The aim is to present the functioning and results of the Catalan Arthroplasty Registry (RACat).
Material and methodThe RACat arose by the initiative of the Catalan Society of Orthopaedic Surgery and Traumatology, the Catalan Health Service (CHS) and the Catalan Agency for Health Information Assessment and Quality. Publicly funded hospitals sent information through the Internet (CHS Applications website) on knee and hip arthroplasties: patient identification, hospital, joint (hip/knee), type (primary/revision), side of operation, date of surgery and prosthesis (manufacturer's name and reference number). The quality of the data is analysed regularly. We estimate the risk of replacement by the Kaplan–Meier method.
ResultsA total of 52 hospitals out of 62 send data to RACat, and information on 36,951 knee and 26,477 hip arthroplasties is available. Data quality improved between 2005 and 2010. In 2010 coverage exceeded 70%, with side of operation 97%, and prostheses identification of 80%. The risk of replacement at three years was 3.3% (95% CI: 3.1–3.6) for knee, 2.9% (95% CI: 2.5–3.3) for total hip, and 2.5% (95% CI: 2.0–3.1) for partial hip.
DiscussionRisk of replacement is higher than that observed in other registers, although data quality and its improvement over time should be taken into account.
ConclusionsThe information available in the RACat will help to establish a standard that will enable hospitals to compare results.
El objetivo de este trabajo es presentar el funcionamiento y los resultados del Registro de Artroplastias de Cataluña (RACat).
Material y métodoEl RACat surgió por iniciativa de la Sociedad Catalana de Cirugía Ortopédica y Traumatología, el Servicio Catalán de la Salud (SCS) y la Agencia de Información, Evaluación y Calidad en Salud. Los hospitales financiados públicamente envían mediante Internet (portal de aplicaciones, SCS) información sobre las artroplastias de rodilla y cadera: identificación del paciente, hospital, articulación (cadera/rodilla), tipo (primaria/recambio), lateralidad, fecha de cirugía y prótesis (fabricante y número de referencia). La calidad de los datos se analiza periódicamente. El riesgo de recambio se estima mediante el método de Kaplan-Meier.
ResultadosEn total 52 hospitales de 62 envían datos al RACat que dispone de información sobre 36.951 artroplastias de rodilla y 26.477 de cadera. La calidad de los datos mejoró entre 2005 y 2010, superando la cobertura el 70%, la información sobre lateralidad el 97% y la identificación de prótesis el 80%. El riesgo de recambio a los 3 años fue del 3,3% (IC 95%:3,1-3,6) para rodilla, del 2,9% (IC 95%:2,5-3,3) para las totales de cadera, y del 2,5% (IC 95%:2,0-3,1) para las parciales.
DiscusiónEl riesgo de recambio es superior al observado en otros registros, aunque es necesario tener en cuenta la calidad de la información disponible y su mejora en el tiempo.
ConclusionesLa información disponible en el RACat permitirá establecer un estándar de referencia que permita a los hospitales evaluar sus resultados.
In Spain, the Health Ministry published a ministerial order (ORDER SCO/3603/2003 December 18th) in the Official State Bulletin of December 26, 2003 which regulated the creation of National Implant Registries, including hip and knee prostheses. The objective was to gather the information contained on implant files: patient identification, centres conducting the interventions, companies marketing the prostheses and descriptions of the components of the implanted prostheses. Despite this, the National Health System does not have a registry of implants and it is only through the initiative of scientific societies in various regions that arthroplasty registers are created (as in the cases of Andalusia, Canary Islands, Madrid and the Basque Country), with the support of their respective health authorities, although with varying degrees of success.1,2
These arthroplasty registries have emerged as a systematic method of obtaining information on the effectiveness and safety of prostheses after marketing.3,4 Such registries also enable a naturalistic approach whereby prostheses results are evaluated under normal use conditions, after implantation by orthopaedic surgeons with different levels of experience and in a variety of hospitals.3,5 In this sense, the registries have been able to identify problems with certain devices, such as the recent case of implants with metal–metal friction coupling.6 These problems had already been pointed out by the Norwegian registry based on the analysis of surface prostheses.7
The Swedish knee arthroplasty registry, created in the 1970s, was the first of its kind and since then several countries have implemented their own, although there are several differences regarding operation and the type of information gathered.8 This requires a consensus on methodological aspects and also with respect to identifying prostheses, in order to ensure that results are comparable.9,10 There are several initiatives which aim to reach a consensus on these aspects: the International Society of Arthroplasties Registries (www.isarhome.org), the International Consortium of Orthopaedic Registries,3 the European Arthroplasty Registry (www.ear.efort.org) and the Nordic Arthroplasty Registry Association.11
Based on the experiences obtained in other countries, in 2005 the Catalan Society of Orthopaedic Surgery and Traumatology (SCCOT), the Catalan Health Service (SCS) and the Agency for Health Information, Evaluation and Quality (AIAQS) signed a collaboration agreement with the intention of developing and implementing a registry of arthroplasties. The aim of this work is not only to present its operation, data quality and characteristics of the prostheses, but also its results in terms of survival for the period 2005–2010.
Material and methodIn 2005 AIAQS was instructed to develop and implement the Arthroplasty Registry of Catalonia (RACat). Its structure was defined by consensus between SCCOT, SCS and AIAQS, and a management committee in charge of overseeing the project was established. This included the Director/President of each institution, an Advisory Committee in charge of monitoring and evaluating project development and analysing relevant information, and a Council representing the collaborators of the registry at each participating centre. From the outset of the project it was decided that it would only include knee and hip prostheses, at least initially, and that the participation of each hospital would be voluntary. Hospitals began to send information towards the end of 2005.
Data collection and data managementIn order to ensure the viability of the project, the data collection system was designed so as to avoid creating an overload for surgeons. To this end, the information available at each centre regarding the implanted prostheses was reviewed to confirm that the minimum data set necessary for the operation of an arthroplasty registry9 was already available in electronic format. This information is sent to RACat periodically, both in the case of primary arthroplasties and prosthetic replacements. It consists of: health card number of each patient, operated joint (knee or hip), type of arthroplasty (primary or replacement), operated side (right or left), date of surgery and data of the prosthesis (manufacturer and reference number; this same information regarding cement is also sent in the case of cemented implants). The data recorded in the information systems of the participating hospitals regarding the implanted prostheses are sent to RACat periodically by their I.T. services in a predetermined format, depending on their Traumatology and Orthopaedic Surgery Services and hospital management, through the online application portal of SCS. Data quality is reviewed 2 times every year and any errors detected in the information submitted are communicated to the registry collaborator at each centre, who is responsible for resolving them.
Health card numbers (unique for each person living in Catalonia) enable the data sent by each hospital to be linked with information contained in the Central Registry of SCS in order to obtain data on each patient. Furthermore, health card numbers also enable the information to be linked with the Minimum Data Set upon Hospital Discharge, thereby yielding information on the main diagnosis (considered the reason for surgery) and comorbidities of each patient. Information on the characteristics of any given prosthesis can be obtained by linking prosthesis data sent by the centre (manufacturer and part number) with the prosthesis catalogue compiled from the information obtained from all manufacturers supplying prostheses in Catalonia. This catalogue includes data on the component type, brand and some structural characteristics (friction coupling and use or not of cement). The information on cement was also included in the catalogue.
