In the last three decades the species of Candida have been of great interest due to the high mortality rates that they cause in immunocompromised and hospitalized patients. These species are opportunistic pathogens and they have inhabited other environments long before colonizing human cells. Among these environments we find wastewater from mines, and water from aquifers and soils that contain high concentrations of precious metals as well as toxic and base metals.
AimsThe aim of this study was to assess whether Candida albicans and Candida glabrata are able to maintain homeostasis in the presence of zinc, copper, cobalt or silver.
MethodsTo achieve the objective, each of the Candida species was exposed to every single metal individually in a salt solution. Subsequently the treated cells were lysed to evaluate the compounds formed by means of Scanning Electron Microscopy-Energy Dispersive X-ray spectroscopy (SEM-EDS).
ResultsWhen analyzing the compounds that both C. albicans and C. glabrata formed in the presence of each of the metals, we found that they had synthesized silver sulfide (Ag2S), cobalt sulfate (CoSO4), zinc phosphate (Zn3(PO4)2), or copper oxide (CuO).
ConclusionsOur results indicate that both C. albicans and C. glabrata have enzymatic and non-enzymatic mechanisms that allow them to achieve homeostasis in a different specific manner for each of the single metals to which they were exposed. To our knowledge, this is the first work reporting that C. albicans and C. glabrata can reduce different metals, with the subsequent formation of sulfides, sulfates, phosphates and oxides. This ability, developed over time by these Candida species, is probably a kind of biochemical mechanism in order to survive and colonize many different environments, from water or soil to humans. For this reason, C. albicans and C. glabrata make up an excellent model of study, both from a medical and biotechnical point of view.
Las especies de Candida han cobrado gran interés en las últimas tres décadas debido a los altos índices de mortalidad que ocasionan en pacientes inmunodeficientes y hospitalizados. Estas especies son consideradas patógenas oportunistas y existen otros medios ambientes que estas levaduras han habitado mucho antes de haber colonizado al ser humano: aguas residuales de minas, agua de mantos acuíferos y suelos que contienen altas concentraciones de metales preciosos, metales tóxicos y metales comunes.
ObjetivosEl objetivo del presente trabajo fue evaluar si Candida albicans y Candida glabrata eran capaces de mantener la homeostasis en presencia de los elementos químicos cinc, cobre, cobalto y plata.
MétodosPara lograr el objetivo, las dos levaduras fueron expuestas a cada uno de los metales elegidos de manera independiente, y posteriormente las células tratadas fueron lisadas para permitir la evaluación por medio de microscopía electrónica de barrido con espectrometría de dispersión de energía de rayos X (SEM-EDS) del compuesto formado.
ResultadosAl analizar los compuestos que tanto C. albicans como C. glabrata formaron en presencia de cada metal, se encontró que habían sintetizado sulfuro de plata (Ag2S), sulfato de cobalto (CoSO4), fosfato de cinc (Zn3(PO4)2), u óxido de cobre (CuO).
ConclusionesNuestros resultados indican que tanto C. albicans como C. glabrata poseen mecanismos enzimáticos y no enzimáticos que les permiten alcanzar una homeostasis de manera específica para cada metal al que son expuestas. A nuestro entendimiento este es el primer trabajo que documenta que C. albicans y C. glabrata pueden reducir distintos metales, con la subsecuente formación de sulfuros, sulfatos, fosfatos y óxidos. Esta habilidad que pudieron desarrollar a lo largo del tiempo estas especies de Candida para poder sobrevivir y colonizar medios ambientes tan diferentes, que van desde el agua o los suelos hasta el ser humano, las convierte en un excelente modelo de estudio, tanto desde el punto de vista médico como biotecnológico.
The species of the Candida genus are yeasts that have been found in different ecosystems, such as water, soil, plants and animals, in addition to being opportunistic human pathogens.9,14,24,44 However, in the last three decades, the species of human pathogens of this genus have been increasingly gaining importance as they can cause candidemia and other forms of candidiasis associated to high mortality rates in hospitalized and immunocompromised patients.43 The pathogenic Candida species have developed several virulence factors to colonize the host causing some type of candidiasis.32–41 However, even though the studies of these fungi have already been focused on the area of health, Candida species have also other characteristics unexplored so far, that make them an excellent model of study. One of these characteristics is their ability to live in water or soils that contain high concentrations of precious and/or heavy and base metals.9,14,24,27 Our work group is studying not only the clinical implications of Candida, but also its response to precious metals (gold and silver) and to heavy metals (lead, mercury and cadmium). Interestingly enough, our work group found that the cells of Candida reach a homeostasis (reduction process) in the presence of these chemical elements.9,27 So far, we know that Candida species reach homeostasis in the presence of precious or heavy metals (through the synthesis of sulfide crystals, for example), however, we still need to find whether they can do so in the presence of other chemical elements. Nonetheless, this ability to reach homeostasis in the presence of the precious and heavy metals mentioned shows the great genomic plasticity of Candida to adapt to diverse ecosystems, thus, the key-point will be to know how the Candida species are able to tolerate the toxic chemicals found in the different reported environments, particularly in mine waters. This type of wastewater, one of the main causes of environmental pollution, seems also to be the source of different precious metals (Au, Ag), base metals (Pb, Zn, Cu, Co), iron (Fe) and steel. In order to evaluate how pathogenic Candida species are able to achieve homeostasis in the presence of some of these chemicals, the aim of this study was to assess whether Candida albicans and Candida glabrata are able to tolerate the presence of Zn, Cu, Co or Ag and reach the electrochemical balance.
