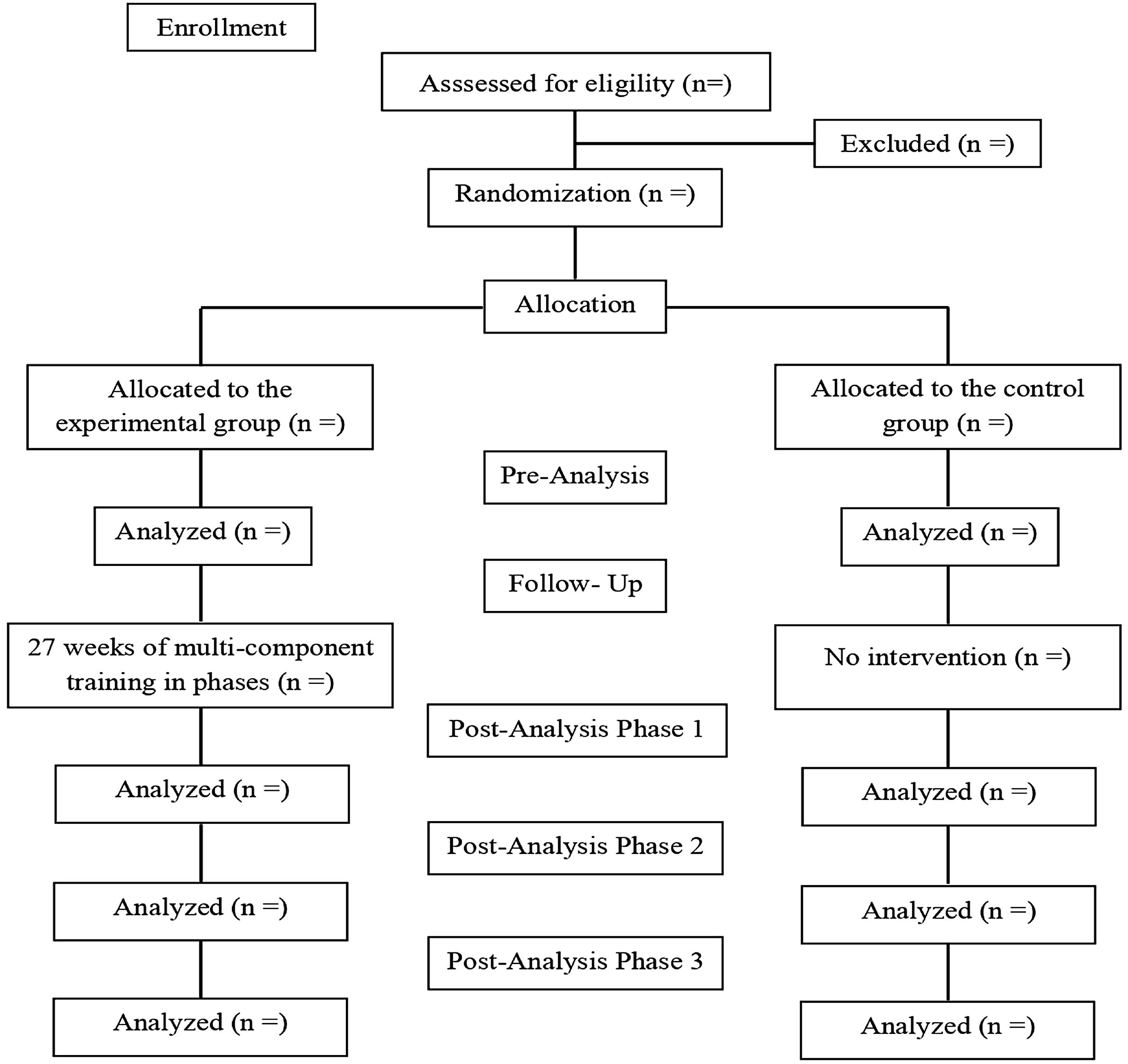
The multicomponent exercise program must be carried out in phases, due to the low tolerance of the old adults to prolonged efforts, since their functional reserve is reduced.