Management of the registry and resources investedRACat is coordinated by AIAQS, a public company of the Health Department ascribed to SCS. In order to limit the use of resources during the implementation of RACat, available resources were reviewed and it was decided to integrate the RACat database within the SCS I.T. system, which is responsible for its maintenance, while AIAQS is responsible for data collection, management and analysis. The registry involves 4 professionals from AIAQS, 1 on a full-time basis (Project Manager) and 3 with part-time schedules (2 epidemiologists and 1 statistician). Data processing taking place at each centre does not have a specific budget. The RACat database is part of the registry of specific conditions and monitoring of healthcare activities of the Health Department, thus ensuring compliance with legal requirements for the treatment of databases containing personal information.
AnalysisThe RACat database structures data analysis into 3 key areas: quality of information, patient characteristics and healthcare process of the prosthetic implants, and risk of replacement of the prostheses.
A set of indicators was developed to track data quality, including: coverage, percentage of cases sent with informed laterality, and percentage of prostheses classified in any of the prosthesis types (Table 1). Coverage was calculated from the percentage of cases sent to the registry versus the total figure of hip and knee arthroplasties performed in Catalonia according to the information available within the Minimum Data Set upon Hospital Discharge (CMBDAH). The CMBDAH was considered to be the gold standard, since all hospitals are required to submit the information required within it for each discharge. With respect to the quality of information of the prostheses, implants could not be classified if there was missing information on a key component or if a reference number was not identified, so the percentage of unclassified prostheses enabled monitoring of the quality of the information sent.
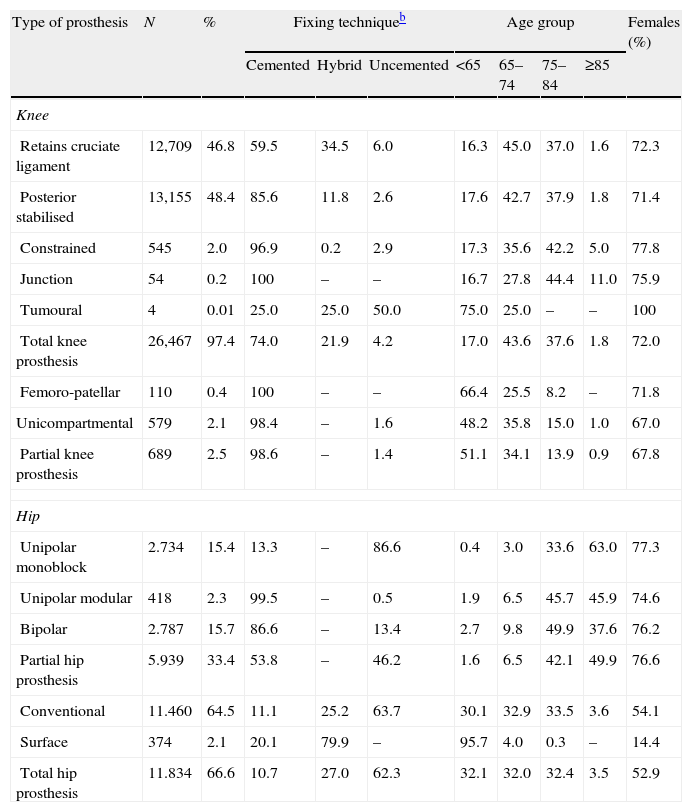
Characteristics of knee and hip arthroplasties.a
| Type of prosthesis | N | % | Fixing techniqueb | Age group | Females (%) | |||||
| Cemented | Hybrid | Uncemented | <65 | 65–74 | 75–84 | ≥85 | ||||
| Knee | ||||||||||
| Retains cruciate ligament | 12,709 | 46.8 | 59.5 | 34.5 | 6.0 | 16.3 | 45.0 | 37.0 | 1.6 | 72.3 |
| Posterior stabilised | 13,155 | 48.4 | 85.6 | 11.8 | 2.6 | 17.6 | 42.7 | 37.9 | 1.8 | 71.4 |
| Constrained | 545 | 2.0 | 96.9 | 0.2 | 2.9 | 17.3 | 35.6 | 42.2 | 5.0 | 77.8 |
| Junction | 54 | 0.2 | 100 | – | – | 16.7 | 27.8 | 44.4 | 11.0 | 75.9 |
| Tumoural | 4 | 0.01 | 25.0 | 25.0 | 50.0 | 75.0 | 25.0 | – | – | 100 |
| Total knee prosthesis | 26,467 | 97.4 | 74.0 | 21.9 | 4.2 | 17.0 | 43.6 | 37.6 | 1.8 | 72.0 |
| Femoro-patellar | 110 | 0.4 | 100 | – | – | 66.4 | 25.5 | 8.2 | – | 71.8 |
| Unicompartmental | 579 | 2.1 | 98.4 | – | 1.6 | 48.2 | 35.8 | 15.0 | 1.0 | 67.0 |
| Partial knee prosthesis | 689 | 2.5 | 98.6 | – | 1.4 | 51.1 | 34.1 | 13.9 | 0.9 | 67.8 |
| Hip | ||||||||||
| Unipolar monoblock | 2.734 | 15.4 | 13.3 | – | 86.6 | 0.4 | 3.0 | 33.6 | 63.0 | 77.3 |
| Unipolar modular | 418 | 2.3 | 99.5 | – | 0.5 | 1.9 | 6.5 | 45.7 | 45.9 | 74.6 |
| Bipolar | 2.787 | 15.7 | 86.6 | – | 13.4 | 2.7 | 9.8 | 49.9 | 37.6 | 76.2 |
| Partial hip prosthesis | 5.939 | 33.4 | 53.8 | – | 46.2 | 1.6 | 6.5 | 42.1 | 49.9 | 76.6 |
| Conventional | 11.460 | 64.5 | 11.1 | 25.2 | 63.7 | 30.1 | 32.9 | 33.5 | 3.6 | 54.1 |
| Surface | 374 | 2.1 | 20.1 | 79.9 | – | 95.7 | 4.0 | 0.3 | – | 14.4 |
| Total hip prosthesis | 11.834 | 66.6 | 10.7 | 27.0 | 62.3 | 32.1 | 32.0 | 32.4 | 3.5 | 52.9 |
In the case of patients, a descriptive analysis was performed of their characteristics upon admission (age, gender, comorbidities according to the classification of Elixhauser et al.12), reason for surgery and length of stay. In the case of the prostheses, not only the distribution of the different types of prosthesis was described, but also the frequency of use of different fixation techniques and the friction coupling in the case of hip prostheses.
Prosthesis survival was the period between the primary arthroplasty and the first replacement. The replacement of a primary arthroplasty was identified from available information regarding the joint, laterality and date of surgery. The replaced component was determined from the components reported by the hospital in replacement surgery. Estimated survival was calculated including the replacement rate represented by the percentage of replacement arthroplasties within a given period versus the total number of joint replacements performed in the same period, indicating a possible accumulation of replacement arthroplasties related to potential problems in prosthesis survival. The replacement risk was calculated through the Kaplan–Meier method, globally and according to the characteristics of the implant. The analysis of possible risk factors for replacement was performed through Cox remission models, adjusting for age and gender. Values of P<.05 were considered as statistically significant.
