Phaeoacremonium parasiticum is considered a rare infectious agent that is part of a heterogeneous group of fungi causing phaeohyphomycosis. This organism is capable of producing subcutaneous infections, eumycetomas, osteomyelitis, arthritis, myositis and also disseminated diseases, such as fungemia and endocarditis.
Case reportWe describe a case of cutaneous infection by P. parasiticum in a kidney transplant patient. The identification of this microorganism was performed by microbiological and histopathological studies and confirmed with the sequence of the gene encoding β-tubulin and a real time panfungal PCR targeting 18S ribosomal RNA gene. The microorganism was correctly identified by phenotypic and molecular methods. The patient was treated with oral antifungal therapy and a debulking surgery and evolved without any complication.
ConclusionsThe diagnosis of this infection is difficult and usually affects kidney transplant patients, but the reasons of this association are still unknown.
Phaeoacremonium parasiticum es considerado un agente infeccioso poco común que forma parte de un grupo heterogéneo de hongos causantes de feohifomicosis. Este microorganismo es capaz de producir infección cutánea, eumicetoma, osteomielitis, artritis, miositis e incluso enfermedad diseminada como fungemia y endocarditis.
Caso clínicoSe describe un caso de infección cutánea por P. parasiticum en un paciente trasplantado renal. Para la identificación del microorganismo se realizaron pruebas microbiológicas e histopatológicas, y se confirmó la identificación con la secuenciación del gen de la β-tubulina y una PCR a tiempo real para la detección del gen 18S rRNA. El microorganismo fue identificado correctamente por métodos fenotípicos y moleculares. El paciente recibió tratamiento con antifúngicos orales y citorreducción quirúrgica, y evolucionó sin ninguna complicación.
ConclusionesEl diagnóstico de esta infección es difícil y se presenta habitualmente en pacientes trasplantados renales. Sin embargo, la asociación de esta infección con este tipo de pacientes no ha sido aún explicada.
Phaeoacremonium species have a wide distribution in the environment. It was thought that they only produced disease in plants,2,8 but in 1974 the first case of cutaneous infection in a kidney transplant patient was described. Phaeoacremonium parasiticum, previously known as Phialophora parasitica, is considered a rare infectious agent although in the last years new cases have been described.2,7,12,13P. parasiticum is part of a heterogeneous group of fungi causing phaeohyphomycosis, a disease that includes a broad spectrum of infections caused by fungi that produce septate hyphae with melanin in the tissue.1,10 This organism produces subcutaneous infections, eumycetomas, osteomyelitis, arthritis, myositis and also disseminated diseases, such as fungemia and endocarditis.17 We describe a case of cutaneous infection caused by P. parasiticum in an immunosuppressed kidney transplant patient.