Materials and methodsStrains and culture conditionsThe reference strains of C. albicans (ATCC 10231) and C. glabrata (CBS 138) were used throughout this study. Yeast strains were cultured on yeast peptone (YP; yeast extract, 1%; peptone, 2% glucose) with 2% agar.6 Zinc sulfate (ZnSO4), zinc nitrate (Zn(NO3)2), copper sulfate (CuSO4), copper nitrate (Cu(NO3)2), silver nitrate (AgNO3) or cobalt nitrate (Co(NO3)2) (Sigma–Aldrich, St. Louis, MO, USA) in a concentration of 1mM were also added independently to the medium.
Lysis of Candida after the formation of compoundsAfter exposing the cultures to the different chemical compounds already described, the cells were lysed in order to evaluate the formation of any related chemical compound produced from homeostasis. Cell lysis was performed according to the protocol proposed by Cuéllar-Cruz et al. and Moreno et al.9,27 Briefly, the cells of Candida were centrifuged at 3500×g for 15min at 4°C. The pellets were washed with sterile deionized water, resuspended in water and the cells were counted. Aliquots of the cell suspension were resuspended at a final OD600nm of 1 in 1ml of lysis buffer containing 50mM Tris–HCl, pH 7.2, 0.8M sorbitol, 0.8M KCl, 10mM MgSO4, 15mM β-mercaptoetanol and 0.25mg/ml lyticase (all reagents from Sigma–Aldrich, St. Louis, MO, USA) and incubated at 37°C. Protoplasts were collected and gently lysed by resuspending in 500μl of sterile deionized water. All Zn, Cu, Co or Ag compounds were first separated from cellular debris. Then, they were thoroughly washed six times with sterile deionized water. The experiments were performed in triplicate.
Scanning electron microscopy (SEM)Zn, Cu, Co or Ag compounds were lyophilized in a Tousimis auto Samdri 815 critical point dryer for 4h. The dried samples were covered with a layer of colloidal gold. Subsequently, the samples were observed in the scanning electron microscope, model EVO HD15, high definition ZEISS®. Finally, the samples were photographed using the secondary electron detector (SE1) at 15kV under high-vacuum conditions at a working distance of 4mm.
Qualitative analysis of elements present in the metals by Energy Dispersive Spectroscopy-(EDS)The particles were observed by SEM and were analyzed qualitatively in order to determine their main components. The percentage of each chemical element in the sample was calculated according to the proportion of the elements found by means of EDS (software Esprit, Bruker Quantax EDS-system, Bruker Nano GmbH, Germany). The analysis of the elements through EDS provides a fast qualitative analysis as well as a semi-quantitative analysis of acceptable accuracy. In a map of quantitative composition (as performed with our samples), under the control of a computer, a complete quantitative analysis is performed to assess the discrete location of each electron beam in the analyzed area.33–39 The X–Y grid of numerical values of concentrations represents the positions of the beam on the sample. This is put in a whole image by using a computed digital processor, which encodes the values of concentration in the corresponding levels of color. The resulting image or compositional map is supported for each element of the image (pixel) by the complete numerical values of the chemical concentration.
The carbon was not considered, as the cellular debris (which is formed by a high percentage of carbon) cannot be completely removed, even though the samples were thoroughly washed six times with sterile deionized water.