The aim of study is investigate the effects of Multicomponent on Progressive Phases Program on functional capacity, fitness, quality of life, dual-task and physiological variables in the elderly.
MethodsThis is a randomized controlled trial protocol with blind examiners. The protocol was registered at clinictrials.gov (protocol number: NCT04118478). The experimental group will participate in a progressive multi-component program of 27 weeks divided into 3 phases of 9 weeks each of them. Primary outcomes will be determined by evaluating functional capacity using the Short Physical Performance Battery (SPPB), gait speed, and Time up and Go test. Fitness will be determined by the handgrip, 2-min step test, chair sit and reach test, and back scratch test. Quality of life will appear with the SF-36 questionnaire and dual-task with the walking-while-talking test. The physiological variables evaluated will be heart rate and blood pressure at rest, autonomic balance and forced spirometry. Secondary outcomes are determined by measuring the level of physical activity, motivation for exercise, and anthropometric variables.
DiscussionThe results derived from this research will increase the knowledge about the effects of a program of this type. The possible discoveries could serve as a guide to encourage future researchers to develop similar protocols. The purpose of the program is to serve as a practical and viable tool for the benefit of older people.
Clinical trial registry protocol: NCT04118478.
Los programas de entrenamiento multicomponente deben realizarse en fases, debido a la baja tolerancia de los adultos mayores a esfuerzos prolongados, ya que su reserva funcional se encuentra reducida.
El objetivo del estudio es investigar los efectos de un Programa Multicomponente en Fases Progresivas sobre la capacidad funcional, capacidad física, calidad de vida, doble tarea y variables fisiológicas en adultos mayores.
MétodosEste es un protocolo de ensayo controlado aleatorizado con examinadores ciegos. El protocolo se registró en clinictrials.gov (número de protocolo: NCT04118478). El grupo experimental participará en un programa progresivo multicomponente de 27 semanas dividido en 3 fases de 9 semanas cada una. Los resultados primarios se determinarán mediante la evaluación de la capacidad funcional utilizando la batería corta de rendimiento físico (SPPB), velocidad de marcha y la prueba Time up and Go. La capacidad física se determinará mediante la prueba de dinamometría manual, pasos en 2minutos, sentarse y extenderse en la silla y rascado de espalda. La calidad de vida se valorará con el cuestionario SF-36 y la doble tarea con la prueba de caminar mientras habla. Las variables fisiológicas evaluadas serán frecuencia cardíaca y presión arterial en reposo, balance autonómico y espirometría forzada. Los resultados secundarios se determinarán midiendo el nivel de actividad física, motivación por el ejercicio y variables antropométricas.
DiscusiónLos resultados derivados de esta investigación incrementarán el conocimiento sobre los efectos de un programa de este tipo. Los posibles descubrimientos podrían servir de guía para animar a futuros investigadores a desarrollar protocolos similares. El propósito del programa es servir como una herramienta práctica y viable en beneficio de las personas mayores.
