To assess the surgical parameters and the clinical and radiological outcomes of revisions of resurfacing shoulder arthroplasty to non-cemented short-stem reverse total shoulder arthroplasty.
Material and methodsA total of 23 revisions from resurfacing shoulder arthroplasty to reverse total shoulder arthroplasty were performed. The mean age was 70.3±11.95 years. The patients included 82.6% (19/23) revised for cuff failure; 13.04% (3/23) cuff failure and aseptic loosening, and 4.35% (1/23) peri-prosthetic fracture. The need for humeral osteotomy or structural allograft, operation length, blood loss, blood transfusions and intraoperative fractures were recorded. Minimum follow-up 25 months.
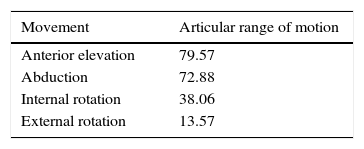
ResultsNo humeral osteotomy or humeral structural allograft was required, and 2/23 (8.69%) required allograft for glenoid reconstruction. The mean operation time was 113.35±21.30min. Intra-operative blood loss was 374±245.09ml. Blood transfusion was required in one case. Intra-operative fracture occurred in 1 case. The Constant score improved from 17.32 to 59.78 (age/sex adjusted, 84). Overall satisfaction improved from 1.37 to 8.04. The range of motion increased 79.57° in forward elevation, 72.88° in abduction, 38.06° in internal rotation, and 13.57° in external rotation. There was no evidence of radiolucency, subsidence, or bone resorption.
ConclusionRevisions of resurfacing implants to non-cemented short-stem reverse prosthesis show good clinical and radiological outcomes, with minimal intra-operative complexities.
Level of evidenceIV, case series.
Valorar los parámetros perioperatorios y los resultados clínicos y radiológicos de la cirugía de revisión de las prótesis de superficie de hombro recambiadas hacia prótesis invertida no cementada con vástago corto.
Material y métodosEntre 2005 y 2012, se realizaron 23 revisiones de prótesis de superficie de hombro a prótesis invertidas. La edad media fue 70,3 años±11,95. Un 82,6% (19/23) de los recambios se realizaron por rotura secundaria del manguito rotador; 13,04% (3/23) por aflojamiento aséptico del componente glenoideo más insuficiencia del manguito y 4,35% (1/23) por fractura periprotésica. Se documentaron: necesidad de ventanas humerales y aloinjerto estructural, duración del procedimiento, pérdidas hemáticas, transfusiones y fracturas intraoperatorias. Seguimiento mínimo de 25 meses.
ResultadosEn ninguno de los casos se necesitó realizar una ventana humeral para la extracción del implante de superficie, así como tampoco aloinjerto estructural. En 8,69% (2/23) de los casos se requirió aloinjerto para reconstrucción glenoidea. La duración del procedimiento fue 113,35±21,30 min. Las pérdidas hemáticas intraoperatorias fueron 374±245,09mls. Se requirió hemotransfusión en un caso. Se produjo una fractura intraoperatoria. El Constant mejoró de 17,32 a 59,78 (ajustado por sexo y edad, 84). La satisfacción general aumentó de 1,37 a 8,04. El recorrido articular aumentó 79,57° en elevación anterior; 72,88° en abducción; 38,06° en rotación interna; y 13,57° en rotación externa. No hubo evidencia de radiolucencias, hundimientos, ni resorción ósea.
ConclusiónLa artroplastia de revisión de las prótesis de superficie de hombro recambiadas hacia prótesis invertida no cementada con vástago corto ofrece buenos resultados clínicos y radiológicos, representando una técnica con complejidades intraoperatorias mínimas.
Nivel de evidenciaIV, serie de casos.
The most frequent indication for revision surgery of a shoulder prosthesis is breakage secondary to insufficiency of the rotator cuff.1 Absence of the depressor effect of the cuff on the humeral head leads it to rise, which finally leads to subacromial impingement, pain and functional impotence. These reasons finally become the indication for revision arthroplasty.
Revision shoulder arthroplasty is a complex procedure with potential complications. Loss of bone stock at the level of the humeral diaphysis and at the level of the glenoid, the involvement of soft tissues and technical matters in connection with the extraction of the implants all make this technique very demanding.
