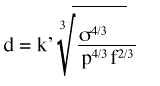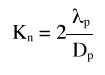Información de la revista
Vol. 18. Núm. 1.
Páginas 21-31 (enero 1998)
Vol. 18. Núm. 1.
Páginas 21-31 (enero 1998)
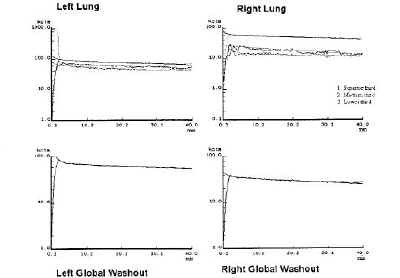
Evaluation of the alveolar-capillary membrane permeability using 99mTc-HMPAO aerosols in severe diffuse interstitial fibrosis
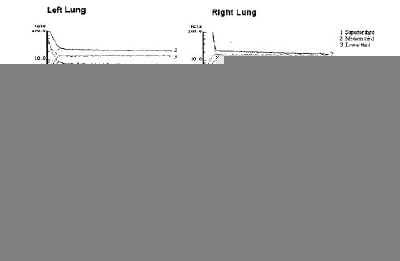
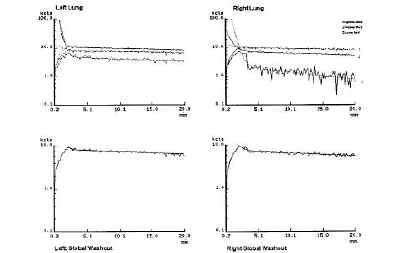
Evaluación de la permeabilidad de la membrana alveolocapilar utilizando aerosoles de 99m Tc-HMPAO en la fibrosis intersticial difusa.
Visitas
1185
Mª F Botelho, João J P de Lima, Manuel D Cerqueira
Este artículo ha recibido
Información del artículo
Opciones para acceder a los textos completos de la publicación Revista Española de Medicina Nuclear e Imagen Molecular
Socio
Si es usted socio de la Sociedad Española de Medicina Nuclear e Imagen Molecular (SEMNIM) puede acceder al texto completo de los contenidos de la Revista Española de Medicina Nuclear e Imagen Molecular desde los enlaces a la revista publicados en la web de la SEMNIN (enlace a https://semnim.es/iniciar-sesion/), previo inicio de sesión como socio. Si tiene problemas de acceso puede contactar con la Secretaría Técnica de la SEMNIM en el correo electrónico secretaria.tecnica@semnim.es o en el teléfono: + 34 619 594 780
Suscriptor
Suscribirse
Contactar
Teléfono para suscripciones e incidencias
De lunes a viernes de 9h a 18h (GMT+1) excepto los meses de julio y agosto que será de 9 a 15h
Llamadas desde España
932 415 960
Llamadas desde fuera de España
+34 932 415 960
E-mail