Aspergillus osteomyelitis of the ribs is relatively uncommon. It is a debilitating and severe form of invasive aspergillosis.
Case reportA 61year-old female presented with spontaneous chest pain on the right side of the rib cage and a palpable soft-tissue mass. FDG-PET/CT scan identified activity in the infected site. The lesion was punctured, and purulent material was sent to the laboratory. Aspergillus complex Flavi was isolated. An antifungal treatment with voriconazole was started. The lesion healed, and no recurrence was observed at 8-month follow-up. Molecular identification of the isolate was based on PCR amplification and sequencing of β-tubulin gene. Aspergillus flavus was identified.
ConclusionsOur case highlights the relevance of microbiological studies in patients with osteomyelitis and the involvement of soft tissue. The FDG-PET/CT scan was found to be a useful tool for revealing the extent of the disease and evaluating the response to the antifungal therapy.
La osteomielitis en la parrilla costal por Aspergillus es una forma debilitante, grave y poco frecuente de aspergilosis invasora.
Caso clínicoMujer de 61 años que presentaba dolor en la parrilla costal derecha y una masa palpable en partes blandas. La FDG-PET/CT identificó actividad en el sitio infectado. Se obtuvo por punción material purulento y se envió al laboratorio. El aislamiento se identificó por cultivo como Aspergillus perteneciente al complejo Flavi. Se indicó tratamiento antifúngico con voriconazol durante 8 meses y la lesión curó sin recurrencia. Mediante la amplificación del gen de la ß-tubulina por PCR y su posterior secuenciación se identificó el aislamiento como Aspergillus flavus.
ConclusionesDestacamos la importancia del estudio microbiológico en pacientes con osteomielitis y con compromiso en los tejidos blandos. El estudio con FDG PET/CT es útil para revelar la extensión de la enfermedad y evaluar la respuesta a la terapia antimicótica.
The patient was a 61year-old female, former smoker with a history of breast cancer in 1991 which required bilateral mastectomy and radiotherapy. She also suffered from chronic obstructive pulmonary disease with centrilobular pulmonary emphysema (treated with indacaterol 110μg/day and glycopyrronium 50μg/day). Mantle cell lymphoma (stage IV B) was diagnosed in January 2016 and treated with 6 cycles of R-CHOP chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone). Six months later, a treatment with rituximab 375mg/m2 and bendamustine 90mg/m2 (3 cycles) was started. In October 2016, a pulmonary nodule was observed and a segmentectomy was performed. It revealed a moderately differentiated squamous cell carcinoma. In February 2017, because of the refractory lymphoma, the patient was treated with rituximab 1400mg (subcutaneous) and ibrutinib 560mg/day.
In May 2017 the patient developed spontaneous chest pain on the right anterior lower chest wall and a palpable mass in the soft tissue. FDG-PET/CT (computed tomography scan with 18F-fluorodeoxyglucose) scan of the chest revealed enhanced soft tissue involving chondrocostal portion of the 10th and 12th ribs (standardized uptake value−SUV−maximum 2.7) suggestive of low-grade malignant tissue neoplasm and without any evidence of involvement of other tissues. In June, a needle puncture and aspiration of the soft tissue were performed. Extensive fibrosis of skeletal muscle and absence of neoplastic cells were observed. The cytokeratin cocktail AE1/AE3 performed was negative.