When a resurfacing shoulder prosthesis fails, revision arthroplasty for an anatomical or reverse prosthesis only involves the extraction of the resurfacing implant from the head of the humerus. The fact that the resurfacing prosthesis lacks a stem avoids the need to create a sarcophagus at the level of the humeral diaphysis. Likewise, the fact that the resurfacing prosthesis does not need to be cemented makes it possible to preserve the bone stock and humerus anatomy.2
The results of revision shoulder arthroplasties using anatomical prostheses are disappointing.3,4 Good medium term clinical results have been described with the use of reverse shoulder prosthesis as the primary implant in arthroplasty secondary to rotator cuff insufficiency.5–7 Several studies have shown that the inverted prosthesis represents an alternative which may improve pain and articular function when it is used as a revision implant.8–10 In spite of the fact that the results of the reverse prosthesis as a revision implant are worse than those obtained when it is used as a primary implant11; and in spite of the fact that information on long-term results is still very limited12; reverse shoulder prosthesis is currently considered to be the best alternative in revision surgery.10
The reported complication rates in connection with revision shoulder arthroplasties to reverse prosthesis vary from 36% to 48%.9,13,14 Complications are associated with the duration of surgical procedures, the need to extract a properly affixed humeral stem (cemented or not) and the need to overcome major impairment of the bone stock. These situations may eventually be detrimental for the clinical result.15 Revision arthroplasty of a shoulder resurfacing prosthesis which has no stem and does not involve the obligatory use of cement may be considered a less aggressive surgical procedure.
The advantages that may arise from a shoulder resurfacing prosthesis are: it avoids stress shielding in the cortical side of the humerus, humeral diaphysis is preserved for future revision arthroplasties and there is less risk of diaphyseal periprosthetic fractures.16 It should also be said that a shoulder resurfacing prosthesis does not alter the metaphyseal anatomy of the humerus, unlike anatomical prostheses with a stem.16
The purpose of this study is to evaluate the perioperative parameters, clinical and radiological results of revision surgery of shoulder resurfacing prostheses replaced with a reverse prosthesis.
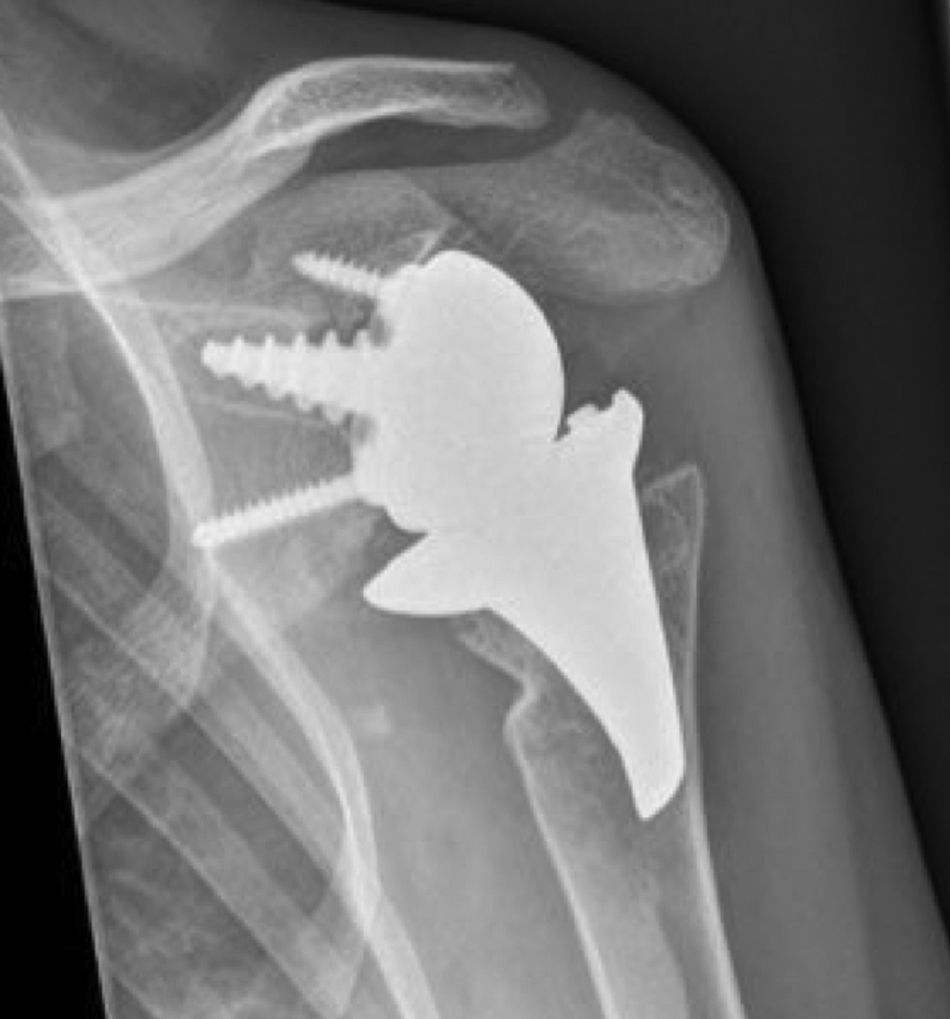
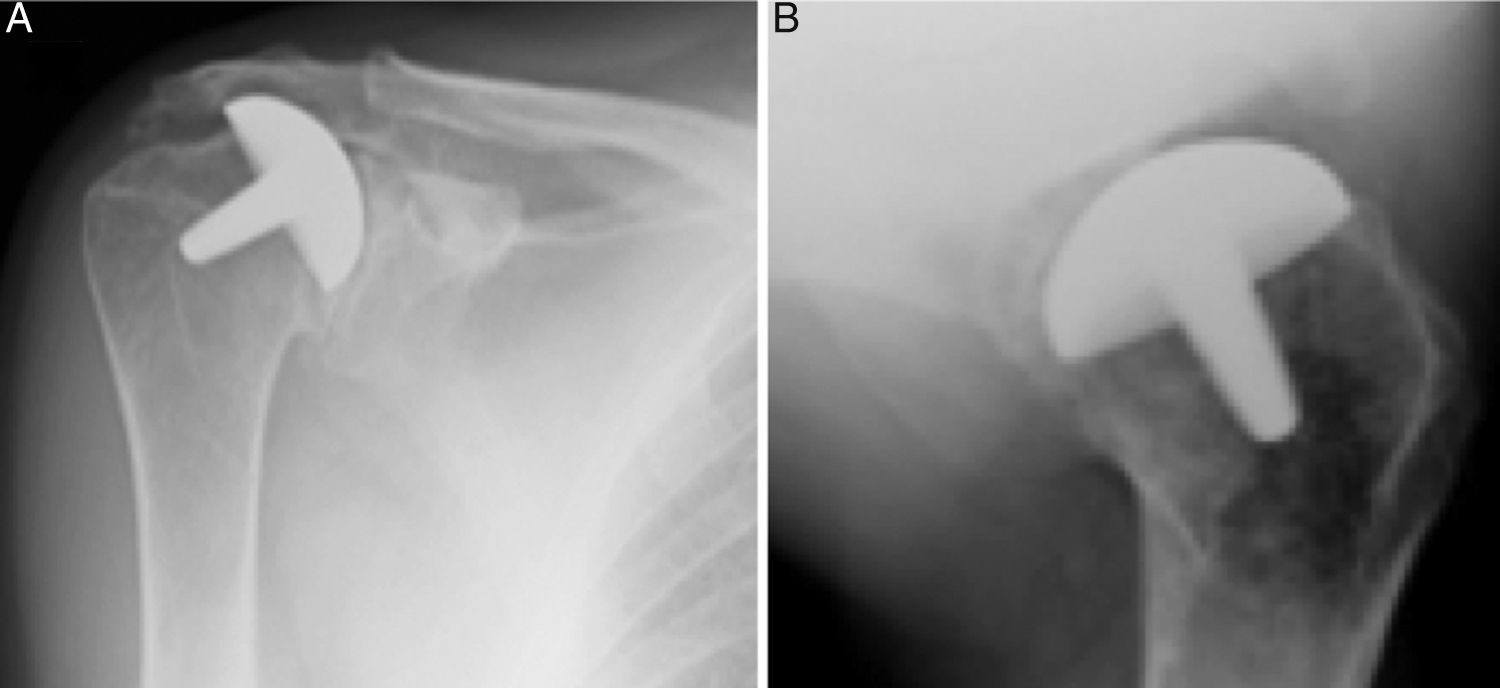
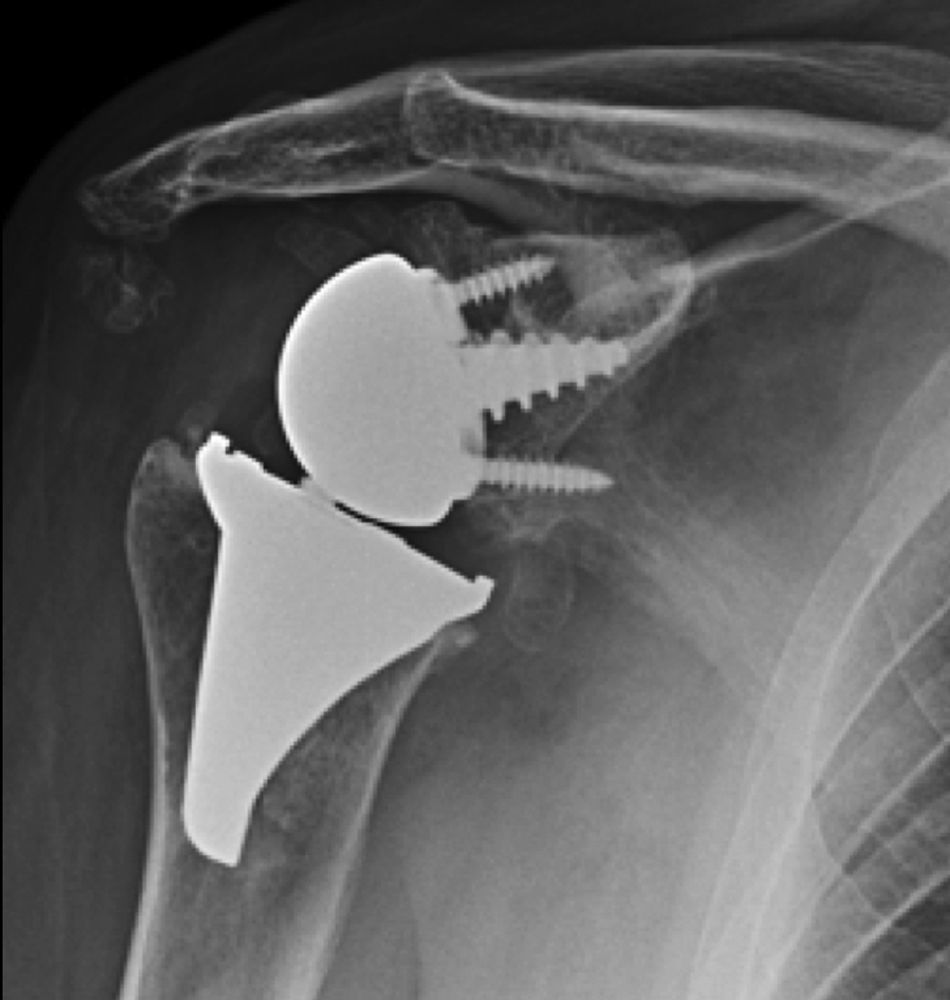
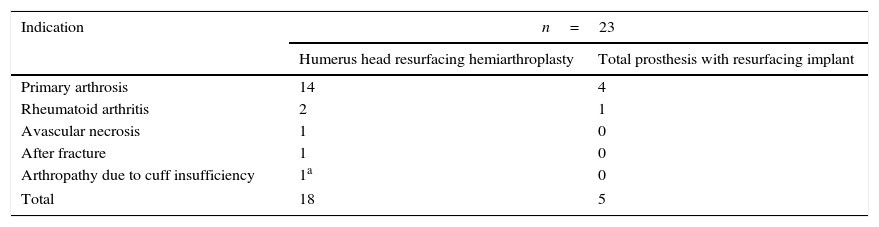
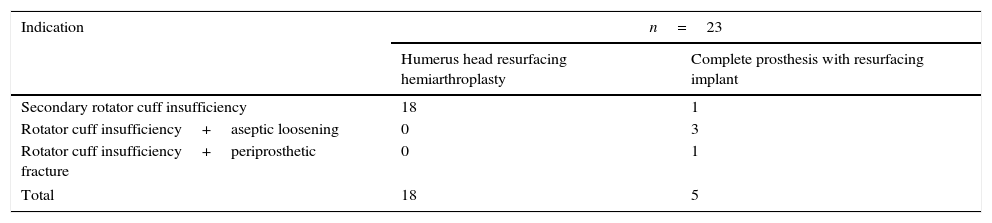
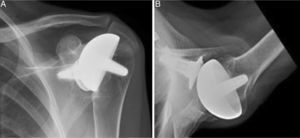
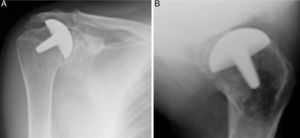
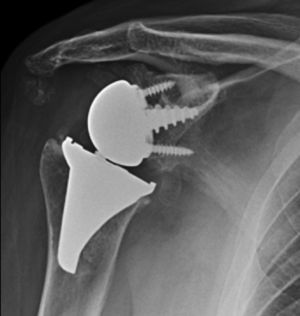