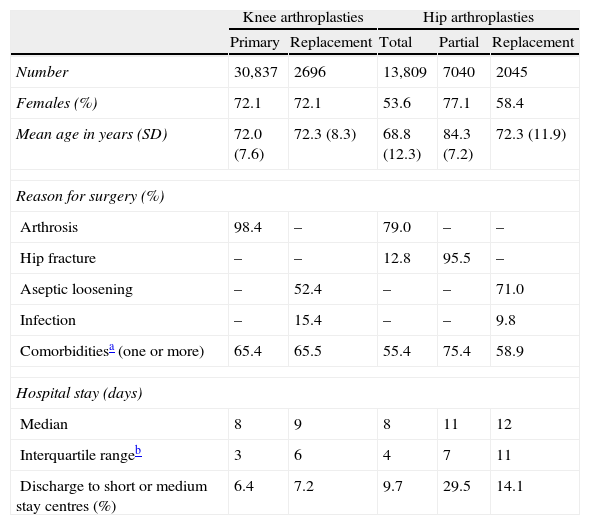
ResultsAt present, 52 hospitals in the public hospital network out of the 62 which perform hip and knee arthroplasties are sending information to RACat. This represents 80–85% of the activity taking place in Catalonia. For the period 2005–2010, RACat contains data regarding 36,951 knee arthroplasties (replacement ratio of 9.0%) and 26,477 hip arthroplasties (replacement ratio of 10.2%). Data quality (coverage, informed laterality and identified prosthesis) improved between 2005 and 2010. In the case of coverage, it increased from 24.4% to 78.3% in knee arthroplasties and from 21.0% to 71.6% in hip arthroplasties. Laterality also improved in both knee and hip replacements, going from 62.3% to 97.8% and from 60.0% to 99.4%, respectively. The percentage of identified prostheses also increased, going from 41.8% to 88.1% for knee arthroplasties and from 41.3% to 80.7% for hip arthroplasties. Patient characteristics are shown in Table 2.
Characteristics of patients and healthcare episodes.
| Knee arthroplasties | Hip arthroplasties | ||||
| Primary | Replacement | Total | Partial | Replacement | |
| Number | 30,837 | 2696 | 13,809 | 7040 | 2045 |
| Females (%) | 72.1 | 72.1 | 53.6 | 77.1 | 58.4 |
| Mean age in years (SD) | 72.0 (7.6) | 72.3 (8.3) | 68.8 (12.3) | 84.3 (7.2) | 72.3 (11.9) |
| Reason for surgery (%) | |||||
| Arthrosis | 98.4 | – | 79.0 | – | – |
| Hip fracture | – | – | 12.8 | 95.5 | – |
| Aseptic loosening | – | 52.4 | – | – | 71.0 |
| Infection | – | 15.4 | – | – | 9.8 |
| Comorbiditiesa (one or more) | 65.4 | 65.5 | 55.4 | 75.4 | 58.9 |
| Hospital stay (days) | |||||
| Median | 8 | 9 | 8 | 11 | 12 |
| Interquartile rangeb | 3 | 6 | 4 | 7 | 11 |
| Discharge to short or medium stay centres (%) | 6.4 | 7.2 | 9.7 | 29.5 | 14.1 |
SD: standard deviation.
During the study period, the posterior stabilised and total conventional types of prostheses were the most common for the knee and hip, respectively, with cemented implants being the most frequent. In primary arthroplasties, the age and gender of operated patients were distributed as would be expected for the epidemiology of osteoarthritis, that is, more frequently among women and with more conservative techniques being applied in younger patients, except for surface prostheses which were primarily implanted in young males (Table 1). The friction coupling used in hip replacements was mainly metal–polyethylene (73.6%), followed by ceramic–polyethylene (12.5%), metal–metal (7.3%) and ceramic–ceramic (6.6%). Patellar replacement was performed in 38.3% of primary total knee arthroplasties performed.
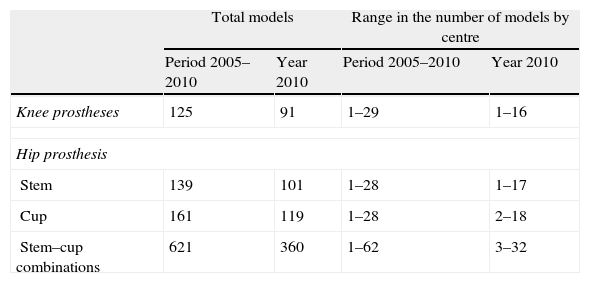
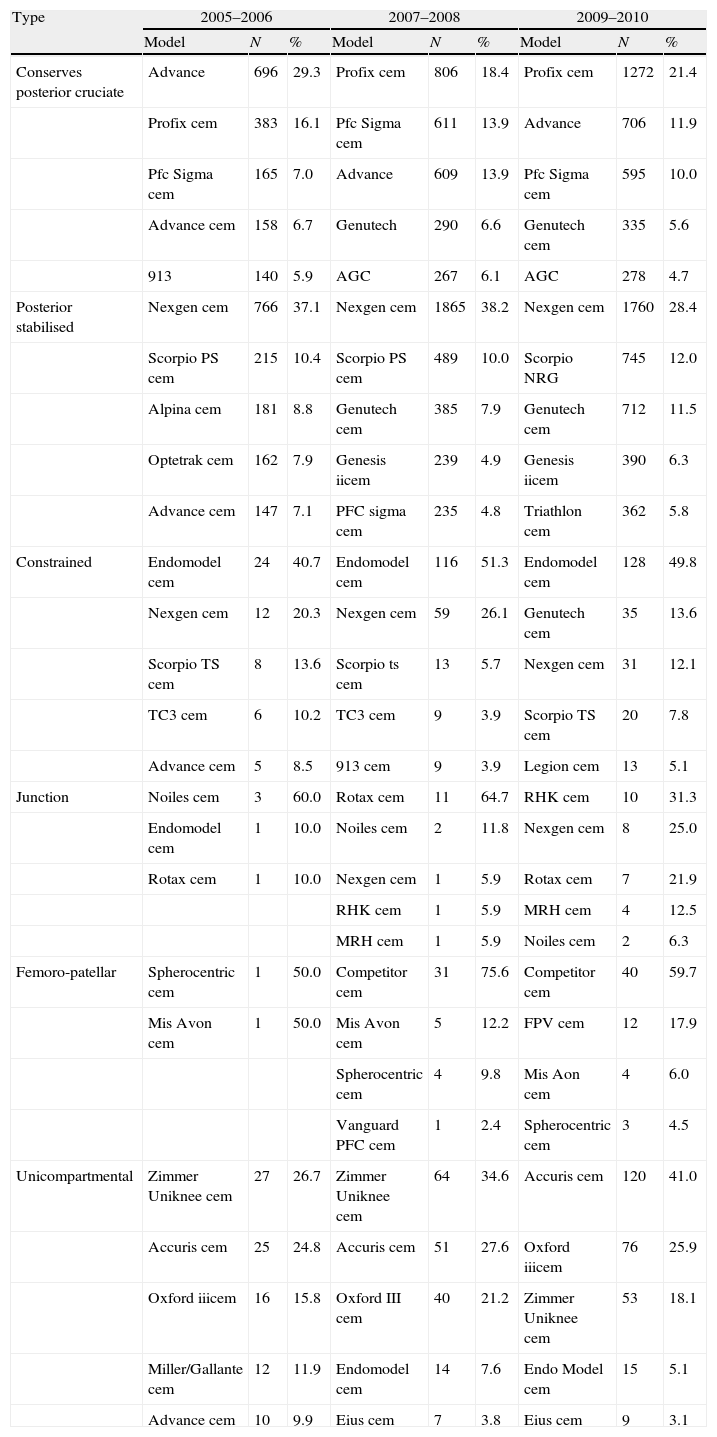
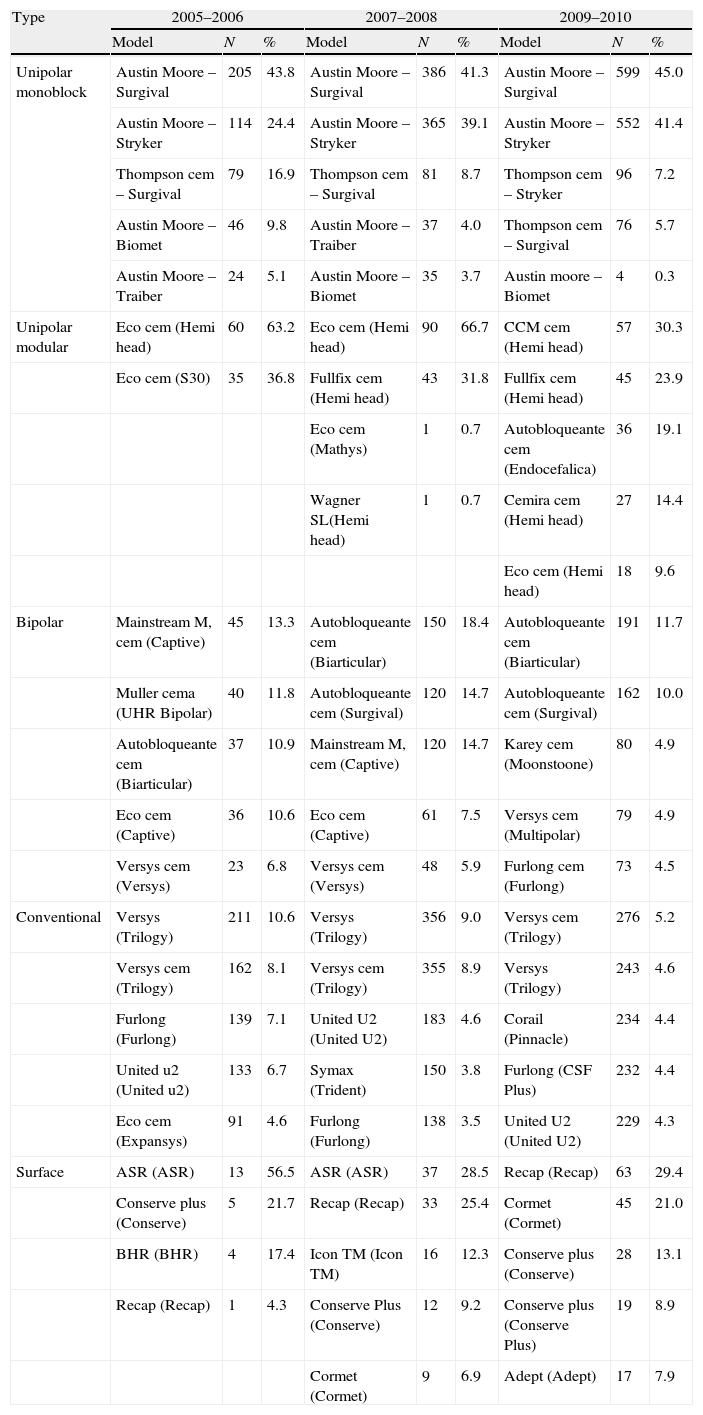
Considering the cemented and uncemented models separately, a significant volume was observed when analysing the number of different models of prosthesis used (trade name or brand). A total of 91 different models of knee prosthesis were implanted in 2010. In the case of hip prostheses, 101 stem models and 119 cup models were employed (Table 3). Regarding the most frequently implanted models, throughout the study period we observed little variation between the 5 most common models of knee prosthesis and a greater variability in primary hip arthroplasties (Tables 4 and 5).