The increase in life expectancy has caused a significant growth in the proportion of people older than 60 years.1 Aging is a process inherent in human life that involves the progressive decrease of all physiological processes of the neuromuscular and cardiorespiratory system.2 Furthermore, muscle disuse because of the drastic reduction in daily physical activity in this age group further accelerates the effects of aging where functional capacity and quality of life are depleted.3 Hence, physical exercise appears as an essential intervention for the treatment and prevention of the effects of aging and frailty, this has been a priority to public health.4 Firstly, because of its low cost and second due to no observable adverse effects.5 Accordingly, strength training in older people delays the effects of sarcopenia, through to the maintenance of the functional reserve of the muscle.6 The increases during the first weeks of resistance training can be 10–30% and even further in the elderly.4 Training programs focused on aerobic endurance produce central and peripheral adaptations that lead to increases in maximal oxygen uptake of 16–19% in older adults.7 Likewise, static/dynamic balance training has resulted in positive effects, showing improvements between 16–42%, which is translated into a revealing reduction in the risk of falls.8 Programs with static stretching exercises and full-range movements demonstrate significant improvements in lower back/hamstring flexibility (+ 25%), spinal extension (+ 40%), and shoulder mobility in 70-year-old men and women.9 In this sense, several studies show that physical activity programs that incorporate different physical capacities in their planning, such as multicomponent training, are key to maintaining and improving health in older people.10 Training programs that combine in the same session, work of strength, resistance, balance and flexibility with a duration of 10–24 weeks show an improvement in maximum strength, oxygen consumption, static/dynamic balance and mobility,11 leading to improvements in functional capacity and quality of life.12 However, older adults with low levels of cardiovascular function may not be able to develop endurance programs and other physical capacities in the same session, due to a low tolerance to large volumes of work on account of a reduced functional muscle reserve, requiring a phase of previous progressive force work.13 For this reason, resistance, balance, and flexibility work should be progressively integrated once adaptations to resistance training have been achieved.13,14 In this sense, few studies in older adults apply a progressive strength program in its early stages that increases the levels of functional capacity. Furthermore, this course allows gradually integrate the development of other physical capacities to continue increasing the autonomy and independence. The present manuscript describes a randomized controlled trial protocol with blind examiners which aims to evaluate the effects of a multi-component training schedule, which is distributed in phases of progressive volume focusing on quality of life, functional capacity, and physiological parameters in older adults.
MethodStudy designA study protocol of a randomized controlled clinical trial with blinded examiners is presented. The research will be carried out in accordance with the CONSORT standards,15 written in accordance with the guidelines for interventionist trials (SPIRIT) and approved by the Research Ethics Committee of the Catholic University of Murcia (Approval number: CE101801), registered at clinictrials.gov (protocol number: NCT04118478). Participants will sign the informed consent, prepared in accordance with the Declaration of Helsinki of the World Health Organization.16 In Fig. 1, the flow chart of the sample is presented.
Eligibility criteriaThe inclusion criteria are: (a) be between 60 and 80 years old, (b) be able to move with or without personal/technical assistance, (c) present autonomy to give consent or otherwise, be assisted by a family member or legal representative.
The exclusion criteria are: (a) terminal illness, (b) severe cardiovascular diseases, (c) fractures in the last 3 months, (d) severe dementia, (e) participate in other physical exercise program (s).
Randomization and blindingParticipants will be electronically randomized (https://www.randomizer.org) into experimental (EG) and control (CG) groups. The investigator performing the randomization will be blinded and will not participate in the recruitment process. Participants and group assignment will be blinded to investigators and technicians performing statistical analysis. Participants will be explicitly informed of their assigned group, without the possibility of modification throughout the program. The evaluation team will be made up of five researchers and technicians, two will apply the different phases of the program, one will randomly assign the participants and two others will oversee the statistical analysis. All the analysis of the results will be gathered by the same researcher. The researcher will neither know which group the participant belongs to, nor the main design of the study or the foreseen changes.