Materials and methodsFrom 2005 to 2012, 23 resurfacing shoulder prostheses were replaced with a reverse non-cemented short stem prosthesis by the senior surgeon OL in Reading Shoulder Unit (Royal Berkshire Hospital and Berkshire Independent Hospital), United Kingdom. Patients of both sexes were included, and the first implant was a shoulder resurfacing prosthesis by Copeland® (Biomet, Swindon, United Kingdom). It was replaced by a Verso® reverse prosthesis (Innovative Design Orthopaedics, London, United Kingdom [previously Biomet, Swindon, United Kingdom]). For the purposes of this study those cases in which the first prosthesis was stemmed were excluded, together with those cases in which a reverse prosthesis was replaced by another one. The average age was 70.3±11.95 years old. There were 19 women and 4 men. The indications for the first implant (resurfacing prosthesis) are shown in Table 1. All of the first resurfacing prostheses were by Copeland®: 18 hemiarthroplasties and 5 total arthroplasties. The average follow-up time after the revision was 43.4 months (ranging from 25 months to 8 years and 5 months). In 86.6% (19/23) of the cases, the indication for revision arthroplasty was breakage secondary to rotator cuff insufficiency. 13.04% (3/23) were changed due to aseptic loosening plus rotator cuff insufficiency (Fig. 1A and B); 4.35% (1/23) of replacements took place after periprosthetic fracture. The indications for revision arthroplasty using a reverse prosthesis are shown in Table 2. In all cases the reverse shoulder prosthesis used was the Verso® system (Fig. 2).
