Candida-associated denture stomatitis is the most common manifestation of oral candidal infection, caused mainly by Candida albicans. Several authors have attempted to add antifungal agents or antiseptics to denture temporary soft lining materials or to denture acrylic resins, without relevant results. Therefore, the investigation of a quaternary ammonium functionalized compound [2-(methacryloyloxy)ethyl]trimethylammonium chloride (MADQUAT), which copolymerizes with methacrylates and which could act as a fungal inhibitor, is of paramount importance.
AimsTo evaluate the in vitro activity of MADQUAT against Candida species.
MethodsThirty-one Candida strains were used to determine the in vitro antifungal activity of this compound. The minimum inhibitory concentrations and minimum fungicidal concentrations of MADQUAT and nystatin were determined.
ResultsMADQUAT showed antifungal properties at concentrations of 6.25 to > 100mg/ml, and fungicidal activity between 25 and > 100mg/ml. The quantitative determinations of the fungistatic and fungicidal activity of MADQUAT showed fungistatic activity against all Candida albicans, Candida krusei and Candida parapsilosis strains, revealing fungicidal activity against some strains of the other species.
ConclusionsMADQUAT has antifungal activity against Candida spp. Moreover, the sensitivity to this substance varies across the different species in terms of MIC values and fungicidal or fungistatic activity.
La estomatitis protética es la forma más común de infección bucal producida por especies de Candida, siendo Candida albicans el agente etiológico más común. Diversos autores han intentado asociar agentes antifúngicos o antisépticos a los materiales de revestimiento blando o a las resinas acrílicas de las prótesis dentales, pero sin éxito. Por ello, se ha investigado un compuesto de amonio cuaternario (2-metacriloil oxietil trimetilamonio [MADQUAT]), que copolimeriza con los metacrilatos y que podría actuar como inhibidor de levaduras.
ObjetivosEl objetivo de este estudio fue evaluar la actividad in vitro del MADQUAT contra especies de Candida.
MétodosSe utilizaron 31 cepas de Candida para determinar la actividad antifúngica in vitro. Se determinó la concentración mínima inhibitoria (CMI) y la concentración mínima fungicida del MADQUAT, así como de la nistatina.
ResultadosEl MADQUAT presentó propiedades antifúngicas en las concentraciones entre 6,25 y > 100mg/ml y actividad fungicida entre 25 y > 100mg/ml. Los estudios cuantitativos de la actividad fungistática y fungicida del MADQUAT demostraron actividad fungistática contra todas las cepas de Candida albicans, Candida krusei y Candida parapsilosis, revelando actividad fungicida contra algunas cepas de otras especies.
ConclusionesEl MADQUAT presenta actividad antifúngica contra Candida spp. Además, la sensibilidad a dicho compuesto es distinta entre las diferentes especies considerando los valores de la CMI y la actividad fungicida o fungistática.
Candida is both a normal commensal and opportunistic pathogen found in warm-blooded animals, including humans. It colonizes the mucosal surfaces of the oral cavity, digestive or genitourinary tract of healthy individuals and causes a variety of infections depending on host susceptibility.6 The prevalence of candidosis has increased due to the larger number of immunocompromised patients, including those on broad-spectrum antibacterial drugs, transplant recipients, and HIV-infected individuals21 and, therefore, fungal infections have been given a lot of attention. One of the first clinical manifestations of candidosis occurs in the oral cavities of prosthesis (acrylic denture) wearers.
Candida species are found in the oral cavity of 25-50% of healthy individuals, in both adults and children. In denture wearers, these rates climb to 60-100%. Candida albicans is the most common species, accounting for almost 70% of the isolates. In addition to Candida albicans, other species including Candida tropicalis, Candida glabrata, Candida krusei, Candida guilliermondii, and Candida parapsilosis are also usually isolated from denture and non-denture wearers.27
Candida adheres directly or via an intermediate layer of plaque-forming bacteria to denture acrylic resin (polymethylmethacrylate).8 Despite antifungal treatment for denture stomatitis, infection recurs soon afterwards, suggesting that denture plaque may serve as a protected reservoir for C. albicans.7 Several antifungal substances, such as triclosan, nystatin3 and zeolite,23 have been added to denture acrylic resins in order to avoid Candida proliferation on prosthetic device surfaces.