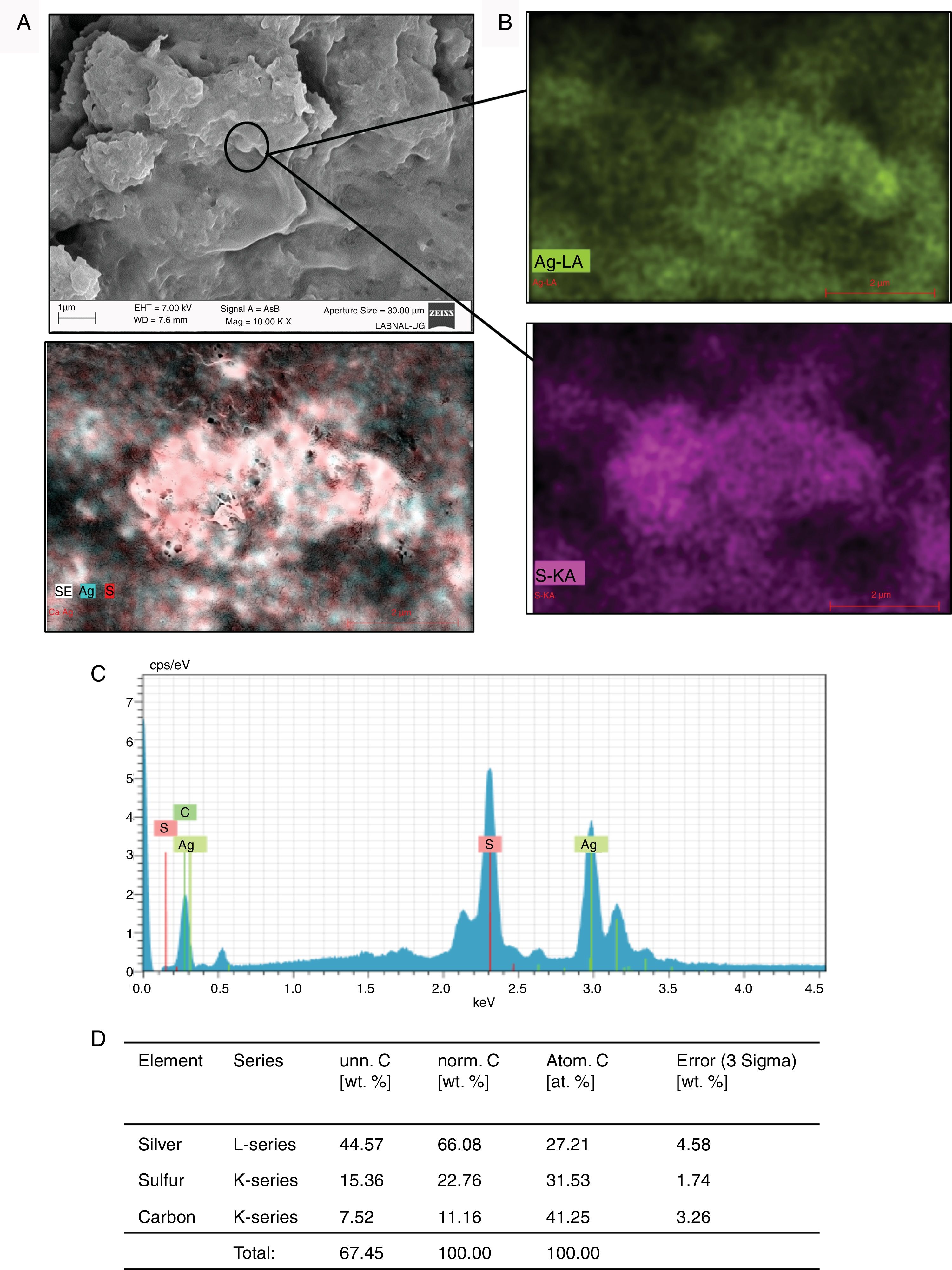
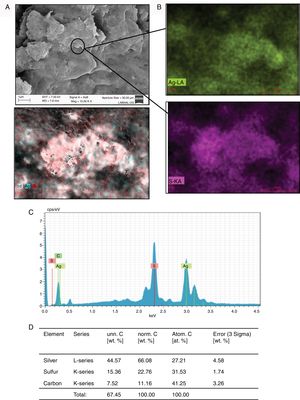
ResultsIn order to evaluate whether C. albicans and C. glabrata were able to use the Ag+ ions present in the culture medium, we emulated the conditions of the wastewater from mining where these fungi are usually found. As shown in Fig. 1, representing the results registered by SEM with C. albicans or C. glabrata in the presence of Ag+, micro-rocks were observed (Fig. 1A). When analyzing these micro-rocks by SEM-EDS we observed that they were formed of Ag and sulfur (Fig. 1B, C). Additionally, the percentage of these elements present in the sample was determined. These elements were the principal ones in the sample (Fig. 1D). Carbon was basically found in the cellular debris present in the samples. Both C. albicans and C. glabrata are able to synthesize Ag2S in the presence of Ag+ (Fig. 1).
Formation of Ag2S by C. glabrata in presence of Ag+. (A) The compound formed was analyzed by SEM. (B) and (C) EDS qualitative analysis of the elements present in the formed compound. (D) Percentage of the elements present in the compound Ag2S was calculated according to materials and methods. The results were the same for both strains.
