While we are used to hearing in the media about new and successful viral vaccine launches, recently in the wake of the COVID-19 pandemic, and we have had a wide range of vaccines against different bacterial diseases for a long time, there is still not any licensed fungal vaccine for use in humans. This is largely due to the molecular complexity of eukaryotic fungal pathogens as immunological targets, together with their ability to evade both naturally acquired and vaccine-induced immunity. However, antifungal resistance is increasing mainly among some species of Aspergillus and Candida, and one strategy to avoid the lack of effectiveness of the antifungal treatments in some mycoses could be the development of fungal vaccines.2 At present, no vaccines against aspergillosis are being developed, and there is little data on the effectiveness of vaccines tested against candidiasis. As far as domestic animals are concerned, only one fungal vaccine has been successfully licensed so far to reduce clinical signs and prevent ringworm in cattle. This vaccination was introduced in the former Soviet Union in the 1960s by the Russian mycologist Sarkisov. In this case an attenuated vaccine containing viable microconidia of Trichophyton verrucosum strain LTF-130 was used. This strain is still used in the current commercial vaccine.
Perhaps this lack of vaccines for the prevention of mycoses may soon change with a promising vaccine against coccidioidomycosis. To develop this live attenuated vaccine, an avirulent mutant strain of Coccidioides posadasii was previously obtained by deleting the CPS1 gene.3 This disease is a systemic mycosis affecting both animals and humans and is contracted through the inhalation of C. posadasii and Coccidioides immitis arthroconidia (Fig. 1). This mycosis is common in arid or semi-arid areas, and endemic to the Southwestern United States, Mexico, Central America and South America. Over half of human infections are asymptomatic and confer disease resistance to future exposure. The rest of the cases suffer a clinical disease, estimated to affect approximately 1% of people annually in the highly endemic areas of Arizona and the Central Valley of California.
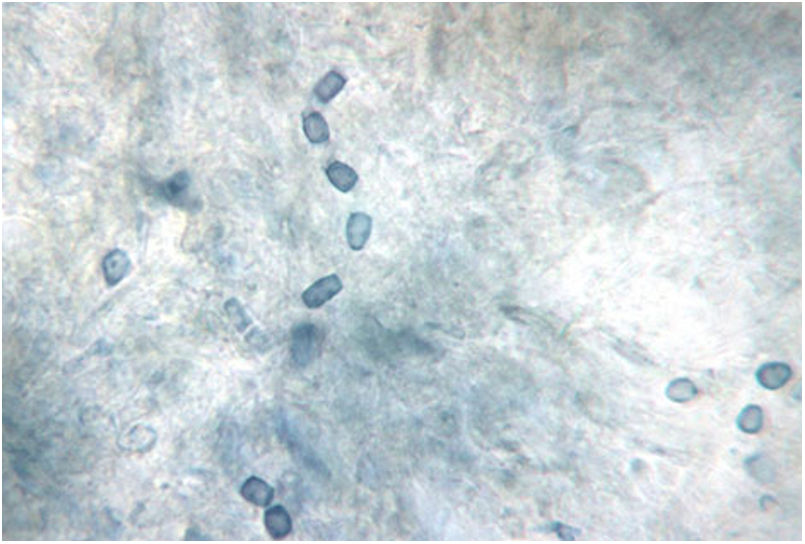
Characteristic barrel-shaped arthroconidia of Coccidioides species, the infectious spores of the mycelial phase. This 3–5μm arthroconidia are easily dispersed in soil and air and inhaled into the deeper airways of the lungs. Fungal culture of the mycelial phase carries a high risk of biological contamination. BSL-3 practices, containment equipment, and facilities are recommended for propagating and manipulating sporulating cultures of these species and for processing soil or other environmental materials known to contain infectious arthroconidia. Lactophenol cotton blue stain. Photo courtesy of Dr. Lisa F. Shubitz. Valley Fever Center for Excellence. The University of Arizona. Tucson, USA.