Indications for a resurfacing prosthesis.
| Indication | n=23 | |
|---|---|---|
| Humerus head resurfacing hemiarthroplasty | Total prosthesis with resurfacing implant | |
| Primary arthrosis | 14 | 4 |
| Rheumatoid arthritis | 2 | 1 |
| Avascular necrosis | 1 | 0 |
| After fracture | 1 | 0 |
| Arthropathy due to cuff insufficiency | 1a | 0 |
| Total | 18 | 5 |
Left shoulder X-rays of a 45 year-old woman (juvenile rheumatoid arthritis), (A) anteroposterior and (B) axillary, showing total Copeland® resurfacing arthroplasty of the shoulder prior to revision surgery due to secondary breakage of the rotator cuff and loosening of the glenoid component.
Indications for revision arthroplasty with a reverse uncemented short stem prosthesis.
| Indication | n=23 | |
|---|---|---|
| Humerus head resurfacing hemiarthroplasty | Complete prosthesis with resurfacing implant | |
| Secondary rotator cuff insufficiency | 18 | 1 |
| Rotator cuff insufficiency+aseptic loosening | 0 | 3 |
| Rotator cuff insufficiency+periprosthetic fracture | 0 | 1 |
| Total | 18 | 5 |
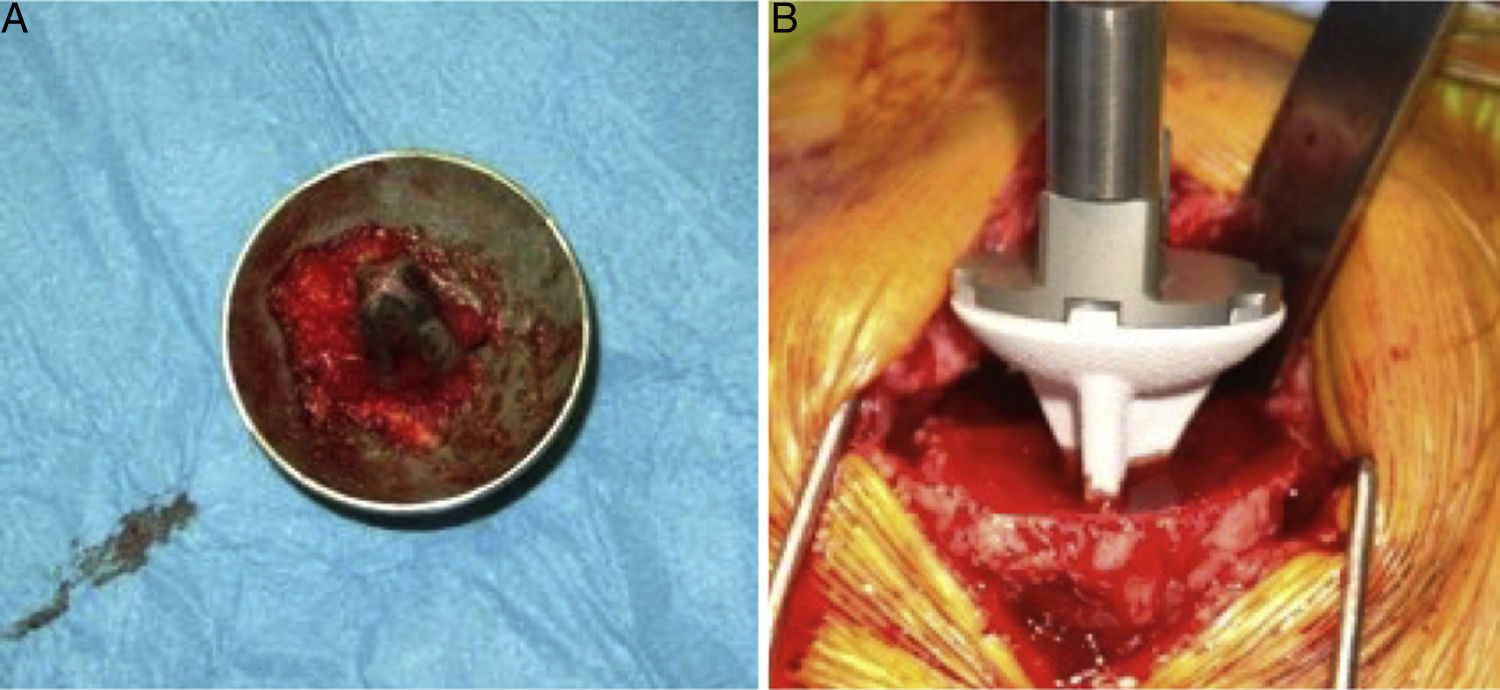
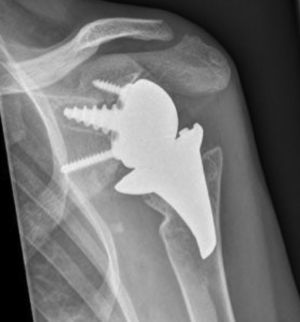
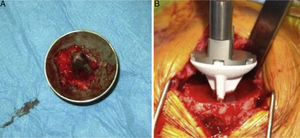
The surgical approach (Neviaser MacKenzie)17 used for implantation of the first implant (resurfacing shoulder prosthesis [Fig. 3A and B]) was the same as that used in the revision arthroplasty (Verso® system of reverse shoulder prosthesis [Fig. 4]). In all cases spongy bone was observed to be affixed to the lower part of the resurfacing implant (Fig. 5A). The Verso® system used in all of the revisions in this series consists of an implant with a mini-stem coated in hydroxyapatite. It is designed to avoid the need to rasp the medullar channel of the humerus and thereby prevent loss of bone stock, and it does not require the use of cement to hold it in place (Fig. 5B). In all cases the bone autograft was impacted at the level of the metaphysis, and it was obtained from the centre of the dried humeral head.