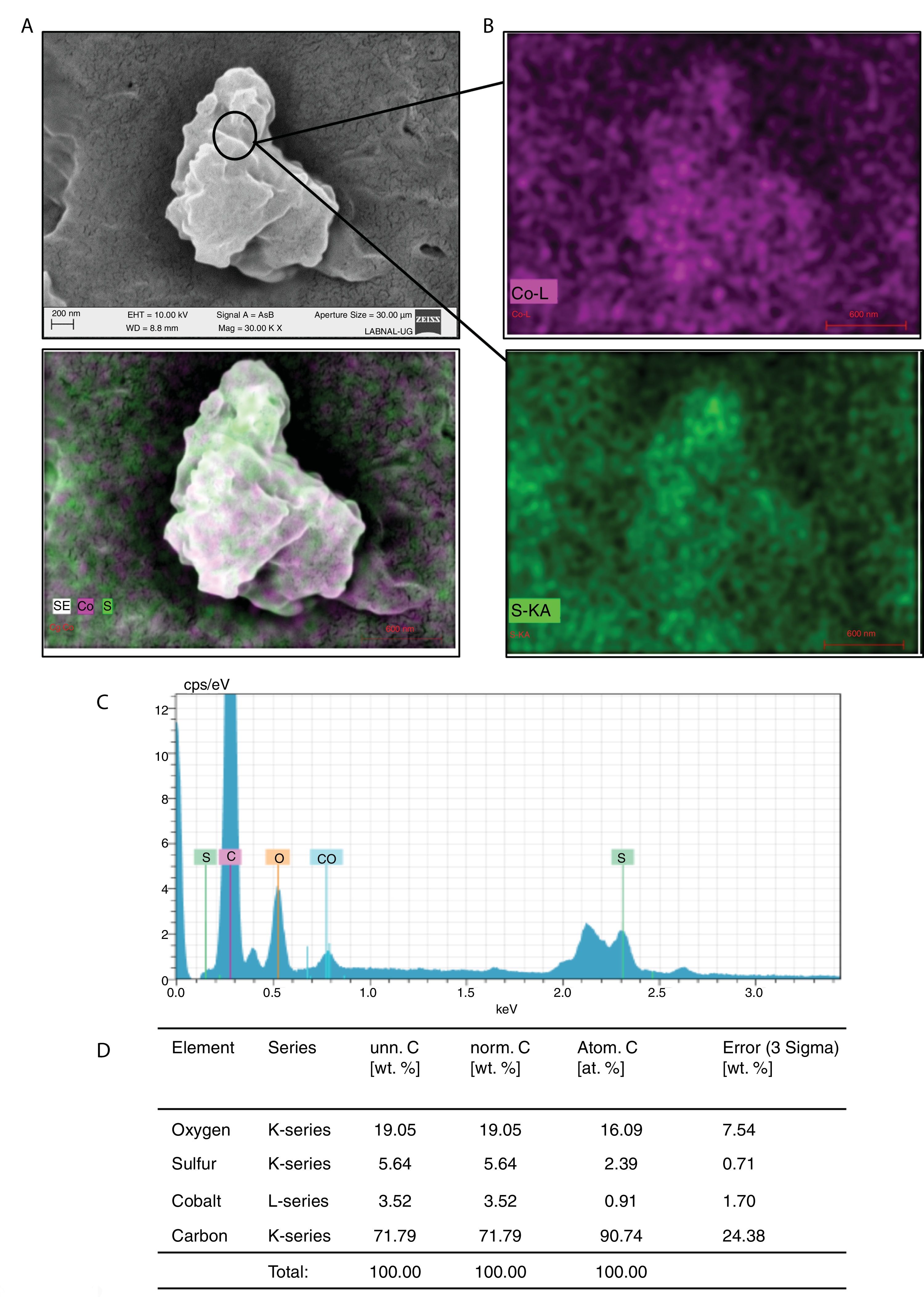
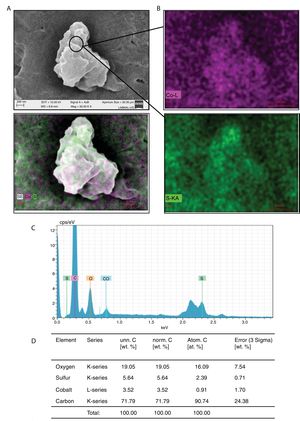
It was also determined if Co can be metabolized by C. albicans or C. glabrata. After being exposed to Co2+, the cells of C. albicans or C. glabrata were lysed, and the compounds were evaluated using SEM: the formation of nano-rock clusters or deposits was observed (Fig. 2A). These clusters were analyzed by SEM-EDS to establish their chemical composition, and it was found that they contained cobalt, sulfur and oxygen (Fig. 2B–D), which suggests that these clusters or nano-rocks are made up of CoSO4.