Number and range of different prosthetic models employed in primary arthroplasties overall and by centre.
| Total models | Range in the number of models by centre | |||
| Period 2005–2010 | Year 2010 | Period 2005–2010 | Year 2010 | |
| Knee prostheses | 125 | 91 | 1–29 | 1–16 |
| Hip prosthesis | ||||
| Stem | 139 | 101 | 1–28 | 1–17 |
| Cup | 161 | 119 | 1–28 | 2–18 |
| Stem–cup combinations | 621 | 360 | 1–62 | 3–32 |
The 5 most frequent prostheses in primary knee arthroplasties according to period and type.
| Type | 2005–2006 | 2007–2008 | 2009–2010 | ||||||
| Model | N | % | Model | N | % | Model | N | % | |
| Conserves posterior cruciate | Advance | 696 | 29.3 | Profix cem | 806 | 18.4 | Profix cem | 1272 | 21.4 |
| Profix cem | 383 | 16.1 | Pfc Sigma cem | 611 | 13.9 | Advance | 706 | 11.9 | |
| Pfc Sigma cem | 165 | 7.0 | Advance | 609 | 13.9 | Pfc Sigma cem | 595 | 10.0 | |
| Advance cem | 158 | 6.7 | Genutech | 290 | 6.6 | Genutech cem | 335 | 5.6 | |
| 913 | 140 | 5.9 | AGC | 267 | 6.1 | AGC | 278 | 4.7 | |
| Posterior stabilised | Nexgen cem | 766 | 37.1 | Nexgen cem | 1865 | 38.2 | Nexgen cem | 1760 | 28.4 |
| Scorpio PS cem | 215 | 10.4 | Scorpio PS cem | 489 | 10.0 | Scorpio NRG | 745 | 12.0 | |
| Alpina cem | 181 | 8.8 | Genutech cem | 385 | 7.9 | Genutech cem | 712 | 11.5 | |
| Optetrak cem | 162 | 7.9 | Genesis iicem | 239 | 4.9 | Genesis iicem | 390 | 6.3 | |
| Advance cem | 147 | 7.1 | PFC sigma cem | 235 | 4.8 | Triathlon cem | 362 | 5.8 | |
| Constrained | Endomodel cem | 24 | 40.7 | Endomodel cem | 116 | 51.3 | Endomodel cem | 128 | 49.8 |
| Nexgen cem | 12 | 20.3 | Nexgen cem | 59 | 26.1 | Genutech cem | 35 | 13.6 | |
| Scorpio TS cem | 8 | 13.6 | Scorpio ts cem | 13 | 5.7 | Nexgen cem | 31 | 12.1 | |
| TC3 cem | 6 | 10.2 | TC3 cem | 9 | 3.9 | Scorpio TS cem | 20 | 7.8 | |
| Advance cem | 5 | 8.5 | 913 cem | 9 | 3.9 | Legion cem | 13 | 5.1 | |
| Junction | Noiles cem | 3 | 60.0 | Rotax cem | 11 | 64.7 | RHK cem | 10 | 31.3 |
| Endomodel cem | 1 | 10.0 | Noiles cem | 2 | 11.8 | Nexgen cem | 8 | 25.0 | |
| Rotax cem | 1 | 10.0 | Nexgen cem | 1 | 5.9 | Rotax cem | 7 | 21.9 | |
| RHK cem | 1 | 5.9 | MRH cem | 4 | 12.5 | ||||
| MRH cem | 1 | 5.9 | Noiles cem | 2 | 6.3 | ||||
| Femoro-patellar | Spherocentric cem | 1 | 50.0 | Competitor cem | 31 | 75.6 | Competitor cem | 40 | 59.7 |
| Mis Avon cem | 1 | 50.0 | Mis Avon cem | 5 | 12.2 | FPV cem | 12 | 17.9 | |
| Spherocentric cem | 4 | 9.8 | Mis Aon cem | 4 | 6.0 | ||||
| Vanguard PFC cem | 1 | 2.4 | Spherocentric cem | 3 | 4.5 | ||||
| Unicompartmental | Zimmer Uniknee cem | 27 | 26.7 | Zimmer Uniknee cem | 64 | 34.6 | Accuris cem | 120 | 41.0 |
| Accuris cem | 25 | 24.8 | Accuris cem | 51 | 27.6 | Oxford iiicem | 76 | 25.9 | |
| Oxford iiicem | 16 | 15.8 | Oxford III cem | 40 | 21.2 | Zimmer Uniknee cem | 53 | 18.1 | |
| Miller/Gallante cem | 12 | 11.9 | Endomodel cem | 14 | 7.6 | Endo Model cem | 15 | 5.1 | |
| Advance cem | 10 | 9.9 | Eius cem | 7 | 3.8 | Eius cem | 9 | 3.1 | |
cem indicates a cemented component.