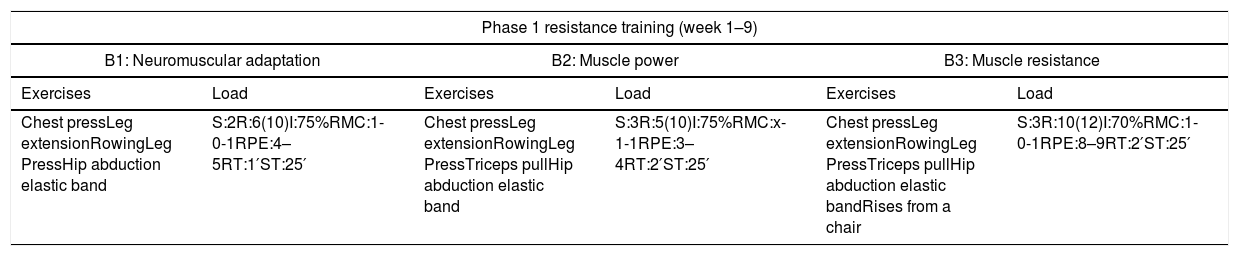
InterventionThe description of the intervention follows the TIDieR checklist. The EG will carry out a 27-week multi-component training program divided into 3 phases of 9 weeks each, where physical qualities will be progressively worked twice a week, on non-consecutive days during the morning session and in groups of 10–15 participants. In each phase the development of a physical quality will predominate. The correspondent programming could result in a progressive improvement in functional capacity and physiological parameters, which can lead to greater autonomy and quality of life. The intervention will take place in a community center for the elderly, equipped with implements for their development. Each session will be supervised by two physical educators with more than 5 years of experience in training the elderly, they will also be instructed for the application of the program two weeks prior to the intervention and supervised weekly by the main investigator. The objective of the first phase will be to develop strength, through variable resistance machines and overload exercises (elastic bands, medicine ball) and, in turn, this phase will be subdivided into 3 blocks of 3 weeks each: Neuromuscular adaptation (block 1), muscular power (block 2), muscular endurance (block 3). Each session will last approximately 45min at an intensity of 70–75% of one maximal repetition (1RM) and with a character of effort from light to maximum. The session will consist of 10min of mobility and muscle activation as a warm-up, 25min of strength exercises and 10min of stretching as a return to calm. The main objective of the second phase will be to develop cardiorespiratory capacity through intermittent gait training in a room measuring 20m long by 10m wide (the walking routes will be demarcated with colored cones) and in turn, It will be subdivided into 3 blocks of 3 weeks each: Static gait (block 1), dynamic gait (block 2) and dynamic gait with changes of direction (block 3). Each session will last approximately 50min with a perception of effort of 6–8. The session will consist of 10min of mobility and muscle activation as a warm-up, 10min of muscle power exercises, 20min of cardiorespiratory resistance and 10min of stretching as a cool down. The last phase will have as a main objective to develop balance and flexibility, by means of the strengthening of the stabilizing muscles and range of movement (bozu, mini bozu, minitramp, fitball). In turn, this phase will be subdivided into 3 blocks of 3 weeks each: Static balance/flexibility (block 1), dynamic balance/flexibility (block 2) and balance/flexibility with dual tasks (block 3). Each session will last approximately 60min with a perception of effort of 6–8. The session will consist of 10min of mobility and muscle activation as a warm-up, 10min of power exercises, 10min of cardiorespiratory resistance, 20min of balance exercises and 10min of stretching as a return to calm). The force loads will be individualized through 10 maximal repetition test (10 RM)17 per exercise every 6 sessions and will be dosed by the character of the effort (CE).18 The loads for cardiorespiratory work, balance, and flexibility will be established through the range of perceived exertion (RPE).19 Both the CE and the RPE will be explained at the beginning of the intervention and reviewed in each session to control the perceived effort in each exercise.
The detailed protocol of the program can be viewed in Table 1. Attendance of participants to the program will be recorded throughout the intervention by the physical educators at the beginning of each session. To promote adherence of the EG to the program, the sessions do not include work on muscle failure, avoiding fatigue and muscle pain that can cause demotivation and abandonment.18 In addition, the setting will be adapted according to tastes and preferences (music, attention from the research team and weekly coexistence meetings).
Multi-component phased training program.