Formation of CoSO4 by C. albicans in presence of Co2+. (A) The compound formed was analyzed by SEM. (B) and (C) EDS qualitative analysis of the elements present in the formed compound. (D) Percentage of the elements present in the compound CoSO4 was calculated according to materials and methods. The results were the same for both strains.
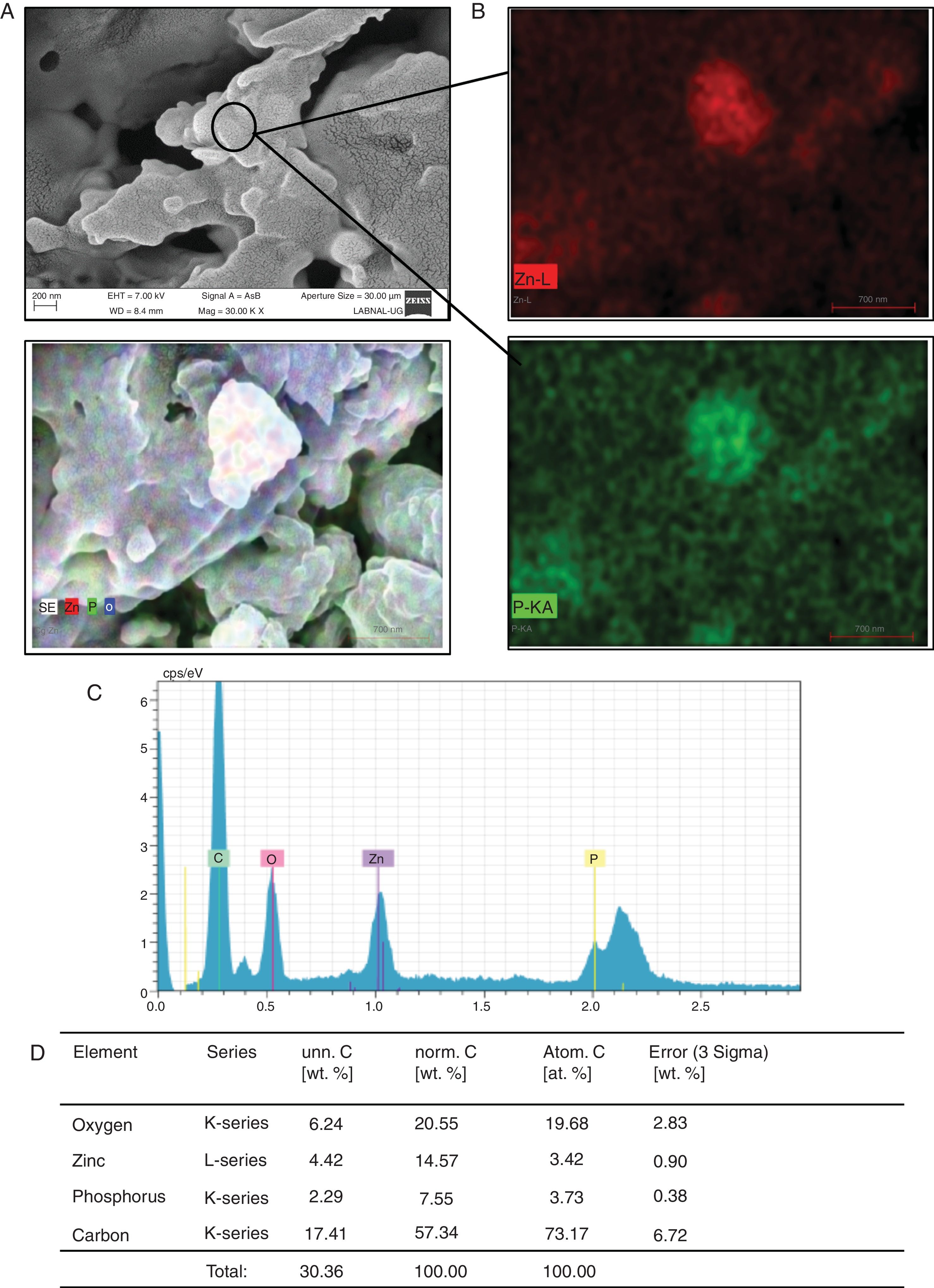
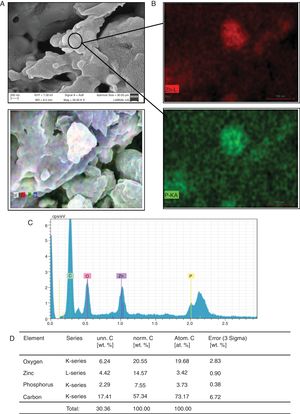
Another transition element of interest in this study was Zn2+. Candida cells, after being exposed to Zn2+ and lysed according to the lysis indicated in materials and methods, were visualized by SEM. As shown in Fig. 3A, deposits that emulate sheets or spliced clusters were found. During the semi-quantitative analysis of EDS it was found that these clusters or sheets are formed by oxygen, Zn and phosphorus (Fig. 3B–D).