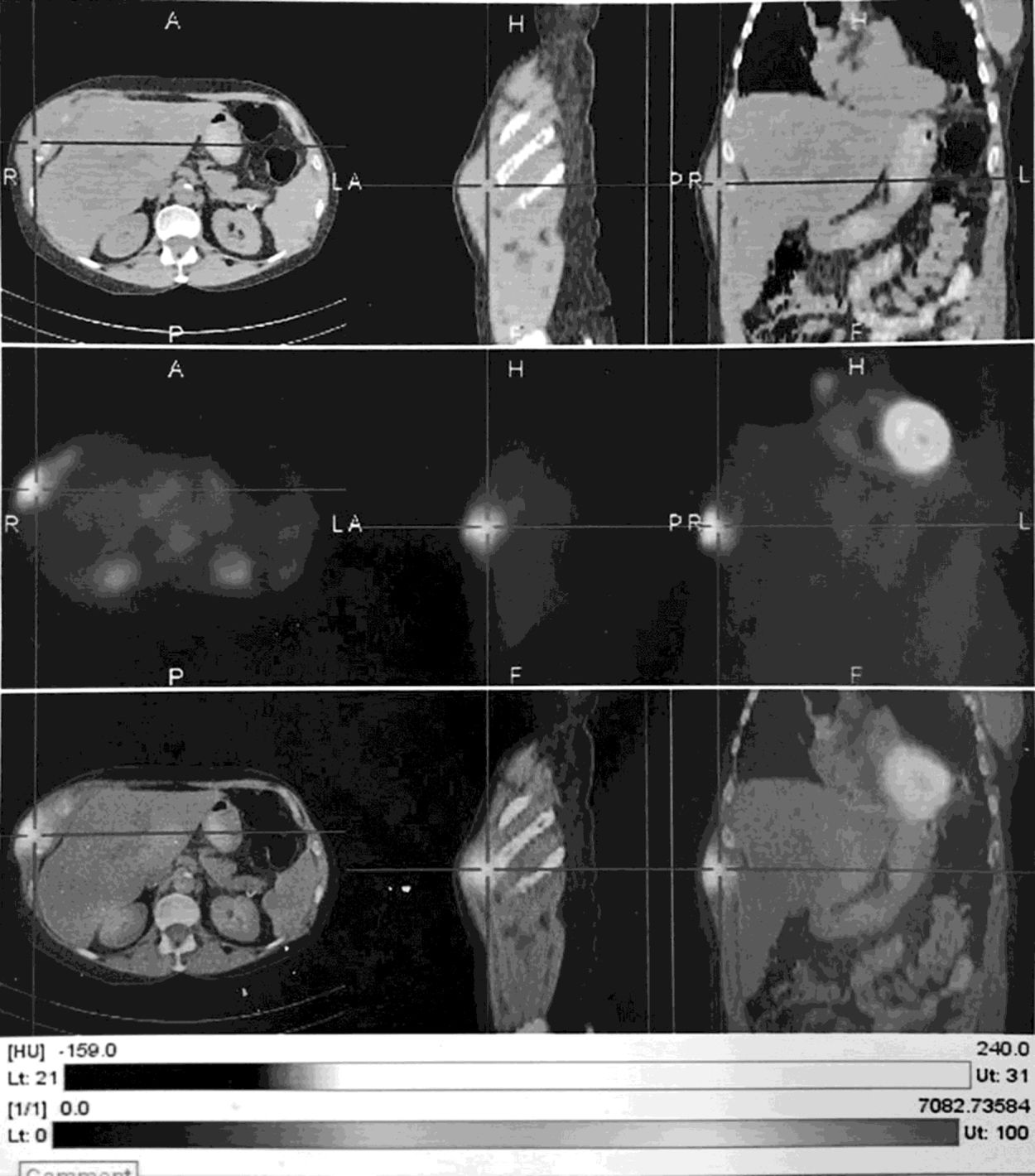
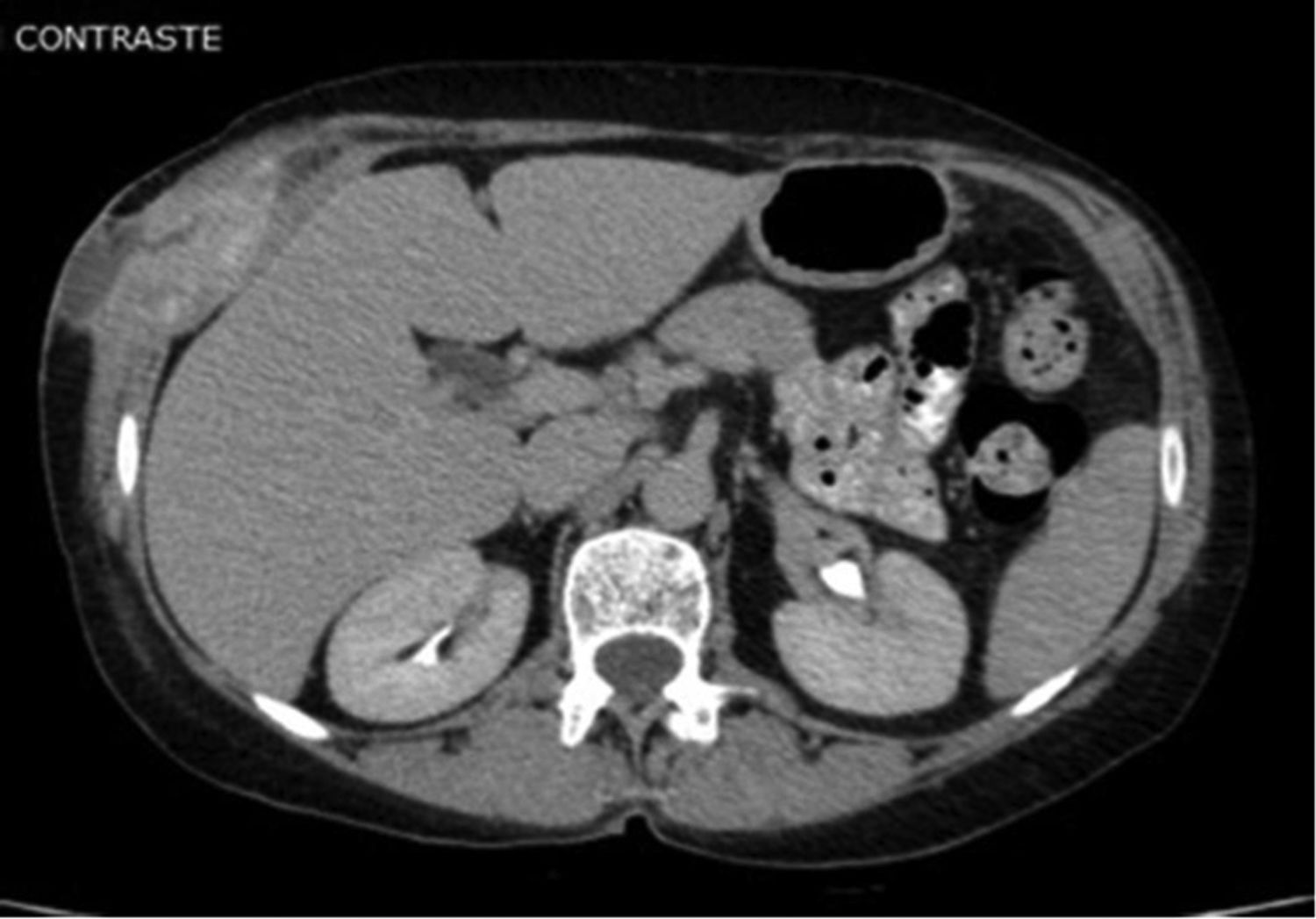
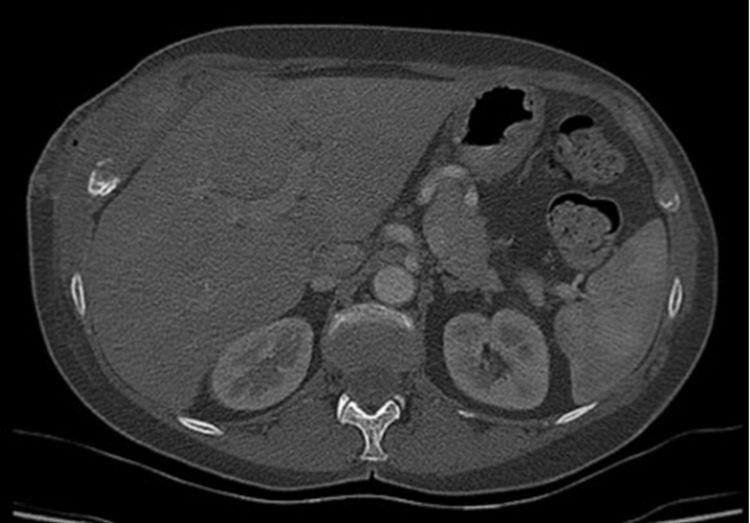
The mass of the chest evolved in an increase in size and softening. In August, FDG-PET/CT scan identified activity at the site infected previously in the soft tissue injury of the right rib cage (SUV max: 5.7) (Fig. 1). Chest and abdomen CT scan revealed the presence of fluid in the thick portion of the external oblique muscle measuring 28×12mm in diameter, with a fistulous trajectory toward the subcutaneous cellular tissue (Figs. 2 and 3). The patient was admitted at the Dr. Julio Mendez Hospital, in the city of Buenos Aires. The lesion in the right anterior lower chest wall was punctured and abundant purulent material was aspirated and sent to the laboratory. It was fresh mounted with and without KOH 10%. The smears showed hyaline and dichotomous branched hyphae. The sample was inoculated onto Lactrimel agar, Brain Heart Infusion agar (BHI, BioKar Diagnosis, Beauvais, France) and Sabouraud dextrose agar with chloramphenicol (Britania®), and plates were incubated at 28 and 37°C. A fungal strain was isolated and its micromorphological characteristics were studied on Czapek-Dox agar (CZD; Becton Dickinson) at 28°C. Aspergillus complex Flavi was the presumptive identification.