The 5 most frequent stem and cup combinations in primary hip arthroplasties according to period and type.
| Type | 2005–2006 | 2007–2008 | 2009–2010 | ||||||
| Model | N | % | Model | N | % | Model | N | % | |
| Unipolar monoblock | Austin Moore – Surgival | 205 | 43.8 | Austin Moore – Surgival | 386 | 41.3 | Austin Moore – Surgival | 599 | 45.0 |
| Austin Moore – Stryker | 114 | 24.4 | Austin Moore – Stryker | 365 | 39.1 | Austin Moore – Stryker | 552 | 41.4 | |
| Thompson cem – Surgival | 79 | 16.9 | Thompson cem – Surgival | 81 | 8.7 | Thompson cem – Stryker | 96 | 7.2 | |
| Austin Moore – Biomet | 46 | 9.8 | Austin Moore – Traiber | 37 | 4.0 | Thompson cem – Surgival | 76 | 5.7 | |
| Austin Moore – Traiber | 24 | 5.1 | Austin Moore – Biomet | 35 | 3.7 | Austin moore – Biomet | 4 | 0.3 | |
| Unipolar modular | Eco cem (Hemi head) | 60 | 63.2 | Eco cem (Hemi head) | 90 | 66.7 | CCM cem (Hemi head) | 57 | 30.3 |
| Eco cem (S30) | 35 | 36.8 | Fullfix cem (Hemi head) | 43 | 31.8 | Fullfix cem (Hemi head) | 45 | 23.9 | |
| Eco cem (Mathys) | 1 | 0.7 | Autobloqueante cem (Endocefalica) | 36 | 19.1 | ||||
| Wagner SL(Hemi head) | 1 | 0.7 | Cemira cem (Hemi head) | 27 | 14.4 | ||||
| Eco cem (Hemi head) | 18 | 9.6 | |||||||
| Bipolar | Mainstream M, cem (Captive) | 45 | 13.3 | Autobloqueante cem (Biarticular) | 150 | 18.4 | Autobloqueante cem (Biarticular) | 191 | 11.7 |
| Muller cema (UHR Bipolar) | 40 | 11.8 | Autobloqueante cem (Surgival) | 120 | 14.7 | Autobloqueante cem (Surgival) | 162 | 10.0 | |
| Autobloqueante cem (Biarticular) | 37 | 10.9 | Mainstream M, cem (Captive) | 120 | 14.7 | Karey cem (Moonstoone) | 80 | 4.9 | |
| Eco cem (Captive) | 36 | 10.6 | Eco cem (Captive) | 61 | 7.5 | Versys cem (Multipolar) | 79 | 4.9 | |
| Versys cem (Versys) | 23 | 6.8 | Versys cem (Versys) | 48 | 5.9 | Furlong cem (Furlong) | 73 | 4.5 | |
| Conventional | Versys (Trilogy) | 211 | 10.6 | Versys (Trilogy) | 356 | 9.0 | Versys cem (Trilogy) | 276 | 5.2 |
| Versys cem (Trilogy) | 162 | 8.1 | Versys cem (Trilogy) | 355 | 8.9 | Versys (Trilogy) | 243 | 4.6 | |
| Furlong (Furlong) | 139 | 7.1 | United U2 (United U2) | 183 | 4.6 | Corail (Pinnacle) | 234 | 4.4 | |
| United u2 (United u2) | 133 | 6.7 | Symax (Trident) | 150 | 3.8 | Furlong (CSF Plus) | 232 | 4.4 | |
| Eco cem (Expansys) | 91 | 4.6 | Furlong (Furlong) | 138 | 3.5 | United U2 (United U2) | 229 | 4.3 | |
| Surface | ASR (ASR) | 13 | 56.5 | ASR (ASR) | 37 | 28.5 | Recap (Recap) | 63 | 29.4 |
| Conserve plus (Conserve) | 5 | 21.7 | Recap (Recap) | 33 | 25.4 | Cormet (Cormet) | 45 | 21.0 | |
| BHR (BHR) | 4 | 17.4 | Icon TM (Icon TM) | 16 | 12.3 | Conserve plus (Conserve) | 28 | 13.1 | |
| Recap (Recap) | 1 | 4.3 | Conserve Plus (Conserve) | 12 | 9.2 | Conserve plus (Conserve Plus) | 19 | 8.9 | |
| Cormet (Cormet) | 9 | 6.9 | Adept (Adept) | 17 | 7.9 | ||||
The stem and the head or cup are presented according to the type of prosthesis in brackets.
cem indicates a cemented component.
aCemented bipolar Muller stem prosthesis by Zimmer.
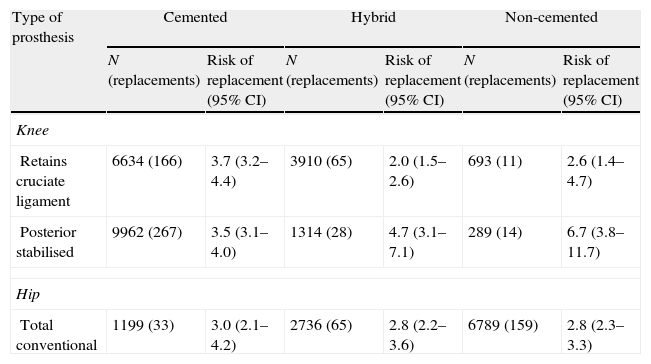
The survival analysis of the prostheses included information on 29,890 knee arthroplasties out of a total 36,951 and on 16,841 hip arthroplasties out of the 26,477 available. The exclusion of these records was related to data quality (absence of laterality, replacement surgery without information on the primary arthroplasty or impossibility in identifying the prosthesis). The median follow-up of knee arthroplasties was 2.4 years (25% of patients were monitored for over 3.9 years and 25% for less than 1.1 years) and 2.0 years for hip arthroplasties (25% of patients were monitored for over 3.4 years and 25% for less than 0.9 years). The cumulative risk of knee arthroplasty replacement was 1.1% (95% CI: 1.0–1.3) at 1 year and 3.3% (95% CI: 3.1–3.6) at 3 years. In the case of total hip arthroplasties, the risk of replacement was 1.9% (95% CI: 1.7–2.2) at 1 year and 2.9% (95% CI: 2.5–3.3) at 3 years. For partial prostheses, this risk was 1.6% (95% CI: 1.3–2.0) at 1 year and 2.5% (95% CI: 2.0–3.1) at 3 years. Table 6 presents the risk of replacement of the most frequent types of prostheses.
