metastases may have one of 3 types of pain: local pain, mechanical pain or radicular pain.
This local pain is tumour-related and occurs as a result of stretching of the periosteum, venous ingurgitation, tumour growth or tumour-induced inflammation and infiltration. Tumours secrete inflammatory mediators that cause pain. When endogenous corticosteroid secretion is reduced at night, patients experience an exacerbation of inflammation that improves dramatically throughout the day. This pain is not relieved even when the patient is lying in bed. It usually responds to non-steroidal anti-inflammatory drugs (NSAIDs), steroids and radiation. The classic clinical signs are non-specific. Sensitivity to local percussion was found in only 43% of patients with CT-positive vertebral metastases, and only 65% with epidural disease. However, when present, the sign helps to localise the metastasis. As a general rule, the site of local pain can help distinguish vertebral metastasis from the more common degenerative back lesion. As a general rule, most degenerative back diseases are cervical or lumbar and cause neck or low back pain, while most epidural spinal cord compressions are thoracic (60–80%)4 and produce local chest pain.
Mechanical pain results from spinal instability, pathologic fractures, or impending vertebral fractures, that is, from injuries that have resulted in significant bone destruction and are at high risk of pathologic vertebral collapse. Pathologic fractures can sometimes be the first sign of metastatic bone disease. They are commonly associated with focal bone loss within lytic lesions arising from breast, lung, kidney, and thyroid cancers. Mechanical pain is usually related to prolonged standing or sitting, and to actions such as changing position from lying to sitting, sitting to standing, and turning in bed. Spinal stabilisation with orthopaedic corsets can markedly improve the quality of life of these patients. Mechanical pain is not relieved by NSAIDs, steroids, chemotherapy, or radiation therapy. Significant pain may require surgical stabilisation or percutaneous augmentation techniques (cementation or radiofrequency).
Neurological involvement in metastasesIntroductionMotor dysfunction is the second most common clinical manifestation in patients with spinal metastases. Pain is usually the first manifestation of those patients with epidural metastases. Authors with Gilbert et al. report that, of 130 epidural metastases detected, pain was the first symptom in 125 of them. It has been published that less time elapses between the onset of pain and the neurological lesion in more aggressive tumours, being less in lung cancer than in breast cancer, for example.19
Root compressionCompression of the nerve roots within the spinal canal, or in the foramina, generates radicular pain. Although compressive radiculalgia usually follows local pain, it sometimes precedes or is independent of it. Radicular pain is present in 80% of patients with cervical lesions. It usually radiates down one arm or sometimes both arms. Of patients with chest compression, 55% characteristically have radiating intercostal pain, in a tight band around the chest or abdomen, often bilateral. 90% of patients with involvement of the lumbar spine have pain that radiates to one or both legs. Most of these patients also have local pain.20
Spinal cord compressionSpinal cord compression usually causes weakness beginning in the legs, regardless of the compression site. The patient often complains of difficulty walking and especially climbing stairs or getting up from low chairs or toilet seats. In upper thoracic or cervical cord injuries, the patient may notice a weak cough, and only late in the development of cervical cord compression do the arms become substantially weak.
Although the weakness is usually bilateral and symmetrical, it can occasionally predominate in one leg or one arm. Examination in the early stages of compression may not reveal spasticity or jumpy reflexes. It is more frequent to observe moderate weakness of the proximal muscles of the lower limbs (iliopsoas and hamstrings), with apparently normal strength in the distal muscles (anterior tibialis, soleus, etc.). Even at this time, the patellar and Achilles reflexes are usually slightly more active than the upper extremity reflexes. As the disease progresses and weakness increases, spasticity, overactive reflexes, and extensor plantar responses develop. These signs are usually bilateral and symmetrical, although on occasion one side may be more affected than the other. It is important to know that, if the patient has received prior chemotherapy, the reflexes may be diminished or absent due to a toxic neuropathy that reduces their localisation value. If the onset of compression is acute, leading to complete paraplegia, most patients are flaccid and areflexic.
Cauda equinaWhen the cauda equina is involved rather than the spinal cord, the weakness reflects lower motor neuron dysfunction. We will find hypotonia, atrophy, fasciculations and areflexia. The weakness is usually more marked distally than proximally, although the gluteal and hamstring muscles are also often affected. Distal weakness begins with foot drop and continues to weakness below the knees. With compression of the cauda equina, sensory loss is usually bilateral, involving the perianal area, the posterior thigh, and the lateral aspect of the leg. The most characteristic autonomic abnormality is bladder dysfunction. Patients often develop painless urinary retention, usually associated with severe weakness and sensory loss.
Sensory impairmentPainless sensory involvement is rare with compression of the spinal cord or cauda equina.21 Sensory abnormalities usually begin at the toes and work their way up in the sock shape, eventually reaching the level of the lesion. The earliest sign is usually a slight decrease in vibration and position sense, followed by pain and loss of thermal sensation. At diagnosis, we found sensory loss for both touch and pinprick to be between one and 5 levels below the actual spinal cord compression site, although it is sometimes more cranial.
Level of evidenceLevel of evidence iv.
Conflict of interestsThe authors have no conflict of interests to declare.