X-rays of the right shoulder of a 58 year-old man in anteroposterior (A) and axillary (B) projections, showing Copeland® resurfacing hemiarthroplasty, prior to revision surgery due to rotator cuff secondary breakage and insufficiency. Figure (A) shows the accentuated rise of the humeral head.
(A) Intraoperative clinical photograph showing an upper view of the Copeland mark-3® resurfacing shoulder prosthesis humeral component, in which the good anchorage can be seen together with the spongy bone of the humeral head in the caudal part of the implant, coated in hydroxyapatite. (B) Intraoperative image showing the insertion of the uncemented hydroxyapatite-coated short stem into the humeral metaphysis.
The concept of this implant involves a direct transfer of force vectors towards the humeral metaphysis, which reduces stress shielding and leads to better bone quality under the implant. The glenoid component too requires no fixing with cement, as the central truncoconical hydroxyapatite coated screw fixes it biologically. The design of the insert includes a low profile medial edge, which gives it a better fit, improved stability and prevents glenoid notching.
Postoperative rehabilitationAll of the patients were immobilised with a sling in internal rotation. This started to be gradually removed in the third week after the operation. Pendular movements commenced from the first day after the operation, and passive unrestricted movements were allowed from the first to the third week after the operation. Work on active mobility commenced from the second to the fourth week after surgery, and work on gaining muscle strength started in the third week.
Intraoperative parameters and complicationsThe parameters measured to evaluate perioperative complications were: the duration of the operation, the amount of blood lost, days of hospitalisation, the need for perioperative transfusion, the need for creating a humeral window to extract the implant, the need to use allograft to replace bone stock, and the creation of intraoperative fractures. Late complications and repeat operations were recorded.
Clinical and radiological evaluationClinical and radiological evaluations took place after a follow-up period of at least 25 months. Clinical and functional assessment before the operation and in the last follow-up visit was based on Constant's test.18 This questionnaire has a potential maximum score of 100 points and in all cases calculation is adjusted for age and sex. The adjusted score is expressed as a percentage of the score foreseen for the age and sex of the patient.19 The level of patient satisfaction is evaluated by subjective measurement on a scale from 0 (completely dissatisfied) to 10 (maximum satisfaction).
Radiological follow-up consisted of radiographic projections 3, 6, 9, and 12 months after the operation, and then annually. The projections used were: anteroposterior, axillary and in internal and external rotations.
Statistical analysisData were analysed using the SPSS 19 (SPSS Inc., Chicago, IL) program. The t-test was used to analyse quantitative variables and χ2 was used for categorical variables.
The χ2-test and variance analysis were used to compare the results before and after the operation. Continuous variables are expressed as averages and standard deviation. Categorical variables are expressed as percentages and frequencies.
The relationship between the variables was analysed using contingency tables for categorical variables, and inference was studied using the χ2-test or Fisher's exact test. Continuous variable inference was calculated using the t-test, and results are expressed with their corresponding standard deviations. The level of significance was set at 5% (α=.05).
ResultsAll of the patients could be contacted to evaluate radiological and functional results.
Intraoperative factorsThe average duration of the surgery to implant the replacement system Verso® was 113.35±21.30min (range 60–145min). Average intraoperative blood losses were 374±245.09ml. In no case was it necessary to perform an osteotomy at the level of the humerus to extract the implant. In 8.69% (2/23) of the revision structural allograft was used at the level of the glenoid. The intraoperative parameters are shown in Table 3.
Intraoperative parameters.
| Variable | |
|---|---|
| Operation duration (min) | 113.35±21.30 |
| Hospitalisation days | 4.90±5.85 |
| Intraoperative blood loss (ml) | 374±245.09 |
| Hb pre-op | 12.83±1.49 |
| Hb post-op | 10.57±1.78 |
| Post-op fall in Hb | 2.26±0.87 |
| Transfusions | 4.38% (1/23) |
| Intraoperative fractures | 4.38% (1/23) |
| Humeral osteotomy | 0% |
| Massive allograft (humerus) | 0% |
| Massive allograft (glenoid) | 8.69% (2/23) |
Intraoperative fractures occurred in 4.30% (1/23) of the revisions. This fracture was at the level of the humeral metaphysis and it was treated by bone suture.