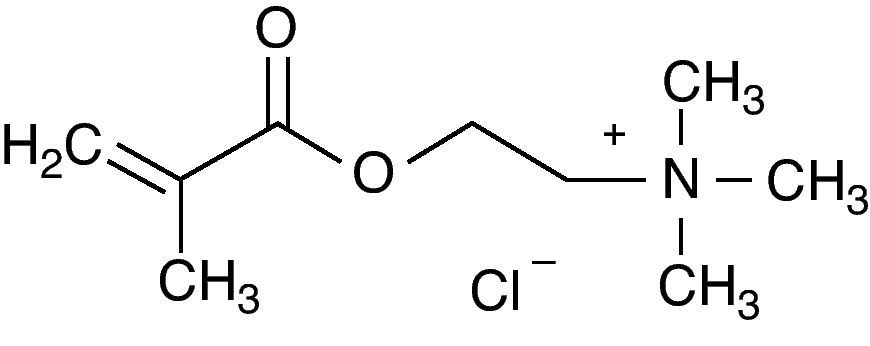
However, the addition of these substances to acrylic resins leads to short-term efficacy due to the leachability of the antifungal agent from the bulk of the polymer. Therefore, the investigation of a quaternary ammonium functionalized compound [2-(methacryloyloxy)ethyl]trimethylammonium chloride –MADQUAT– (fig. 1), which copolymerizes with methacrylates and which could act as a fungal inhibitor, is of paramount importance.
The aim of this study was to evaluate the activity of MADQUAT against Candida species, determining the minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC).
Material and methodsThirty-one Candida strains were used to determine the in vitro antifungal activity: C. albicans (American Type Culture Collection - ATCC 10231, ATCC 18804, ATCC 28367, 0050-L, 0051-L, MG), C. dubliniensis (22, 23, 25, 27, 28, 29, ATCC 7987), C. glabrata (0030-L, 993, ATCC 2001, MG), C. krusei (ATCC 6258, ATCC 20298, 0037-L, 219, 990, MG), C. parapsilosis (ATCC 22019, 0052-L, 0053-L, 0054-L, MG) and C. tropicalis (0056-L, ATCC 750, 0055-L).
Minimum inhibitory concentrations of MADQUAT and nystatin were determined using the Clinical and Laboratory Standards Institute M27-A3 methodology.4 The strains were subcultured onto Sabouraud dextrose agar at 35°C for 24h. The inoculum was suspended in saline solution and adjusted to a final concentration of 0.5 x 103–2.5 x 103 in RPMI 1640 medium (Sigma, St Louis, MO, USA) buffered to pH 7.0 with 165 mmol l-1 morpholinopropanosulfonic acid (MOPS; Sigma).
Nystatin (Jansen-Cilag) was used as positive control. Stock solutions of nystatin and MADQUAT were prepared in dimethyl sulfoxide (DMSO; Vetec) and diluted in RPMI 1640 medium. The final concentrations of the antifungal agents ranged from 0.0312 to 16μg/mL for nystatin and from 0.20 to 100mg/ml for MADQUAT.
Sterilized round-bottomed 96-well microtiter plates (Cral Plast) were used, with addition of 100μl of each drug to columns 1 to 10; 100μl of RPMI 1640 medium were added to columns 11 and 12, which were used as growth positive and medium sterile controls, respectively. Aliquots of 100μl of the standardized inoculum were added to the wells and the microtiter plates were incubated at 35°C for 24h. After incubation, the MIC was determined visually by comparison with the drug-free growth control well. The MIC was defined as the lowest concentration of the antifungal agent preventing visible fungal growth.
In order to determine the MFC, 100μl of all wells with 100% of growth inhibition were seeded into culture tubes with 2ml of Sabouraud dextrose broth medium. The tubes were incubated at 35°C for 3 days to determine fungal growth. The MFC was the minimum fungistatic concentration that prevented fungal growth.10
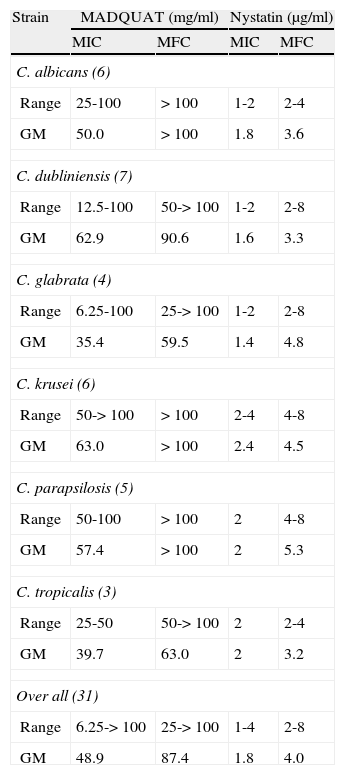
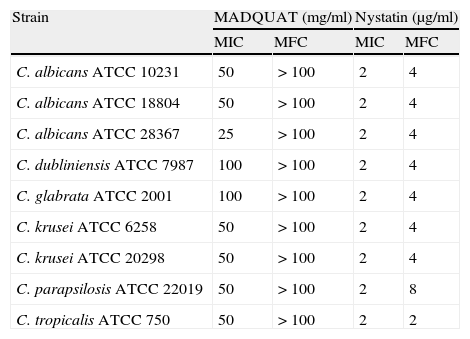
Results and discussionAccording to table 1, MADQUAT showed antifungal activity with a geometric mean (GM) MIC of 48.9mg/ml (230μM) whereas nystatin had a GM antifungal activity of 1.8μg/ml. The quantitative determinations of the fungistatic and fungicidal activity of MADQUAT showed fungistatic activity against all C. albicans, C. krusei and C. parapsilosis strains, revealing fungicidal activity against some microorganisms of the other species. The nine reference strains of Candida spp. used in the present study demonstrated fungistatic activity (table 2). Nystatin showed fungicidal activity against all strains (GM 4.0μg/ml).