Dogs are also highly susceptible to coccidioidomycosis and have a higher infection rate than humans. The forms of the disease are similar, but complications in dogs approach 25% of the cases, which often receive long-term treatment with antifungal drugs. The cost of these treatments can be an excessive burden to owners, which may lead to euthanasia or abandonment of the animals. Therefore, the morbidity and mortality of this disease are significant in dogs and humans, and an effective vaccine to prevent coccidioidomycosis would be beneficial for both. This promising vaccine appears to be well tolerated in dogs and protects them significantly from experimentally induced coccidioidomycosis. The public per capita health expenditure related to coccidioidomycosis in the endemic regions of the United States is similar to that for polio, measles, mumps or rubella before effective vaccines were available.
In dogs, as well as in human beings, the infection may be asymptomatic or show up as a primary respiratory disease or a disseminated disease.1 However, a high rate of dissemination is observed in these animals and may involve almost all tissues of the body, with bone being the most common site. As in humans, pulmonary coccidioidomycosis is the most frequent form of canine coccidioidomycosis, and the affected dogs develop an acute to chronic cough, variable fever, anorexia and weight loss. Dogs with disseminated coccidioidomycosis may have skin lesions, osteomyelitis, central nervous system disease and/or pericardial involvement. Clinical signs of pulmonary or disseminated disease may be present for days to years before dog owners seek veterinary care. With regard to treatment, fluconazole is the main drug used to treat all forms of coccidioidomycosis in dogs, as it is cheaper and has a very high oral bioavailability and relatively low toxicity. Other azoles or amphotericin B are usually selected when the disease does not respond to fluconazole, worsens or spreads.
The fungi causing these mycoses are dimorphic. The arthroconidia formed in the mycelial phase are inhaled and, thereafter, they turn out within the host tissues into spherules filled with endoconidia, the parasitic forms (Fig. 2). Infected small desert-dwelling mammals are the reservoir of Coccidioides species in endemic areas, which is a very important fact from the epidemiological point of view of this disease.
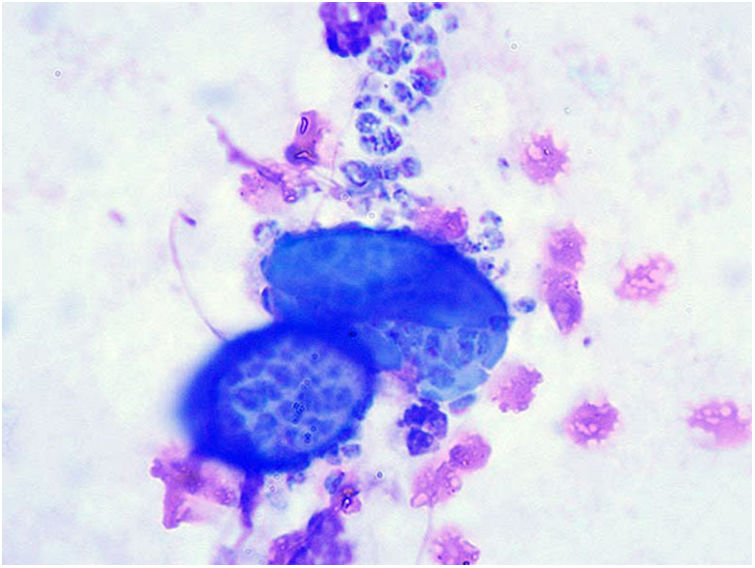
Cytology of a sample from a dog with coccidioidomycosis showing spherules containing numerous endoconidia. These endoconidia can remain in the lung tissue or disseminate to multiple body sites. Diff-Quik stain. Photo courtesy of Dr. Lisa F. Shubitz. Valley Fever Center for Excellence. The University of Arizona. Tucson, USA.
Author has no conflict of interest.
Financial support came from Servei Veterinari de Bacteriologia i Micologia of the Universitat Autònoma de Barcelona.
These Mycology Forum articles can be consulted in Spanish on the Animal Mycology section on the website of the Spanish Mycology Association (https://aemicol.com/micologia-animal/).