Late complications and repeat operationsThe overall rate of re-revision was 8.7% (2/23). Two of the patients required 2 re-revisions of the humeral component. One of the patients operated again had presented 2 episodes of luxation (the first episode was treated by closed reduction) due to a medial remnant osteophyte that caused impingement at the said level. The treatment consisted of changing and redirecting the humeral insert. The other patient presented a traumatic periprosthetic fracture and the repeat operation consisted of implanting a reverse Verso® prosthesis with a stem.
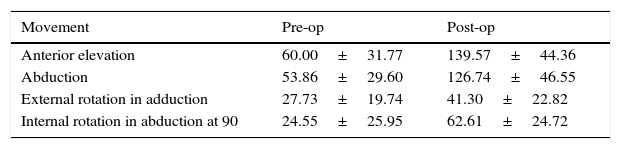
Functional resultsThe postoperative clinical results are shown in Table 4. Improvements can be seen in almost all of the parameters. The gain in articular range of motion (post-op and pre-op) is shown in Table 5. Constant's test and general satisfaction are shown in Table 6.
No radiolucencies, subsidence or metaphysis osteolysis indicative of stress shielding were detected. There was evidence of glenoid notching (Nerot-Sirveaux)20 in 8.7% (2/23) of the patients: grade 1 in one case and grade 2 in the other. In the latter case the patient had juvenile rheumatoid arthritis and in the revision arthroplasty retentive polyethylene was used.
DiscussionThe main finding of this study is that revision surgery of shoulder resurfacing prostheses to replace them with reverse prosthesis does not necessarily involve the creation of a bone window at the level of the humeral metaphysis to extract the implant, nor the use of a structural bone allograft to restore the bone stock.
The indications for shoulder resurfacing arthroplasty are the same as those for an anatomical prosthesis with a stem: pain and dysfunction secondary to primary arthrosis, rheumatoid arthritis and other types of inflammatory arthritis, posttraumatic arthritis, avascular necrosis and arthropathy due to instability.21 The results described in the series of cases in which primary degenerative arthropathy of the shoulder was treated using a resurfacing implant are comparable to the results using an anatomical prosthesis with a stem.22 The revision surgery rate described after 6 years for shoulder resurfacing prosthesis is 5%.21 Huguet et al. concluded that following an average follow-up of 63 patients treated using a shoulder resurfacing prosthesis during 45 months is that the preliminary results indicate a functional improvement that is comparable to that which is obtained by using third and fourth generation stemmed implants.23 In the same way, Habermeyer et al. describe the results in a series of 78 patients with an average follow-up of 72 months. They are similar to those obtained with the latest generation of stemmed implants used in cases of primary and post-traumatic arthrosis.16 These authors state that the rate of loosening of these implants may be lower than those corresponding to latest generation implants with a stem.16 A resurfacing prosthesis is considered as the first option for all of the patients who arrive at the Reading Shoulder Unit with an indication of shoulder arthroplasty. The implantation of an anatomical prosthesis with a stem is only considered for patients with severe loss of bone stock, with fractures, or with pseudoarthrosis.
It has been said that the majority of shoulder (hemi- or total) arthroplasty failures are due to secondary insufficiency of the rotator cuff.24 The cranial migration of the humerus head is the main cause of pain. In our series 91.3% (21/23) of the revisions were performed because of secondary insufficiency of the rotator cuff. Other causes may be connected with the glenoid component, or the native glenoid. Favard et al. state that the rate of surgical revisions due to failure of the glenoid component was 5.6%, while for painful glenoiditis in hemiarthroplasties it was 7.4%.25 In our series 60% (3/5) of the patients in the total prosthesis group required replacement for both reasons: secondary insufficiency of the rotator cuff and aseptic loosening of the glenoid component.
Revision shoulder arthroplasty is considered a technically complex surgical procedure that potentially may lead to perioperative complications. It functional results are also far less predictable than those of primary shoulder reverse arthroplasties. Austin et al. retrospectively revised the results of 28 revision reverse shoulder prostheses, comparing them with a group of 28 primary shoulder reverse prostheses.8 These authors state that clinical results in the group of revision reverse shoulder prostheses were poorer and that the complications rate was higher, at 35.7%.8 In spite of the fact that the functional results obtained following the implantation of a revision reverse shoulder prostheses are clearly inferior to those of the primary reverse prosthesis,5–7,26 reverse prosthesis is considered to be the best alternative for revision surgery.9,10
Respecting perioperative complications with revision shoulder arthroplasty, Flury et al. described an intraoperative complications rate of 43% and a postoperative rate of 38%.15 Wall et al. reported a complications rate at 40 months of 36.7%.27 Boileau et al. described a rate of postoperative complications of 47%,5 while Levy et al. mention 48%.28 Walker et al. subsequently cite a lower rate of complications (22.7%) in a retrospective series of 24 revision reverse shoulder prostheses.29 Subsequently Patel et al. found complications in only 10.7% (3/28) of replacements in their series.30 The gradual fall over time in the rate of perioperative complications in revision reverse shoulder arthroplasties may be a reflection of the experience that shoulder surgeons have gained since the approval of the said implant by the Food and Drug Administration in 2004.30 The overall rate of complications in our series stood at 13% (3/23): 4.35% intraoperative (1/23) and 8.7% postoperative (2/23). We believe that the low rate of perioperative complications recorded in our series may be the result of using implants designed for this purpose: primary resurfacing implants that do not require the use of a stem or cement, and a reverse revision prosthesis with a low medial profile insert to prevent notching and wear, as well as the liberation of particles and the consequent loosening.