Risk of replacement at 3 years of the most frequent types of prosthesis according to the fixation technique.
| Type of prosthesis | Cemented | Hybrid | Non-cemented | |||
| N (replacements) | Risk of replacement (95% CI) | N (replacements) | Risk of replacement (95% CI) | N (replacements) | Risk of replacement (95% CI) | |
| Knee | ||||||
| Retains cruciate ligament | 6634 (166) | 3.7 (3.2–4.4) | 3910 (65) | 2.0 (1.5–2.6) | 693 (11) | 2.6 (1.4–4.7) |
| Posterior stabilised | 9962 (267) | 3.5 (3.1–4.0) | 1314 (28) | 4.7 (3.1–7.1) | 289 (14) | 6.7 (3.8–11.7) |
| Hip | ||||||
| Total conventional | 1199 (33) | 3.0 (2.1–4.2) | 2736 (65) | 2.8 (2.2–3.6) | 6789 (159) | 2.8 (2.3–3.3) |
95% CI: 95% confidence interval.
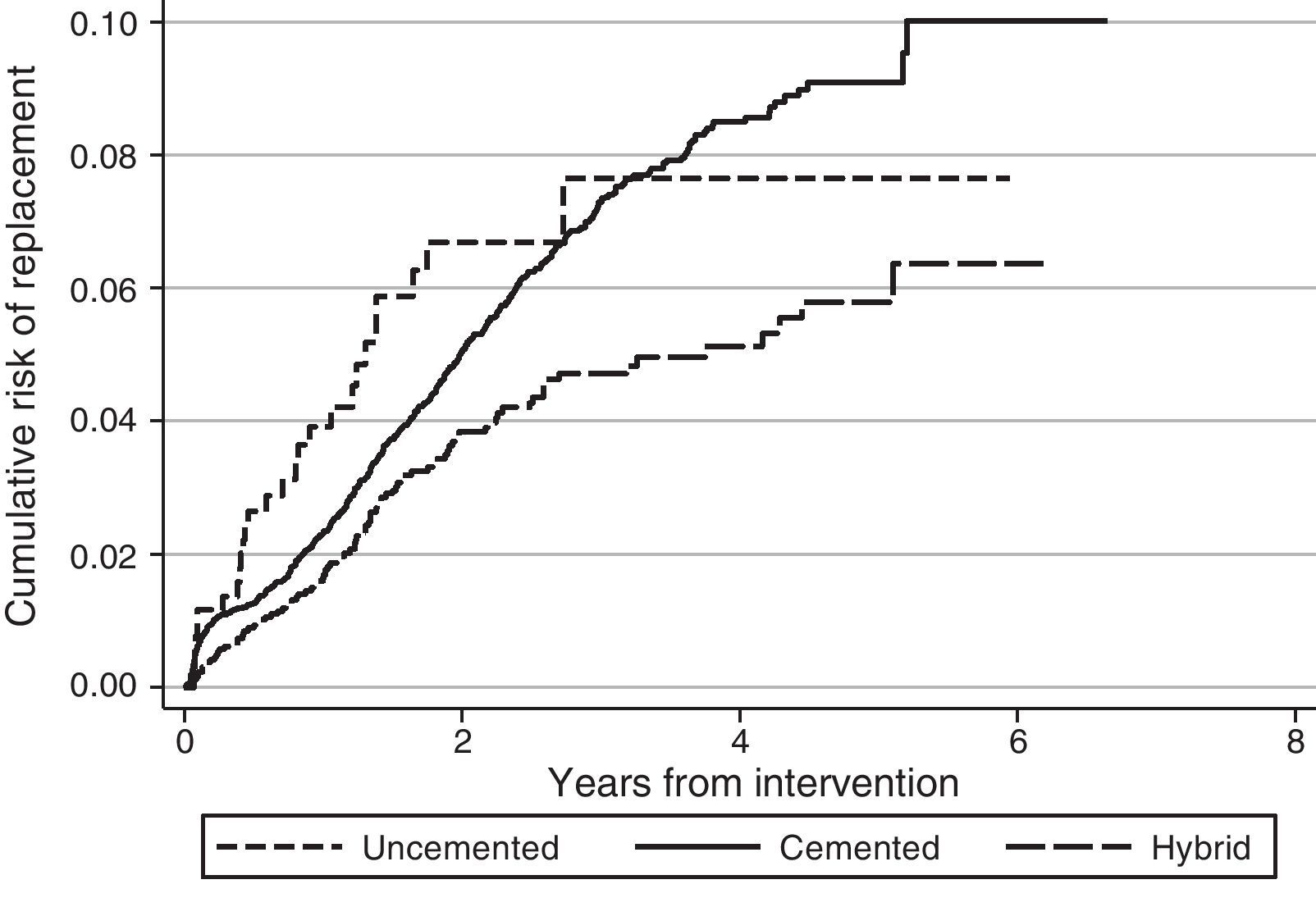
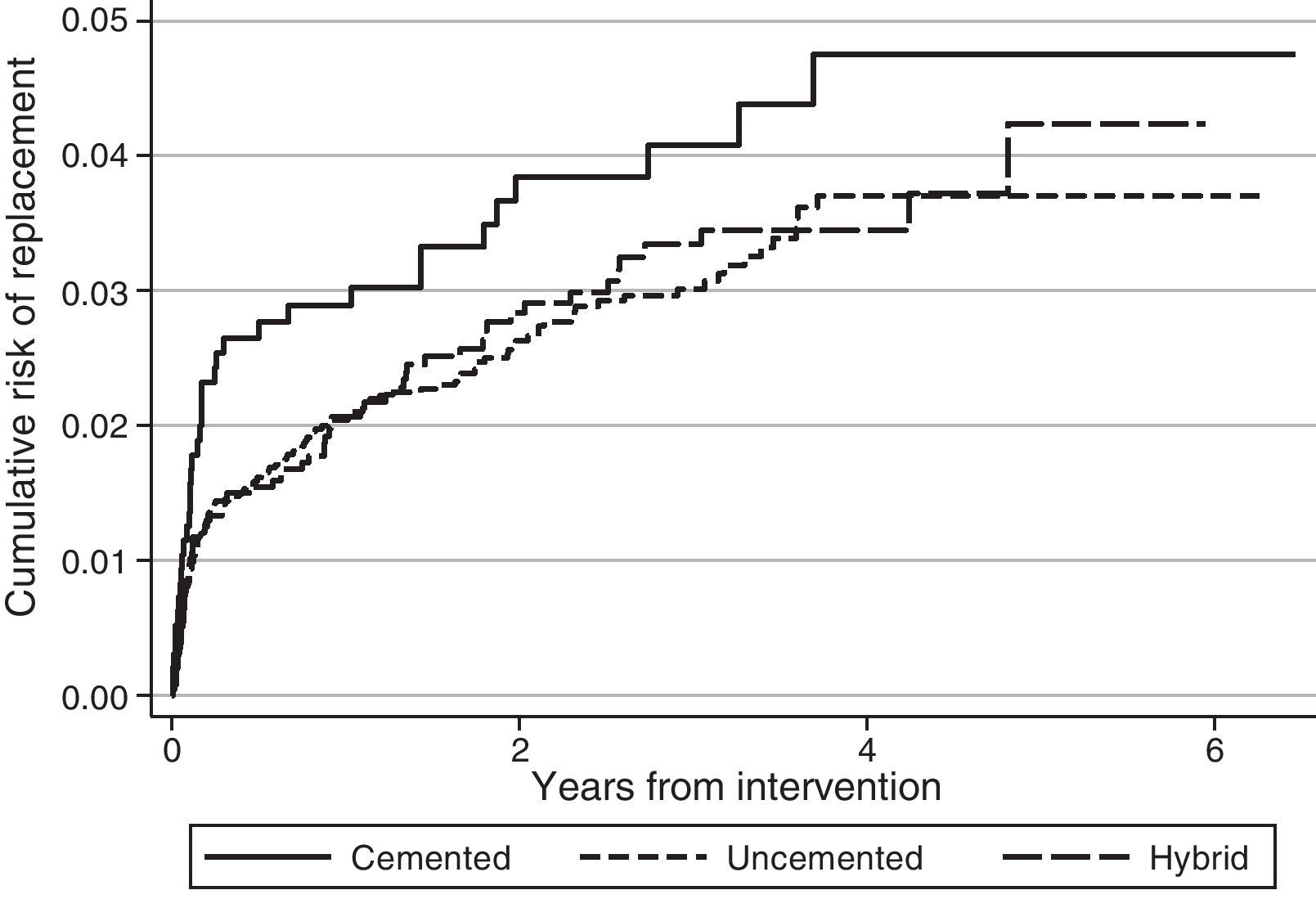
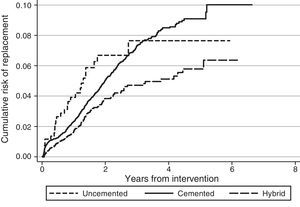
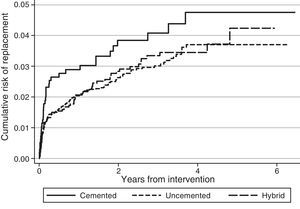
Regarding the risk of replacement adjusted by age and gender according to the fixation technique, no differences were observed between cemented and uncemented prostheses in the case of primary knee arthroplasties (hazard ratio [HR]: 1.2; 95% CI: 0.8–1.8), whereas it was higher for cemented prostheses compared with hybrid ones (HR: 0.7; 95% CI: 0.5–0.8) (Fig. 1). In the case of hip prostheses, no differences were observed in the risk of replacement between the cemented and uncemented implants (HR: 0.7; 95% CI: 0.5–1.1) or between the cemented implants and hybrid ones (HR: 0.8; 95% CI: 0.5–1.2) (Fig. 2). The friction coupling in hip prostheses was not associated with an increased risk of replacement, with the cumulative risk varying between 1.2% (95% CI: 0.6–2.4) for metal–metal and 2.0% (95% CI: 1.7–2.3) for metal–polyethylene at 1 year, and 2.4% (95% CI: 1.5–3.7) for ceramic–polyethylene and 2.9% (95% CI: 2.5–3.3) for metal–polyethylene at 3 years.
DiscussionRACat facilitates the analysis of clinical practice by providing information about the prosthetic models employed and their characteristics, as well as their short, medium and long term results. Upon analysing the data available in the registry it has been observed that the risk of replacement at 3 years (knee: 3.3% [95% CI: 3.1–3.6]; hip: 2.9% [95% CI: 2.5–3.3]) in RACat is higher than that reported by the registry of England and Wales (knee: 2.68% [95% CI: 2.61–2.74]; hip: 2.32% at 3 years [95% CI: 2.25–2.38]), and it is expected that after 7 years of follow-up it will also be higher than those of the Norwegian registry (5.2% [95% CI: 4.8–5.6]) and the registry of the U.S.A. insurer Kaiser Permanente (3.7% [95% CI: 3.4–4.0]).13 In fact, at present the replacement rate of knee arthroplasties in RACat (9.0%) is higher than those of the Italian (6.4%), England and Wales (6.0%) and Swedish (5.2%) registries.14–16
The fact that replacement rates are higher in RACat than in other registries may be associated with the style of practice. In Norway, 94.7% of the total knee arthroplasties were performed without patellar replacement, while the registry of Kaiser Permanente reports replacements in 98.3% of cases, with a similar use of uncemented implants, showing longer survival in Kaiser Permanente.13 In the case of other European countries, patellar replacement took place in 76% of cases in Denmark and only in 11% and 14%, respectively,11 in Norway and Sweden, and in 32% in England and Wales.14 In knee arthroplasty, uncemented or hybrid fixations were also more common in Denmark (22%) than in Norway (14%) and Sweden (2%), which was the country with the lowest risk of replacement.11 In England and Wales, uncemented or hybrid implants accounted for 15% of the total.14