Range of antifungal activities of [2-(methacryloyloxy)ethyl]trimethylammonium chloride and nystatin against clinical isolates of Candida spp.
| Strain | MADQUAT (mg/ml) | Nystatin (μg/ml) | ||
| MIC | MFC | MIC | MFC | |
| C. albicans (6) | ||||
| Range | 25-100 | > 100 | 1-2 | 2-4 |
| GM | 50.0 | > 100 | 1.8 | 3.6 |
| C. dubliniensis (7) | ||||
| Range | 12.5-100 | 50-> 100 | 1-2 | 2-8 |
| GM | 62.9 | 90.6 | 1.6 | 3.3 |
| C. glabrata (4) | ||||
| Range | 6.25-100 | 25-> 100 | 1-2 | 2-8 |
| GM | 35.4 | 59.5 | 1.4 | 4.8 |
| C. krusei (6) | ||||
| Range | 50-> 100 | > 100 | 2-4 | 4-8 |
| GM | 63.0 | > 100 | 2.4 | 4.5 |
| C. parapsilosis (5) | ||||
| Range | 50-100 | > 100 | 2 | 4-8 |
| GM | 57.4 | > 100 | 2 | 5.3 |
| C. tropicalis (3) | ||||
| Range | 25-50 | 50-> 100 | 2 | 2-4 |
| GM | 39.7 | 63.0 | 2 | 3.2 |
| Over all (31) | ||||
| Range | 6.25-> 100 | 25-> 100 | 1-4 | 2-8 |
| GM | 48.9 | 87.4 | 1.8 | 4.0 |
GM: geometric mean; MFC: minimum fungicidal concentration; MIC: minimum inhibitory concentration; (): number of isolates.
Antifungal activities of [2-(methacryloyloxy)ethyl]trimethylammonium chloride and nystatin against reference strains of Candida spp.
| Strain | MADQUAT (mg/ml) | Nystatin (μg/ml) | ||
| MIC | MFC | MIC | MFC | |
| C. albicans ATCC 10231 | 50 | > 100 | 2 | 4 |
| C. albicans ATCC 18804 | 50 | > 100 | 2 | 4 |
| C. albicans ATCC 28367 | 25 | > 100 | 2 | 4 |
| C. dubliniensis ATCC 7987 | 100 | > 100 | 2 | 4 |
| C. glabrata ATCC 2001 | 100 | > 100 | 2 | 4 |
| C. krusei ATCC 6258 | 50 | > 100 | 2 | 4 |
| C. krusei ATCC 20298 | 50 | > 100 | 2 | 4 |
| C. parapsilosis ATCC 22019 | 50 | > 100 | 2 | 8 |
| C. tropicalis ATCC 750 | 50 | > 100 | 2 | 2 |
MFC: minimum fungicidal concentration; MIC: minimum inhibitory concentration.