Case reportA 77 year-old man went to a local hospital with an injury in his right hand caused with plants of his garden. The wound was cleaned and a daily antiseptic cleaning was prescribed. However, after several weeks the wound did not improve. He came back to the same centre, and at the time of admission the patient explained that he was under immunosuppressive treatment due to a kidney transplant in 2015.
In the culture from the sample taken from the lesion, a filamentous fungi, identified as Exophiala dermatitidis by microscopic identification, grew. The patient started an antifungal treatment with voriconazole, but after four days the patient stopped the treatment due to of intolerance.
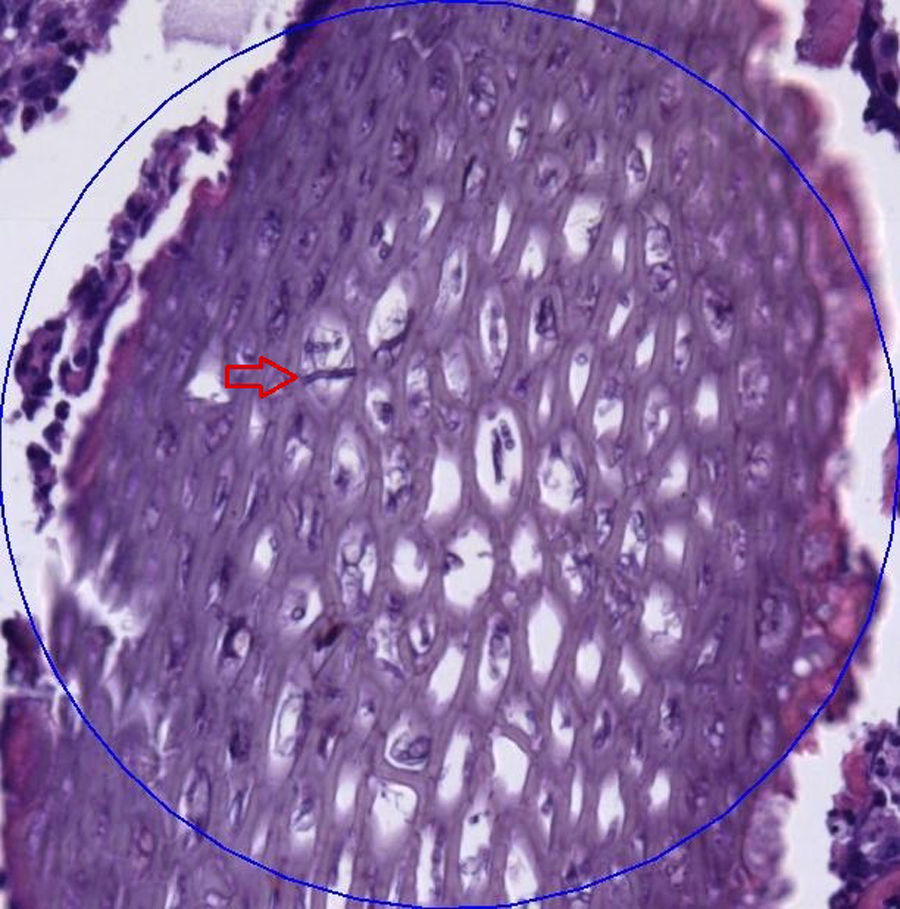
After 10 months, time in which the lesion was still present, the patient went to the dermatology department of our hospital. Physical examination revealed a nodular lesion in the right hand without any systemic symptoms. Oral treatment with itraconazole was started and a debulking surgery was performed. During the surgery, samples for culture and histopathological study were taken. The histopathological examination of the tissue showed aggregates of neutrophils forming microabscesses, a moderate lymphoid infiltrate and numerous granulomas. In the centre of one granuloma, a material of vegetal appearance with a fungal structure consistent with non-septate hyphae was observed (Fig. 1).
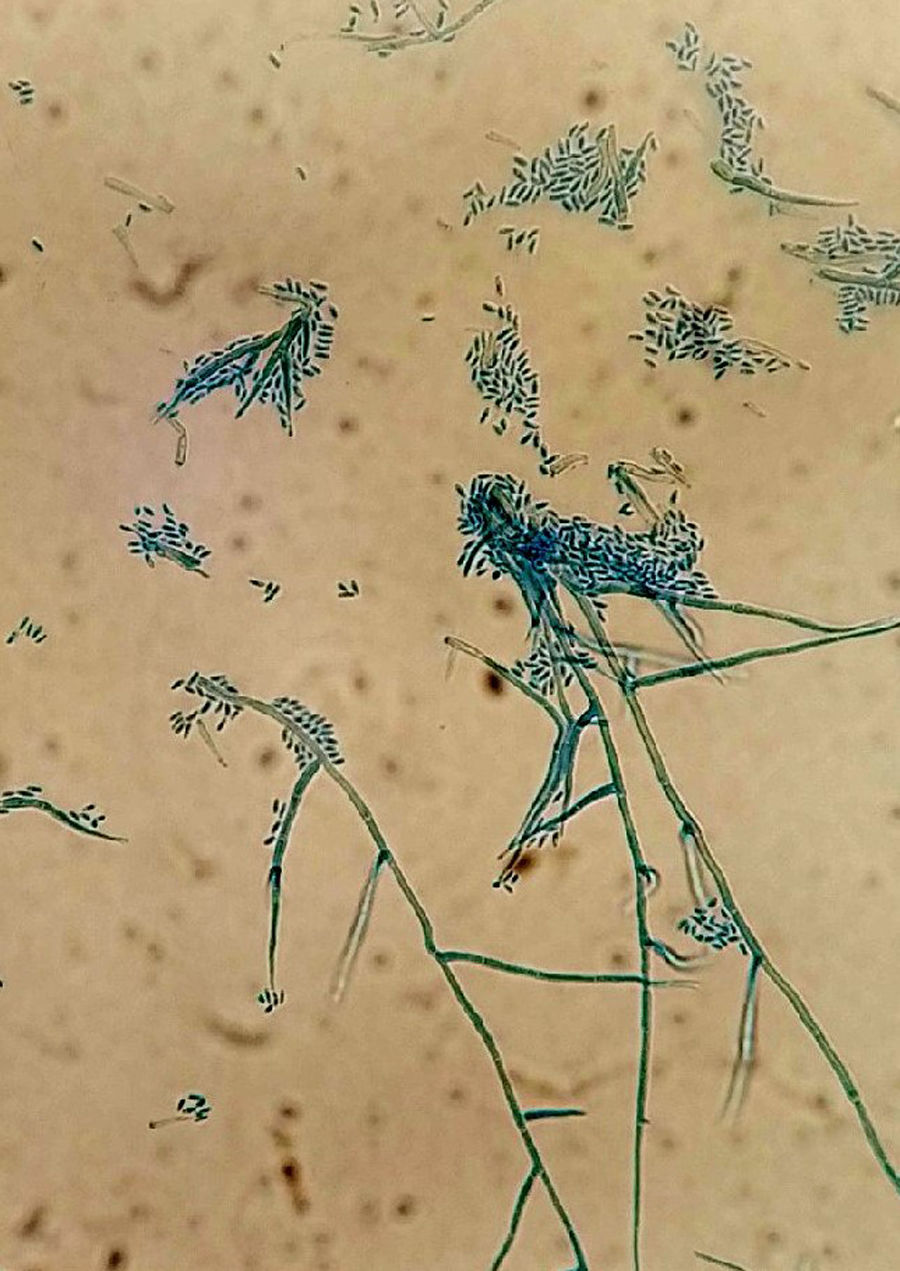
Samples were plated on potato dextrose agar and incubated at 30°C. After 7 days of incubation, colonies of fluffy appearance with irregular borders and olive-grey colour were observed. A direct microscopic examination of the culture with lactophenol cotton blue staining showed hyaline hyphae, thin-walled phialides tapering towards the tip with small funnel-shaped collarettes and hyaline conidia in balls. This morphology is consistent with Phaeoacremonium genus (Fig. 2). The identification of the isolate was confirmed by the analysis of the sequence of the gene encoding β-tubulin and a real time panfungal PCR targeting 18S ribosomal RNA gene. DNA extraction was performed as described by Turenne15 with some changes.16 For the extraction, vortexing with glass beads to improve the lysis of fungal cells wall was performed. Amplification was performed in an automated PCR-System Smartcycler (Cepheid, USA) with cycles of 95°C for 120s, 45 cycles at 95°C for 10s, 52°C for 30s and 72°C for 10s, using Sybergreen to detect the amplified products (Sensifast SYBR Hi-Rox Kit, Bioline, UK). A melting curve analysis was performed in both methods.
The PCR products of both targets were sequenced (BigDye, Applied Biosystem) and the sequences obtained were compared with those available on GenBank using a BLAST search. P. parasiticum species identification was obtained; the sequences obtained with the two amplification assays matched two sequences with accession numbers KU375504 and KX268647, with 100% and 99% of similarity respectively.
Antifungal susceptibility testing was performed on Sensititre YeastOne panel (Thermo scientific diagnostic systems, UK) following EUCAST guide.4 The minimal inhibitory concentration (MIC) values obtained were the following: voriconazole 0.06μg/ml, itraconazole 0.12μg/ml, posaconazole 0.06μg/ml and amphotericin B 8μg/ml. The isolate was considered sensitive to azoles and had a decreased sensitivity to amphotericin B. The patient continued with oral itraconazole and progressed favourably.