In serum, Aspergillus galactomannan was detected by ELISA (Platelia Aspergillus; Bio-Rad) in a concentration of 0.85 ng/ml; subsequent intra-treatment controls tested negative. Abnormal laboratory tests included white blood count 3170cells/mm3 with a normal neutrophil count of 2948cells/mm3, hemoglobin 8.8g/dl, and hematocrit 28%. The diagnosis was osteomyelitis of the right rib cage involving the soft tissue surrounding the chondrocostal portion of the 10th and 12th ribs. The treatment was intravenous voriconazole (200mg every 12h) for 14 days. The patient had a good clinical response and was discharged with oral voriconazole (400mg/day). After 8 months on antifungal treatment, FDG-PET/CT scan was performed and no abnormal findings were observed.
The molecular identification of the isolate was based on PCR amplification and sequencing of the β-tubulin gene. DNA extraction was performed14 and a part of β-tubulin gene was amplified by PCR and sequenced for accurate identification.2 The nucleotide sequence obtained (478bp) was compared with those available in the GenBank database: the sequence amplified had 99% of identity and 99% of query cover with the sequence of partial β-tubulin genes of Aspergillus flavus AY 017536. Furthermore, the sequence of β-tubulin from our isolate was deposited in the GenBank database as MH404255.
DiscussionAspergillus osteomyelitis of the rib cage is a relatively uncommon entity, with an incidence rate of only 9%.6 Early recognition of Aspergillus osteomyelitis depends on a high level of suspicion, particularly in vulnerable patients who present with osseous tenderness, pain, and/or sinus tract drainage. History of past surgical interventions, especially orthopedic and thoracic surgeries, may serve as a source of direct inoculation.5,7 Most of the reported Aspergillus osteomyelitis had a history of surgical procedures.1,15 In the present case report, osteomyelitis of the right rib cage and soft tissue surrounding the chondrocostal portion of the 10th and 12th ribs was diagnosed 6 months after the segmentectomy was performed, which could have been the cause of a direct inoculation of A. flavus. Even though our patient had a scar close to the scapula, hematogenous seeding of bone tissue could not be ruled out as she had suffered from numerous comorbidities and had been neutropenic on several occasions. Nonetheless, Aspergillus osteomyelitis was the first manifestation of aspergillosis in this patient.7 Few data are available regarding galactomannan assays in the diagnosis of fungal osteomyelitis and joint infection. Only four of 47 case reports detected positive galactomannan antigenaemia.12,13,17,18
Although this diagnosis can generally be easily achieved, clinicians often encounter substantial challenges in detecting and localizing the exact infection sites. The introduction of whole-body imaging techniques such as the FDG-PET/CT scan opened new means of research for examining glucose metabolism. However, even if useful, FDG is a nonspecific tracer that can be kept at the infection sites.19 Although the FDG-PET/CT scan can raise the suspicion of fungal infection, culture or biopsy are required for specific diagnoses. The major role of the FDG-PET/CT scan in fungal infections is to reveal the extent of the disease, and it can also provide useful guidance in monitoring treatment response to antifungal therapies.16 In the present case report, diagnosis was proven by aspiration and culture of the localized collection.
Before making the diagnosis, the patient was treated with ibrutinib and rituximab for 3 months. Rituximab was discontinued and ibrutinib was kept at a lower dose because of ibrutinib-voriconazole interactions.4 Early-onset invasive Aspergillus infections, with an onset median time of three months, have been reported in ibrutinib-treated patients, especially in those who were neutropenic, or on concomitant rituximab or corticosteroid.1,8 Voriconazole, which has also been used successfully in Aspergillus osteomyelitis treatment,7 has an acceptable bioavailability and can be administered in parenteral or oral formulation. The lesion healed with no recurrence at 8-month follow-up, as observed in the FDG-PET/CT scan control. The optimal duration of a treatment for Aspergillus osteomyelitis is not known.5,11
The most common species recovered is Aspergillus fumigatus followed by A. flavus.7 Hedayati et al. reviewed the A. flavus complex and included 23 species or varieties, comprising two sexual species.10 It is difficult to achieve a final identification of Aspergillus complex Flavi as taxonomy is constantly evolving. An international Aspergillus working group3 proposed the following recommendations: the term “species complex” as an alternative to “section,” the use of sequences from the ITS region for the identification of Aspergillus isolates to the species complex level, and doing comparative sequence analyses of the β-tubulin region for the species identification within a complex.9
Our case highlights the increasing incidence of this rare disease, which is a reflection of an increase in the number of people living with immunosuppression. Thus, a patient with osteomyelitis and soft tissue infections must undergo a microbiological examination to avoid a misdiagnosis. This is especially important in patients who are elderly or otherwise debilitated.
This research was supported by research grants from University of Buenos Aires UBACyT20020150100158BA.