| Phase 1 resistance training (week 1–9) | |||||
|---|---|---|---|---|---|
| B1: Neuromuscular adaptation | B2: Muscle power | B3: Muscle resistance | |||
| Exercises | Load | Exercises | Load | Exercises | Load |
| Chest pressLeg extensionRowingLeg PressHip abduction elastic band | S:2R:6(10)I:75%RMC:1-0-1RPE:4–5RT:1′ST:25′ | Chest pressLeg extensionRowingLeg PressTriceps pullHip abduction elastic band | S:3R:5(10)I:75%RMC:x-1-1RPE:3–4RT:2′ST:25′ | Chest pressLeg extensionRowingLeg PressTriceps pullHip abduction elastic bandRises from a chair | S:3R:10(12)I:70%RMC:1-0-1RPE:8–9RT:2′ST:25′ |
| Phase 2 endurance training (week 10–18) | |||||
|---|---|---|---|---|---|
| B1: March in place | B2: Dynamic gait | B3: Dynamic/multi-directional gear | |||
| Chest pressLeg extensionRowingLeg Press | S:2R:4(10)I:75%RMC:x-1-1RPE:3–4RT:1′ST: 10′ | Chest pressLeg extensionRowingLeg Press | S:2R:4(10)I:75%RMC:x-1-1RPE:3–4RT:1′ST: 10′ | Chest pressLeg extensionRowingLeg Press | S:2R:4(10)I:75%RMC:x-1-1RPE:3–4RT:1′ST:10′ |
| Intermittent static gear | S:3×6′WT:10″RT:20″Dn: 1:2P:1′RPE:6ST: 20′ | Dynamic intermittent gait | S:3×6′WT:15″RT:15″Dn: 1:1P:1′RPE:7ST: 20′ | Dynamic ride with changes of direction | S:3×5′WT:20″RT:10″Dn: 2:1P: 2:30′RPE:7ST: 20′ |
| Phase 3 Flexibility and balance (week 19–27) | |||||
|---|---|---|---|---|---|
| B1: Static balance-flexibility | B2: Dynamic balance/flexibility | B3: Dual task dynamic balancing/flexibility | |||
| Chest pressLeg extensionRowingLeg press | S:1R:7(10)I:75%RMC:x-1-1RPE:3–4RT:2’ST: 10′ | Chest pressLeg extensionRowingLeg press | S:1R:7(10)I:75%RMC:x-1-1RPE:3–4RT:2′ST: 10′ | Chest pressLeg extensionRowingLeg press | S:1R:7(10)I:75%RMC:x-1-1RPE:3–4RT:2′ST: 10′ |
| Dynamic intermittent gait | S:2×4′WT:10″RT:20″Dn: 1:2P:2minRPE:6ST: 10′ | Dynamic ride with changes of direction | S:2×4′WT:15″RT:15″Dn: 1:1P:2minRPE:7ST: 10′ | Dynamic march with changes of direction on the demarcated line and with manipulation of the globe. | S:2×4′WT:15″RT:15″Dn: 1:1P:2minRPE:8ST: 10′ |
| Hip abduction and static unipodal thrustCross balanceActive stretching:Upper and lower limb and trunk. | S:4R:15″RPE:6–8RT:1′ST: 20′ | Bozu squat.Static running in minitrampStanding and sitting of the chair in semitandemActive stretching: Upper limb, Lower limb and trunk. | S:4R:15″RPE:6–8RT:1′ST: 20′ | Balance in bozu manipulating a balloonStraight march on demarcated line naming the vowelsActive stretching:Upper and lower limb and trunk. | S:4R:15″RPE:6–8RT:1′ST: 20′ |
B: block; S: series; R: repetitions; I: intensity; RM: maximum repetition; C: cadence; RPE; range of perceived exertion; RT: rest time; ST: spent time; WT: working time; Dn: density; P: pause.
Seniors assigned to the CG will not do any training schedules. They will only attend the measurements and it will be verified through telephone contact with the director of the department of older adults of the commune as a way to ensure that participants are not taking part in other programs.