Formation of Zn3(PO4)2 by C. albicans in presence of Zn2+. (A) The compound formed was analyzed by SEM. (B) and (C) EDS qualitative analysis of the elements present in the formed compound. (D) Percentage of the elements present in the compound Zn3(PO4)2 was calculated according to materials and methods. The results were the same for both strains.
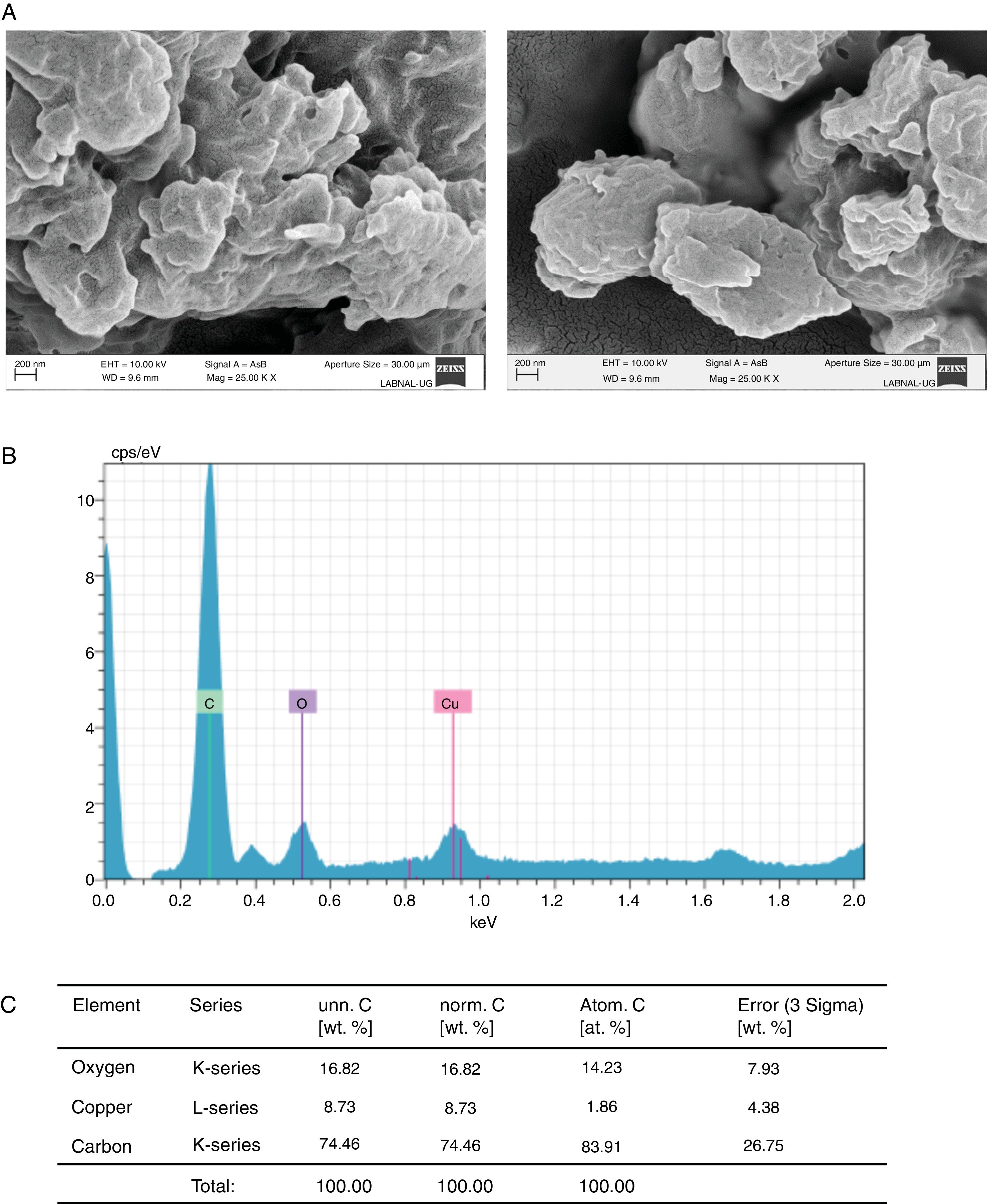
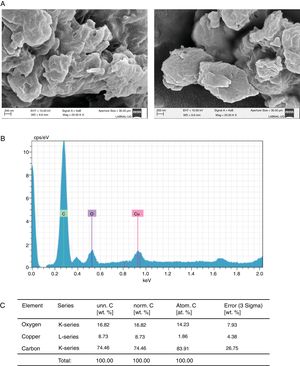
Concerning the study of Cu2+, in the samples treated with this element we found, using SEM, clusters of material with undefined shape (Fig. 4A). A semi-quantitative analysis of this material showed that copper and oxygen were present in the sample (Fig. 4B, C), thus suggesting copper oxide (CuO) was the molecule formed.