P. parasiticum is an infrequent microorganism that causes infection especially in transplant patients receiving immunosuppressive treatment.6,8,11,13,14 There are few reports of infections caused by this microorganism, but some reviews suggest an association with kidney transplantation. Up to 36% of infections occurred in kidney transplant patients,1,3,10 as our case, and in some cases an invasive infection was observed.1,6 However, the cause of this association is not yet clear. Patients with several immunocompromising haematological diseases, stem cell transplantation, or rheumatoid arthritis treated with infliximab have also been recognized as a risk group for infections by P. parasiticum.8,11 Although a disseminated infection is rare it can be fatal in some cases. No death related to localized infection has been reported.11
Traumatic implantation (i.e. injuries caused by plants) is the main infective route, taking into account that species of Phaeoacremonium are ubiquitous in nature.11 After the trauma, dermis is the mainly affected tissue and, in some cases, the subsequent lesion relapses.11 The aetiology of phaeohyphomycosis involves a broad spectrum of microorganisms such as Acrophialophora, Alternaria, Cladosporium, or Exophiala, among other genera, included Phaeoacremonium, being the latter a very rare cause.1,3 Due to the similar morphology of these microorganisms it is challenging to obtain a reliable identification only under a microscope.2,6,9,12 Therefore, the sequencing of certain regions of the β-tubulin gene and 18S ribosomal RNA gene is recommended to reach a definitive diagnosis.2,6 The performance of only microscopic identification may lead to misdiagnoses as happened in our case at the very beginning, when the fungus was identified as E. dermatitidis. The combination of molecular and morphological diagnosis is the most appropriate way to achieve a reliable identification.6
Antifungal susceptibility testing for Phaeoacremonium is not standardized yet, thus caution must be taken when interpreting the results. Although a specific treatment against this microorganism is still unknown, it is believed that azoles can give optimum results. A study of the in vitro activity of several antifungals against P. parasiticum showed a MIC range of voriconazole 0.125–2μg/ml, mean 0.55μg/ml, amphotericin B range 1–16μg/ml, mean 3.08μg/ml, and itraconazole range 0.25–32μg/ml, mean 6.17μg/ml.8 Some reports describe a lower activity of itraconazole.1,3,5,8 However, in our study the strain seemed to be sensitive to itraconazole, and this antifungal was used as part of the patient treatment.
In conclusion, the diagnosis of this infection is difficult. However, there is a need to continue investigating, especially on kidney transplant patients. The combined treatment of surgery and antifungal therapy seems to be the best option to treat this infection.