In revision surgery it may be necessary to perform osteotomies and create humeral windows to extract humeral stems.31 In the series of Sperling et al., in 9.95% (20/201) of revision arthroplasties it was necessary to perform a humeral osteotomy to extract the implant (the 201 cases in the series consisted of humeral implants with a stem). However, in our series (although it was composed of fewer cases) no humeral osteotomy was necessary to extract any of the resurfacing implants. Extracting a properly affixed humeral stem, regardless of whether it is cemented or not, involves technical complications and may require a long surgical operation. As far as we know, apart from our series there are not other studies which have recorded the average duration of revision shoulder arthroplasty.
Intraoperative loss of bone stock at the level of the proximal humerus and especially in the larger tuberosity may expected when technical difficulties arise in connection with the extraction of cement. Reduction in the bone stock may give rise to the need to use structural allograft. In the revision arthroplasty series of Walker et al., bone allograft had to be used in 62.5% (15/24) of cases, in which it was structural in 41.7% (10/24).29 In our series structural allograft was only used in 8.69% (2/23) of the revisions, at glenoid level.
The main intraoperative complication in revision shoulder arthroplasty is fracture of the humeral diaphysis, which leads to a prolonged surgical procedure with major loss of blood. Boileau et al. describe a series of revision reverse shoulder arthroplasties in which intraoperative fractures occurred in 10.53% (2/19). In our series we recorded fractures in 4.34% (1/23) cases. This difference may be because the primary implants that were replaced in our series had no stem or cement. Given that the results of primary implants that use a stem and cement have been reported to be comparable to those of primary resurfacing implants,16 we believe that whenever possible a resurfacing prosthesis should be used instead of an anatomical prosthesis with a stem for primary arthrosis of the shoulder.
Revision reverse shoulder arthroplasties have been said to be associated with a high risk of infectious complications.32 Boileau et al. cite a 16% infection rate at 40 months.5 In our series no infection was recorded in an average follow-up time of 43.4 months. We believe that the fact that the primary implant was not cemented and had no stem leads to a potentially shorter duration of surgery, so that this may reduce the possibility of developing an infection in the postoperative period.
The use of cement in primary and revision shoulder arthroplasties may affect bone density. Neither the Copeland® shoulder resurfacing prosthesis nor the design of the Verso® inverted prosthesis requires the use of cement for implantation, so that both systems may be considered implants that preserve bone. In replacement arthroplasties the incomplete extraction of the cement may lead to the loosening of the revision components because the remaining cement would hinder bone growth towards the hydroxyapatite.29 We therefore believe that the use of cement and implants with a stem should be avoided as far as possible in primary glenohumeral arthroplasty and in cases of rotator cuff insufficiency.
Melis et al. report a re-revision rate of 22%,12 while the rate recorded in our study is 8.7%. This difference may be due to the design of the implants used in our series. A low medial profile insert that limits notching and wear, as well as the liberation of particles and the resulting loosening; together with an uncemented glenoid component with a central coated truncoconical screw that ensures a very strong primary anchorage. The use of a reverse prosthesis with a short stem and no cement keeps possibilities open in case of revisions being required in the future.33,34
This study is not free of limitations. It is a retrospective revision. There was no control group for the purposes of this study. Nevertheless, the results shown in this study may be compared to those described in the series of patients treated with an implant with a stem.
We conclude that revision shoulder arthroplasty from resurfacing to non-cemented short-stem reverse prosthesis revision offers excellent clinical and radiological results, and that it is a technique of minimum intraoperative complexity.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments in human beings or animals were performed for this research.
Confidentiality of dataThe authors declare that no patient data appears in this paper.
Right to privacy and informed consentThe authors obtained the informed consent of the patients and/or subjects referred to in this paper. This document is held by the corresponding author.
Conflict of interestsLuis Natera MD, Juan Bruguera MD PhD and Ehud Atoun MD have no conflict of interests to declare in connection with this study.
Ofer Levy, MD MCh(Orth) FRCS receives royalties from Innovative Design Orthopaedics® (IDO) for patent rights as a surgeon involved in the design of the implants covered by this study.
Please cite this article as: Natera L, Bruguera J, Atoun E, Levy O. Artroplastia de revisión de prótesis de superficie de hombro hacia prótesis invertida no cementada con vástago corto. Rev Esp Cir Ortop Traumatol. 2016;60:175–183.