Patellar replacement in RACat is within the range of other countries (38.3%), although it may not be a relevant factor for the survival of the prosthesis.17 Nevertheless, uncemented and hybrid implants are more common (26%) and this fact could influence the risk of replacement since survival is usually less in uncemented implants compared to cemented ones.18 However, these differences have not been observed in RACat. Another aspect that may influence the results is the elevated number of prosthesis models employed. Among other factors, the Swedish hip registry attributed an improvement of its results to a limitation of the number of different prosthesis models employed,19 although it would be necessary to confirm this hypothesis with further studies. Despite these results, the differences observed should be interpreted with caution since they are raw comparisons, so that aspects related to the characteristics of patients in each registry or the information collected and its interpretation are not taken into account.11
Data quality in RACat has improved throughout the study period. However, coverage is not as high as in other registries and the lack of information on laterality and data enabling prosthesis identification have led to various cases being excluded from survival analysis. This could affect estimates, although the tendency towards improvement of data quality will limit this effect in the future. In the case of Australia, coverage reached 93% by the year 2010,20 England and Wales reached 99.1% that same year,14 and the Swedish records (hip and knee) also exceeded 95% coverage,15,21 while coverage in RACat was 78.3% for knees and 71.6% for hips in 2010. This could limit the representativeness of the results. However, it should be noted that, for the moment, only those hospitals in the public network of Catalonia are participating in the project, and these only account for 80–85% of the total surgical activity. Furthermore, the available monitoring time may currently represent a limitation to evaluate the results of the prostheses, which could partly explain the lack of differences in risk of replacement of the metal–metal friction coupling, for which a higher risk of replacement was expected.6 Other factors that may have influenced this result are the small number of surface prostheses implanted and differences in the selection of prostheses. In Catalonia, 2 of the 3 surface models with less survival,6 Bionik® (Orthodynamics, Lübeck, Germany) and Icon® (International Orthopaedics, Geisingen, Germany), are not used and the third, DePuy Articular Surface Replacement® (ASR), has only been implanted in 50 patients. In the case of femoral head size, this could not be analysed as detailed in the published works, since there is currently no information on it.
The significant volume of hip and knee arthroplasties performed by the National Healthcare System22 and the impact that this type of procedures have for the healthcare system, call for the creation of a registry of arthroplasties. Knowing the results throughout the National Healthcare System could help to improve the quality of healthcare by providing information to support decision-making by physicians, hospital managers and health planners. In this regard, it should be noted that in Spain there is a lack of relevant information about the results of knee and hip arthroplasties. A comprehensive literature review of articles published by Spanish researchers on hip and knee arthroplasties with a longitudinal design, with a minimum of 6 months follow-up and with a sample size of over 30 patients, yielded 36 works for the period 1996–2006.1 As a possible result of this, prostheses with worse than expected results could go unnoticed. In addition, ongoing evaluation enables the detection of problems not only at the level of the prostheses but also of the hospitals or professionals performing the intervention, as in the case of the registry of England and Wales.14
Making the most of existing experiences in the National Healthcare System could facilitate a consensus on the structure and function of a joint implant registry, which should include the minimum information required by the International Society of Arthroplasty Registries.9 An arthroplasty registry could work as a tool enabling a system of continuous assessment and improvement of the quality of healthcare through a common protocol.23 In turn, this could become an instrument which would provide the information necessary to establish quality standards. Thereupon, obtaining results below a reference standard would not be a problem, but would instead offer an opportunity for improvement.