Candida species frequently cause oral infections, including denture-related stomatitis, a chronic inflammatory condition associated with the oral mucosa and that affects 30-60% of patients wearing removable dental prostheses.28 While several factors have been implicated in the etiology of denture stomatitis, such as poor denture hygiene, mechanical irritation, diet, use of antibiotics or allergic reaction to denture base material, Candida species have been recognized as the primary agents.18,34
Microbial growth ensues from the adhesion of microbial cells to rough surfaces and from adhesive interactions between Candida species and oral bacteria, mostly streptococci.28,29 The adhesion of Candida cells is a critical step in the colonization of human mucosal surfaces, on which the yeast lives as a commensal, causing disease whenever an opportunity arises. In addition, fungal adhesins are recognized virulence factors that contribute to pathogenesis.2
Several authors have attempted to add antifungal agents (nystatin, miconazole) or antiseptics (zeolite, triclosan) to denture temporary soft lining materials or to denture acrylic resins, but almost to no avail.3,7,9,23 Quaternary ammonium compounds are age-old and well-known antiseptics with a favorable safety profile, and have been added to a variety of personal hygiene products. These compounds have shown MIC values between 0.25 and > 12,800μg/ml against some bacteria such as Enterococcus faecalis, Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli.13,30,32 Other compounds showed microbiological activity against bacteria, but did not inhibit C. albicans.25,30 However, several bisquaternary ammonium salts were investigated against C. albicans, showing 50% growth inhibition (GI50) values between 2.2 and > 350μM. The most potent compound –1,12-bis(tributylammonium)dodecane dichloride– contained a tributyl head group which seemingly has a direct effect on biological activity, considering that unsubstituted compounds have lower activity. Conversely, the compound contained a trimethyl head group with a GI50 of 175μM.22 MADQUAT also contains this head group, but our results were more consistent as MADQUAT thoroughly inhibited microbial growth at 230μM. Thus, it is possible to infer that the alkyl 2-(methacryloyloxy)ethyl chain of MADQUAT enhances antifungal activity compared to dodecane. Nevertheless, in order to improve the activity of this compound in the future, the trimethyl group of MADQUAT can be replaced with tributyl. Another important issue to be investigated is the ideal pH for use of this polymer. Tapia et al.31 showed that clinical isolates treated with the same polymer had different susceptibility profiles at different pH levels. Thus, this aspect must also be evaluated.
Quaternary ammonium compounds also have intrinsic detergent and anti-adhesive properties, especially against gram-positive bacteria.12,14,24,26 These products reduce surface tension and have stronger attraction for negatively charged surfaces such as bacteria. These characteristics promote the adsorption of these products onto bacterial surfaces. Their mode of action is not associated with surface activity only, and thus cytolytic damage is the primary lesion caused by such cationic surfactants and a major contribution towards cell death. Consequently, there is a well-established relationship between cytolytic action and surface tension.19
In the study carried out by Caillier et al.1 on quaternary ammonium compounds with the lowest inhibition concentration, the MFC is equal to the MIC. In all other cases, MIC and MFC values are very similar, ranging from 14.3 to > 2,000μM. According to these authors, the results are in perfect agreement with the mechanism of action of quaternary ammonium in the first phase of inhibition of cell multiplication with relatively weak concentration (MIC) followed by a second phase of eradication, with higher concentrations (MFC). Our results follow this trend for C. tropicalis and C. glabrata. Although C. dubliniensis showed MIC values greater than the GM obtained for all strains, MADQUAT exhibited fungicidal activity against this species. On the other hand, MADQUAT did not show any fungicidal activity against C. albicans, C. krusei and C. parapsilosis strains at the concentrations analyzed.
In the experiment carried out by Codling et al.,5 a quaternary ammonium compound, myristamidopropyl dimethylamine –MAPD–, possibly induced plasma membrane damage in C. albicans, but did not induce lysis of spheroplasts. According to Vieira and Carmona-Ribeiro,33 the mechanism of antifungal action of quaternary ammonium compounds, hexadecyltrimethylammonium bromide (CTAB) and dioctadecyldimethyl ammonium bromide (DODAB), does not involve fungal cell lysis, but a change in cell surface charge from negative to positive instead.
Probably, this different profile of antifungal activity exhibited by MADQUAT is related to its mechanism of action and to the structure-activity relationship. Two factors known to be important in determining the antimicrobial activity of insoluble polymeric ammonium salts are the positive charge (ammonium group) density and the length of the substituent chain.15,16 High positive charge density is believed to enhance the interaction of the ammonium group with the cytoplasmic membrane, while the long substituent chain may increase the hydrophobicity of the quaternary group, strengthening the interaction with the cytoplasmic membranes.15 Moreover, with an increased hydrophobic surface, less cell adhesion17,20 and a lower long-term degradation11 of polymers are expected. Therefore, more studies are necessary to elucidate the mechanism of action and implications for the polymer properties of MADQUAT.
Our data indicate that MADQUAT has antifungal activity against Candida spp. Moreover, the sensitivity to this substance varies across the different species in terms of MIC values and fungicidal or fungistatic activity.
FinancingThe authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS) for the financial support.






![Chemical structure of [2-(methacryloyloxy)ethyl]trimethylammonium chloride. Chemical structure of [2-(methacryloyloxy)ethyl]trimethylammonium chloride.](https://static.elsevier.es/multimedia/11301406/0000002900000001/v1_201305061404/S1130140611000374/v1_201305061404/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