Formation of CuO by C. glabrata in presence of Cu2+. (A) The compound formed was analyzed by SEM. (B) and (C) EDS qualitative analysis of the elements present in the formed compound. (D) Percentage of the elements present in the compound CuO was calculated according to materials and methods. The results were the same for both strains.
This is the first study that reports the Candida species mechanism for synthesizing Ag2S. However, other microorganisms, such as the bacterium Shewanella oneidensis, have been reported to be capable of producing Ag2S nanoparticles (Ag2S-NPs) in the presence of AgNO3 and Na2S2O3.10 Other bacteria that have been reported as able to produce Ag2S-NPs are Aeromonas sp. SH10, Klebsiella pneumoniae, Lactobacillus, Pseudomonas stutzeri AG259, Corynebacterium sp. SH09 and Enterobacter cloacae.18 The mechanism that some of these bacteria use to synthesize Ag2S-NPs is the reduction of aqueous Ag+ ions through the nitroreductase enzyme.50 In other bacteria such as Aeromonas and Corynebacterium it is proposed that the compound [Ag (NH3)2]+ is formed; it reacts with the anion OH− to form Ag2O, which is metabolized, and Ag+ is reduced to produce Ag2S-NPs.28 The species of fungi capable of synthesizing Ag2S-NPs are Phanerochaete chrysosporium, Verticillium, Aspergillus flavus, Aspergillus fumigatus, Fusarium oxysporium and Fusarium semitectum.18 However, the species of the Candida genus have not previously been reported as producers of Ag2S (Fig. 1). The reported mechanism by which fungi reduce the Ag+ ion to Ag2S is attributable to NADH-dependent reductase.3 In this way, F. oxysporium has been reported to secrete nitrate reductase into the medium, allowing the formation of Ag2S-NPs.22 Additionally, it has been shown that, in other fungi, other non-enzymatic factors like pH, the presence of compounds like proteins, organic acids, polysaccharides, and the presence or absence of glucose, contribute to the bio-reduction of Ag+ to Ag2S.21,22,47 In the case of C. albicans and C. glabrata, the mechanism that reduces Ag+ to Ag2S must be similar to those reported in the mentioned fungi, since they have the required enzymes. Ag2S-NPs are of special biotechnological interest due to their unique electrical, optical and biological properties as semiconductors.19,31,53 These allow them to be used in nanodevices, catalysis, in the detection of viral structures (SERS and silver nanorods), as coating for hospital textiles or even catheters for cerebrospinal fluid drainage, and as an additive to polymerize dental materials, among others.18,19,31,58
Biosynthesis of CoSO4 by C. albicans and C. glabrataCo is a transition element and, as well as Cu, Ni and Zn is used at trace concentrations by microorganisms as micronutrient, bio-element or enzymatic cofactor.2,12 There are microorganisms capable of metabolizing Co, and the facultative phototrophic anoxygenic proteobacterium Rhodobacter sphaeroides is one of them.55 In a study with this microorganism in which Co-resistant mutants were chosen, it was interestingly found that those Co-resistant mutants showed a severe down-expression of an ABC sugar transporter.55 In another microorganism, Synechocystis PCC 6803, the P1-type ATPase, named CoaT, responsible for transporting Co in the cell, has been identified.8,11,46 Even though some of the genes involved in the transport of Co have been described, the mechanism by which this element is assimilated by the microorganisms is still unknown; a mechanism of reduction in the water of mines where iron is present has been reported.30Candida species are found in different environments, including water, therefore assuming Candida tolerates this metal,9,14,24 a fact that lead us to evaluate how these fungi are able to achieve homeostasis in the presence of Co2+. In order to describe the chemical reactions by which C. albicans and C. glabrata synthesize CoSO4 in the presence of Co2+ (Fig. 2), we suggest a mechanism that favors the synthesis of compounds containing that metal embedded in the cell wall (CW): a coordinated covalent bond is formed between the metal ion and one of the components of the CW. In this way, an acidic pH is favored13 and one of the chemical reactions that take place in the CW is the production of H2S. Cobalt sulfide (CoS) is obtained from the H2S. This sulfide can be oxidized by Candida species via a direct mechanism through the following reaction: CoS+2 O2=CoSO4. To our understanding, this is the first report showing that C. albicans and C. glabrata in the presence of Co2+ reach homeostasis by synthesizing CoSO4 (Fig. 2).