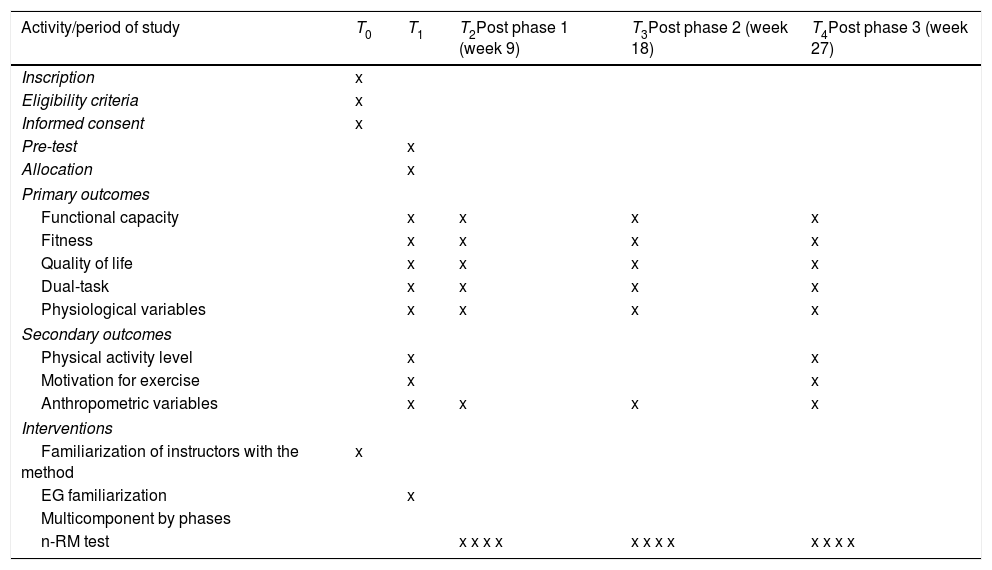
OutcomesThe primary and anthropometric measurement tests will be applied at week 1, 9, 18 and 27, while the secondary measurement tests will only be applied at the beginning and at the end of the intervention. All tests will be administered by two members of the evaluation team and supervised by the main investigator. The physical tests will be performed in a room within the neighborhood unit divided into independent zones. These tests will take place between 8:00 and 14:00 and there will be a 5-minute break between measurements. The timeline for the application of the tests can be seen in Table 2.
Study timeline.
| Activity/period of study | T0 | T1 | T2Post phase 1 (week 9) | T3Post phase 2 (week 18) | T4Post phase 3 (week 27) |
|---|---|---|---|---|---|
| Inscription | x | ||||
| Eligibility criteria | x | ||||
| Informed consent | x | ||||
| Pre-test | x | ||||
| Allocation | x | ||||
| Primary outcomes | |||||
| Functional capacity | x | x | x | x | |
| Fitness | x | x | x | x | |
| Quality of life | x | x | x | x | |
| Dual-task | x | x | x | x | |
| Physiological variables | x | x | x | x | |
| Secondary outcomes | |||||
| Physical activity level | x | x | |||
| Motivation for exercise | x | x | |||
| Anthropometric variables | x | x | x | x | |
| Interventions | |||||
| Familiarization of instructors with the method | x | ||||
| EG familiarization | x | ||||
| Multicomponent by phases | |||||
| n-RM test | x x x x | x x x x | x x x x | ||
Regarding measure functional capacity, The Short Physical Performance Battery (SPPB) will be applied. This battery covers different functional levels and the score ranges between 0 and 12 points (between 4 and 9 points suggests fragility).20 It is composed of three subtests: one of balance (balance feet together, semi-tandem and tandem), push legs (get up and sit on a chair five times as fast as possible) and measure the walking speed at a normal pace over 4 and 6 meters. It has validity and sensitivity in different populations (ICC: 0.87).20
Walking speed 6m: It will be measured by calculating the time it takes the person to travel a distance of 6m, being a speed lower than 0.8m/s an indicator of fragility and risk of falling.21 It is proposed as the simplest and most valid way of functional assessment in the elderly (ICC: 0.87).21
Timed up and go: It will measure the time in seconds it takes for the person to get up from a chair, walk 3 meters, turn, walk back at a normal pace and sit down; to evaluate the mobility and function of the lower limb, proving to be practical and reliable (ICC: 0.95).22 A time ≥13.5s is associated with an increased risk of falling in older people.23
FitnessGrip strengthThe CAMRY EH101® digital dynamometer will be used to record the maximum isometric strength of the upper limb, being a simple and reliable marker (ICC: 0.95).24 The test will be performed by sitting with the elbow flexed 90° and at the signal, the participant will press the device for 5s. 2 attempts will be made with both hands and the highest value will be recorded. In older adults, values<21kg (men) and <15kg (women) are considered indicators of frailty.25
Two-minutes step testThe greatest number of steps will be measured by marching in place for two minutes, each knee reaching an intermediate point between the patella and the anterior superior iliac spine, the number of times that the right knee reaches the determined height.26 A score of less than 65 steps indicates that the subject has poor functional capacity27 and shows high reliability for older adults (ICC: 0.91).27
Chair sit and reach testIt will measure the flexibility of the hamstring and lower back when sitting in a chair to extend the arms to the tip of the foot, while one leg is extended. The distance between the fingertips and the toes will be assigned.28 This test is widely used in older adults, presenting high reliability (ICC: 0.99).29