Level of evidenceLevel of evidence i.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace regarding the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare having obtained written informed consent from patients and/or subjects referred to in the work. This document is held by the corresponding author.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors wish to thank the companies involved for their collaboration in supplying information for the prostheses catalogue of RACat.
Manufacturing companies: aap Implantate AG; Active Implants Corporation; Adler Ortho; Amplitude; Arthrosurface; Aston Medical; B. Braun Surgical, SA; Bioimpianti; Biomet; Biomed; Biotechni; CGDB SRL; Conformis, Inc; ConMed Corporation; Corin Medical; Cousin Biotech; De Soutter Medical; Dedienne Sante; Downs Surgical; Earthy Medical Iternational; ESKA Implants AG; European Medical Contract M.; F.I.I. FH Orthopedics; Finsbury Orthopaedics; Global Medical Implants, SL; Groupe Lépine; Hit Medica; I Ceram (Sa Mil); Implants Industrie; Io, J.R.I.; Johnson & Johnson, SA; Lafitt; Lima Implantes, SL; Mathys; Medin, SA; Merete; New2DM; OHST Medizintechnik AG; Omni Life Science, INC; Orthodynamic Orthopedics; OTHESIO Implants; Permedica; PETER BREHM GMBH; PSB Exactech; Sanortho; Scanos Medical España; Science et Médecine; Serf, SA; Small Bone Innovations, Inc; Smith & Nephew, SA; Socinser 21, SA; Somepic Technologie; Stanmore Implants; Stryker Ibérica; Surgical Medibérica; Surgival CO, SA; Symbios; Tantum; Tecres; Teknimed; Tornier España, SL; Traiber España, SA; Transysteme, SA; United Orthopedic Corporation; Waldemar Link España, SA; Wright Medical Technology; Zimmer, SA.
Distribution companies: 3M España, SA; A&T Soluciones Médicas, SL; A2C; Acuña y Fombona, SA; Alomedic, SL; Bio-implants Medical, SL; Bosch Ortopèdics, SL; Catimp; Distrauma, SL; Euroimplant Medical; Eurotrauma, SL; Grifols; Hospitak, SL; Hospitrauma, SL; HR Fungibles, SL; Intermedic; Karey Ortho, SA; Kinetics Plus, SL; Lifante; M. Kor; Material Médico, SL; MBA; Medcomtech; Medical Service; Meditram Orthopaedic, SL; Orbimed, SA; Palex Medical, SA; Polymedic 2000, SA; Prim Suministros; Prognomed, SA; Scanos Medical España; Stemcup Medical Products AG; Subministraments Medics Lleida, SL; Sucesores de Pedro Molina, SA; Técnicas Médicas MAB, SA; Tramedic, SA; Transplant Services Foundation; Vortrom, SRL; Wescott Medical.
Management Committee. Deputy Manager for CatSalut: Dr. Francesc Brosa; President of the Catalan Society of Orthopaedic Surgery and Traumatology (SCCOT), Chief of Orthopaedic Surgery and Traumatology (COT) at Hospital de Bellvitge: Dr. Federico Portabella; Director of Evaluation, Agency for Information, Self-evaluation and Quality in Health (AIAQS): Dr. Joan Escarrabill.
Advisory Committee. Chairman of the Advisory Committee, Head of COT, Hospital Vall d’Hebron, Plan for Rheumatic and Locomotor System Diseases: Dr. Joan Nardo; Head of COT, Hospital de Mataró: Dr. Jaume Auleda; Clinical Director for COT, Hospital Vall d’Hebron: Dr. Enric Cáceres; Head of COT, Hospital Joan XXIII of Tarragona: Dr. Josep Giné; Head of COT, Hospital de Blanes: Dr. Ramon Oller; Head of COT, Hospital Sta. Maria of Lleida: Dr. Francesc Pallisó; Head of COT, Hospital Clínic of Barcelona: Dr. Santiago Suso; COT Service, Hospital de Granollers: Dr. Alejandro Yunta; Purchase Management and Evaluation of Healthcare Services-CatSalut: Dr. Josep M. Argimon; Waitlist Coordinator for the Division of Request and Activity Records-CatSalut: Dr. Silvia Cutillas; Head of Request and Activity Records Management-CatSalut: Dr. Montse Bustins; Head of Healthcare Purchasing Services and Population Allocation Division-CatSalut: Dr. Carme Casas; Manager of the Healthcare Consortium of Barcelona: Dr. Jaume Estany; Deputy Manager of Healthcare Quality-AIAQS: Mireia Espallargues; Researchers for AIAQS: Vicky Serra-Sutton, Cristian Tebé, Alejandro Allepuz; Project Manager-AIAQS: Olga Martínez.
Participating hospitals. Centre Hospitalari-ALTHAIA, Clínica Girona, Clínica Plató, Fundació Privada, Clínica de Ponent, Corporació Sanitària Parc Taulí, F.G.S. Hospital de la Santa Creu i Sant Pau, Fundació Privada Hospital de Mollet, Fundació Sanitària d’Igualada F.P., Fundació Sant Hospital de la Seu Urgell, H. Sant Joan Despí-Moisès Broggi, H. Universitari Vall d’Hebron, Hospital Clínic i Provincial de Barcelona, Hospital Comarcal Móra d’Ebre, Hospital Comarcal d’Amposta, Hospital Comarcal de Blanes, Hospital Comarcal de l’Alt Penedès, Hospital Comarcal del Pallars, Hospital Dos de Maig-CSI, Hospital General L’Hospitalet-CSI, Hospital General de Granollers, Hospital General de Vic, Hospital Municipal de Badalona, Hospital Mútua de Terrassa, Hospital Provincial Santa Caterina, Hospital Residència Sant Camil, Hospital Sant Bernabé, Hospital Sant Jaume d’Olot, Hospital Sant Joan de Déu de Martorell, Hospital Sant Rafael, Hospital Santa María, Hospital Universitari Arnau de Vilanova, Hospital Universitari Germans Trias i Pujol, Hospital Universitari Sagrat Cor, Hospital Universitari Sant Joan de Reus, Hospital Universitari de Bellvitge, Hospital Universitari de Girona Dr. Josep Trueta, Hospital Universitari de Tarragona Joan xxiii, Hospital de Campdevànol, Hospital de Figueres, Hospital de Mataró, Hospital de Palamós, Hospital de Puigcerdà, Hospital de Sant Boi-Parc Sanitari St Joan de Déu, Hospital de Sant Celoni Fundació Privada, Hospital de Sant Jaume Calella, Hospital de Sant Joan de Déu d’Esplugues Llobregat, Hospital de Sant Pau i Santa Tecla, Hospital de Terrassa, Hospital de Tortosa Verge de la Cinta, Hospital de Viladecans, Hospital de l’Esperit Sant, Hospital del Vendrell, IMAS and Pius Hospital de Valls.
Please cite this article as: Allepuz A, et al. Los registros de artroplastias como sistemas de vigilancia poscomercialización: el Registro de Artroplastias de Cataluña. Rev Esp Cir Ortop Traumatol. 2013;57:27–37.
The names of the band members Arthroplasty Registry of Catalonia (RACat) and participating hospitals are listed in Annex 1.