Formation of Zn3(PO4)2 by Candida speciesC. albicans and C. glabrata can reach homeostasis in the presence of Zn2+ through the synthesis of Zn3(PO4)2. The formation of Zn3(PO4)2 has been described in several microorganisms. Yeasts in particular have a butterfly-like microstructure that functions as biotemplate.16,42,57 In the formation of Zn3(PO4)2 by several microorganisms, numerous gene clusters capable of detoxifying the cell through mechanisms such as complexation, efflux or reductive precipitation have been described.23 The mechanism by which C. albicans and C. glabrata possibly synthesize Zn3(PO4)2 is also similar to that described in microalgae.48 The synthesis of Zn3(PO4)2 would take place on the CW. The metal ion binds to some of the CW compounds, such as polysaccharides and proteins that contain polyphosphate. Subsequently, the metal can form complexes with polyphosphates leading to Zn3(PO4)2 formation. To our understanding, this is the first report that shows that Candida species, which have so far been studied for their pathogenicity in humans, are also capable of forming compounds of biotechnological interest. These Candida species can also be used as microorganisms for bioremediation. C. albicans and C. glabrata should now be considered, like other microorganisms, good producers of Zn3(PO4)2. This compound has several types of applications such as ion exchanger, chelating agent, coatings resistant to corrosion, glass technology, biomedical research, cements, high-quality fertilizers, pigments for coating products, and the automotive industry.5,7,49,56 Due to the large number of applications that Zn3(PO4)2 has, structures made up of particles of well-defined size could be produced. This is a characteristic way of synthesis performed by microorganisms.
C. albicans and C. glabrata produce CuO from Cu2+The oxides of some metals, such as CuO, are of particular interest for their unique optical, electronic and magnetic properties,1 which allow them to be used in solar energy, magnetic storage media, electronic, gas sensors and catalysis, among other applications.4,20,26,45,51 The biogenic formation of CuO has been reported in organisms ranging from algae to microorganisms, however, the number of reports is few in comparison with other nanoparticles.17 Bacteria such as Escherichia coli, Streptomyces and Serratia have been reported to produce CuO nanoparticles.15,52,54Penicillium aurantiogriseum, Penicillium citrinum and Penicillium waksmanni are also capable of forming CuO nanoparticles.17 Nonetheless, in C. albicans and C. glabrata, the formation of CuO has not yet been reported. These yeasts, having several habitats, are likely to possess the enzymatic and non-enzymatic mechanisms that allow them to metabolize Cu2+. The mechanism by which microorganisms form CuO has not yet been fully elucidated. However, several alternatives have been proposed. For example, the cells of Serratia in stationary phase suffer oxidative stress; when these cells were later exposed to Cu2+, a high quantity of reactive oxygen species was produced, and Cu was reduced to its metallic form.15 Subsequently, the copper nanoparticles were oxidized thanks to the oxidizing environment, thus leading to the formation of CuO. In that work the authors also mention that the formation of CuO nanoparticles were favored by cell death.15 In the case of fungi, the proposed mechanism suggests that different biomolecules are responsible for the synthesis of CuO nanoparticles. The cell secretes numerous reducing and capping agents that make the formation of nanoparticles possible.17,25,29C. albicans and C. glabrata possess the biomolecules proposed in the previously described mechanisms. However, further researches must be carried out in this regard to know the mechanism by which Cu2+ favors the formation of CuO.
ConclusionsWhile C. albicans and C. glabrata have been studied as part of the microbiota in healthy individuals and as opportunistic pathogens in immunocompromised and hospitalized patients, they have not yet been explored in other areas where could also be useful. Our data indicate that both yeasts have enzymatic and non-enzymatic mechanisms that allow them to sense different metal elements, metabolize them and achieve homeostasis in a particular and specific way for each of the metals to which they are exposed. C. albicans and C. glabrata can reduce different metals, with the subsequent formation of sulfides, sulfates, phosphates and oxides. This survivable ability to colonize different environments evolved in these Candida species over time, and make them an excellent model of study both from the medical and biotechnological point of view.
Conflict of interestThe authors declare that they have no competing interest.
Mayra Cuéllar-Cruz thanks the sabbatical support from SEP-PRODEP (registration No. 511-6/18-5929). This work was carried out with the financial support granted to Dr. M. Cuéllar-Cruz by Proyecto-Institucional-IDCIIC-44/2018 from University of Guanajuato, México. We are grateful to Dr. Ricardo Navarro and Dra. Paulina Lozano-Sotomayor from the National Laboratory, University of Guanajuato, México, for the facilities and technical assistance with the SEM photographs. The authors acknowledge the final English style correction performed by Ms. Antonia Sanchez Marin and Prof. John Dye.