Back scratch test: It will measure the flexibility of the shoulder joint when trying to bring both hands toward the middle of the back.30 The result will correspond to the distance between the tips of the heart fingers of both hands, registering the distance as negative if the fingers do not touch, and positive if the fingers overlap; in the event that the fingers only touch each other, it will be scored as zero.30 The test demonstrates high reliability in older adults (ICC: 0.99).28
Quality of lifeThe SF-36 health questionnaire will be applied, which represents a generic scale that provides a profile of the state of health and is applicable to the general population, having validity for research and clinical practice (ICC: 0.7).31 It includes 36 items grouped into 8 scales: physical functioning, physical performance, bodily pain, emotional performance, mental health, vitality, general health, social functioning, and change in health over time. The SF-36 scales are ordered in such a way that the higher the score, the better the health status.31
Dual-taskIt will be measured with Walking While Talking Test. The walking speed under the condition of talking along 6 meters, proving to be a reliable instrument for measuring walking speed and cognitive ability in older people (ICC: 0.53–0.92).32 The time taken and the number of errors made in the test will be recorded. A slower walking speed and/or a greater number of stops and errors are considered a marker of frailty and cognitive deterioration.
Physiological variablesResting heart rate and blood pressure will be recorded with the Omron M6 digital arm blood pressure monitor (HEM 7001-E®). The person must be seated and relaxed for 3–5min before starting the measurement, the arm being extended and resting on a firm surface.33 The device meets the validation criteria of the international protocol for blood pressure measurement in different populations.
Heart rate variability will be recorded using the HRV Expert Cardiomood® smartphone application, which is capable of recording RR intervals for analysis of HRV indices with a high level of reliability (r=1.0) and the model POLAR H7® heart rate monitor capable of recording these intervals (r=0.99) as an electrocardiogram (ECG) examination.34 The participant will remain in rest and supine position for 5min with the heart band on the chest and linked to the application, recording the RR intervals during that time.34
Forced spirometry will be measured with the CMS-SP10® manual spirometer, which has good reproducibility and a degree of correlation with lung function (ICC: 1.0).35 The variables considered will be the forced expiratory volume in one second (FEV1ml/sg) and the maximum expiratory flow (PEF L/sg).36 The participant will insert the mouthpiece into his mouth once the maximum inspiration has been made, and then have a sustained exhalation for 6s.36 Values less than 1.5L/s indicate poor lung function.
Secondary outcomesPhysical activity levelPhysical activity level will be measured by two tests. Physical Activity Vital Sign (PAVS) assessed by Moderate to Vigorous Physical Activity (MVPA) which compares by two questions the physical activity performed in the past week and the physical activity performed in a regular week in which the respondent performs MVPA.37 It presents a high correlation (r=0.71) with the modifiable activity questionnaire (MAQ), being applicable for the general population.37 On the other hand, Stanford Brief Activity Survey (SBAS) assesses the levels of physical activity at work and leisure, using five brief descriptions of physical activity profiles,38 ranging from poor to high levels of exercise commitment it should be mentioned that SBAS has been widely used in older adults (r: 0.62).38
Motivation for exerciseThe Exercise Behavior Regulation Questionnaire (BREQ-3) will be used, headed by the phrase “I do physical exercise ⋯” in 23 items that evaluate: intrinsic, integrated, identified, introjected regulation, external and demotivation (ICC=0.70–0.88).39 Its application is valid in older adults.
Anthropometric variablesAbdominal circumference will be measured by a SECA S201® tape which has an accuracy of 0.1cm. It will measure the midpoint between the inner edge of the last intercepted rib with the anterior axillary line and the iliac crest, verifying that the person has not inhaled or that the person has had a forced exhalation. The result will be recorded in centimeters.
Electrical impedance with OMRON HBF-514® device will be used, which provides an anthropometric profile based on the weight/height ratio, % fat/% muscle mass and biological age. This device is a safe, reproducible, and a reliable tool to assess body composition in people up to 80 years (Men r: 0.94–Women r: 0.89).40
Participant timelineOlder adults between 60 and 80 years of Til Til commune who wish to participate in the study must present themselves in August 2019. The volunteers will be evaluated to participate in the study according to the eligibility criteria during the same month.
Sample sizeRegarding the standard deviation established for gait speed in previous studies, an estimated error of 0.07s and a significance level setting at α=0.05; a valid sample size for a confidence interval of 95% will be 85.37.
In the interest of stablishing the sample size, Rstudio 3.15.0 software will be used.
RecruitmentTo reach target sample size, the study will be conducted in Unión Comunal del Adulto Mayor (UCAM) with a high number of older clubs and members. All the participant will be asked to be enrolled in the study. A dropout rate of 15% is assumed based on previous investigations. Therefore, a minimum of 99 participants will be considered for recruitment.
Statistical analysisKolmogorov–Smirnov test will be used to analyze the normality of the variables. It will be reported as (standard deviation) or median (Inter-quartile range, IQR) as appropriate for continuous variables, percentages, and number for categorical variables. A two-way ANCOVA (adjusted for basal value of the primary outcomes) with repeated measurements of one factor (time) will be used to analyze inter- and intra-group differences and to analyze the interaction between groups and time. The post hoc Bonferroni test will be used to evaluate statistical significance of parametric variables. For nonparametric variables, the Wilcoxon signed-rank test will be used to check for intragroup changes, and the Mann–Whitney test to check for intergroup differences. The size effect will be calculated using partial eta-squared (η2p) for variance analysis, and it will be defined as small: ES≥0.10; moderate: ES≥0.30, large: ES≥1.2; or very large: ES≥2.0, and an error of p≤0.05 is set. The statistical analysis is performed using the statistical package SPSS 21.0 for Windows.
DiscussionThe results derived from this research will increase the knowledge about the effectiveness of a training program to improve the functional capacity of the elderly, which could provide this population with greater autonomy and independence. This trial proposes the application of a multicomponent program divided into phases with a predominance of one physical capacity over the others, starting from strength work and later integrating cardiorespiratory resistance, balance, and flexibility in blocks of progressive volume. Although, without neglecting the development of other physical capacities such as autonomy and independence. It is our hypothesis that an intervention like this will improve functional capacity, parameters of the physiological state and quality of life in the study participants.
This protocol uses robust research methodologies being a blinded examiner who performs a randomized controlled trial. The present study may have some limitations since some of the instruments used for both primary and secondary measurements are not the gold standard. However, each of the instruments and measurements used have high reliability and sensitivity for this population. In addition, the long duration of the program makes it unfeasible to avoid losses in the sample, either due to lack of time, age-related conditions, or the geographic limitations of the locality.
The result of this study will not be conclusive, since there are only a small number of studies that apply this type of programs without the need to reach muscle failure.
The possible findings could guide and encourage future researchers to develop this type of protocol in the community of older adults due to its practicality and feasibility.
Trial statusParticipant recruitment started in June 2019, and is expected to end in July 2019. The intervention is expected to start in August 2019. The intervention is expected to end in February 2020.
Ethical approvalUniversidad Católica San Antonio de Murcia. Spain. Committee (protocol: CE101801).
FundingClub de Adultos Mayores de la Villa San José y La Corporación Nacional de Cobre de Chile (CODELCO) División Andina. The funding agencies mentioned had no role in the design, data collection, analysis or interpretation of the study.
Conflict of interestsThere are no conflicts of interest.