Nannizzia nana is a zoophilic dermatophyte that affects animals like pigs, boars and, exceptionally, humans, in whom it causes tinea capitis, as well as tinea corporis and onychomycosis.
Case reportCase 1. A previously healthy 8 year-old boy presented to our clinic with a 1-month evolution dermatosis that affected scalp, developing a pseudoalopecic tumor lesion with abundant seropurulent material. The patient had worked in a pig farm. Case 2. A previously healthy 6 year-old girl, sister of the aforementioned child, presented to our clinic with a dermatosis characterized by multiple erythematous-scaly plaques that affected her face, trunk and arms. N. nana was the fungus isolated on culture in both cases. The children were treated with oral griseofulvin and topical ketoconazole that led to clinical and mycological cures.
ConclusionsN. nana dermatophytosis, although being rare in humans, can be treated as other cases of dermatophytosis.
Nannizzia nana es un dermatofito zoófilo que habitualmente afecta a animales, como el cerdo, el jabalí y, excepcionalmente, al ser humano, en el cual provoca tiña de la cabeza, tiña corporal y onicomicosis.
Casos clínicosCaso 1: niño sano de 8 años, con dermatosis de un mes de evolución que afectaba al cuero cabelludo con una lesión tumoral seudoalopécica y abundante material seropurulento. Como antecedente, el niño había trabajado en una granja porcina. Caso 2: niña sana de 6 años y hermana del niño del caso anterior, con dermatosis que le afectaba a la cara, el tronco y los brazos con numerosas placas eritematoescamosas. En ambos casos se aisló en cultivo Nannizzia nana. El tratamiento administrado a los niños fue griseofulvina por vía oral y ketoconazol tópico. Se consiguió la curación clínica y micológica.
ConclusionesLas dermatofitosis por N. nana, aunque son raras en el ser humano, pueden ser tratadas como otras dermatofitosis más habituales.
Tinea is a dermatophyte infection with different clinical presentations according to the causative organism and the specific host T-lymphocyte inflammatory response. Nannizzia nana (Syn.: Microsporum nanum) is a zoophilic and geophilic fungus which affects animals, like swine, and exceptionally humans.1,2,6,7,17,21N. nana is the common cause of ringworm in the pig, accounting for 27% of the pigs of the same flock.17 However, Microsporum canis, Nannizzia gypsea, Trichophyton verrucosum and Trichophyton mentagrophytes have also been reported.3 In humans, N. nana causes characteristic lesions of tinea capitis and tinea corporis.3,11 According to the new multilocus phylogenetic taxonomy for dermatophytes recently proposed by de Hoog et al., 5M. nanum is now called N. nana. We present two clinical cases of dermatophytosis caused by this fungus in two siblings.
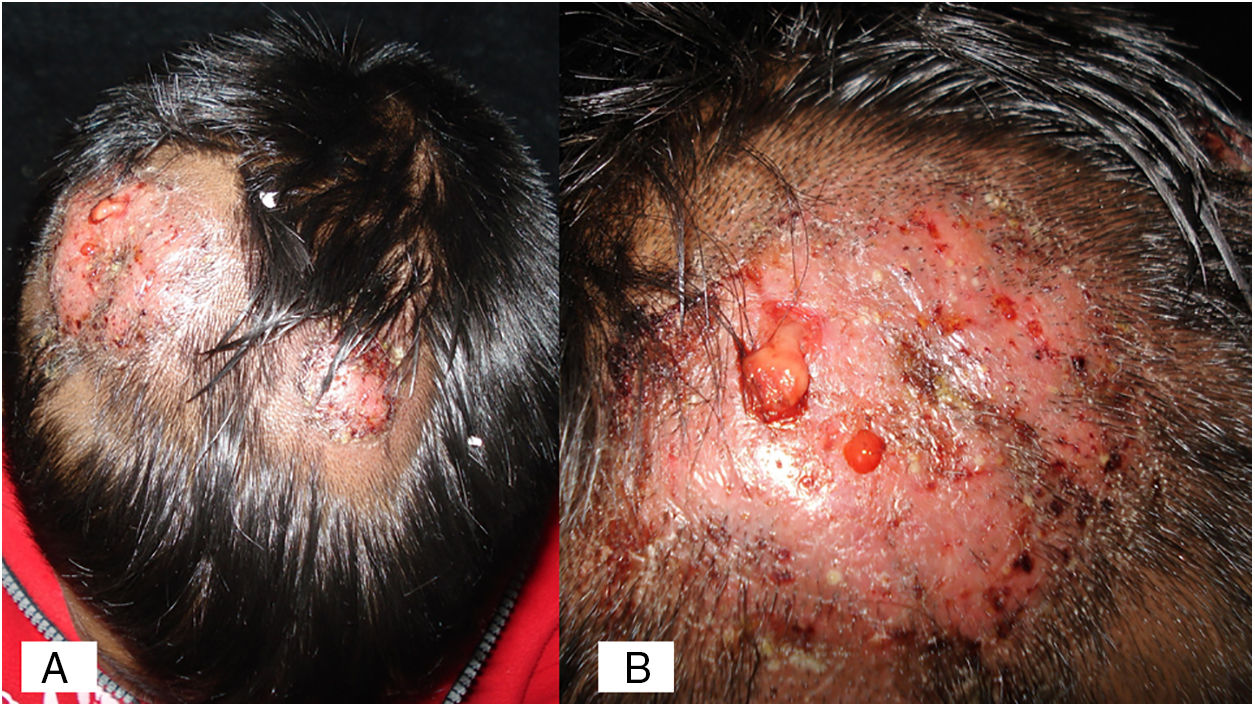
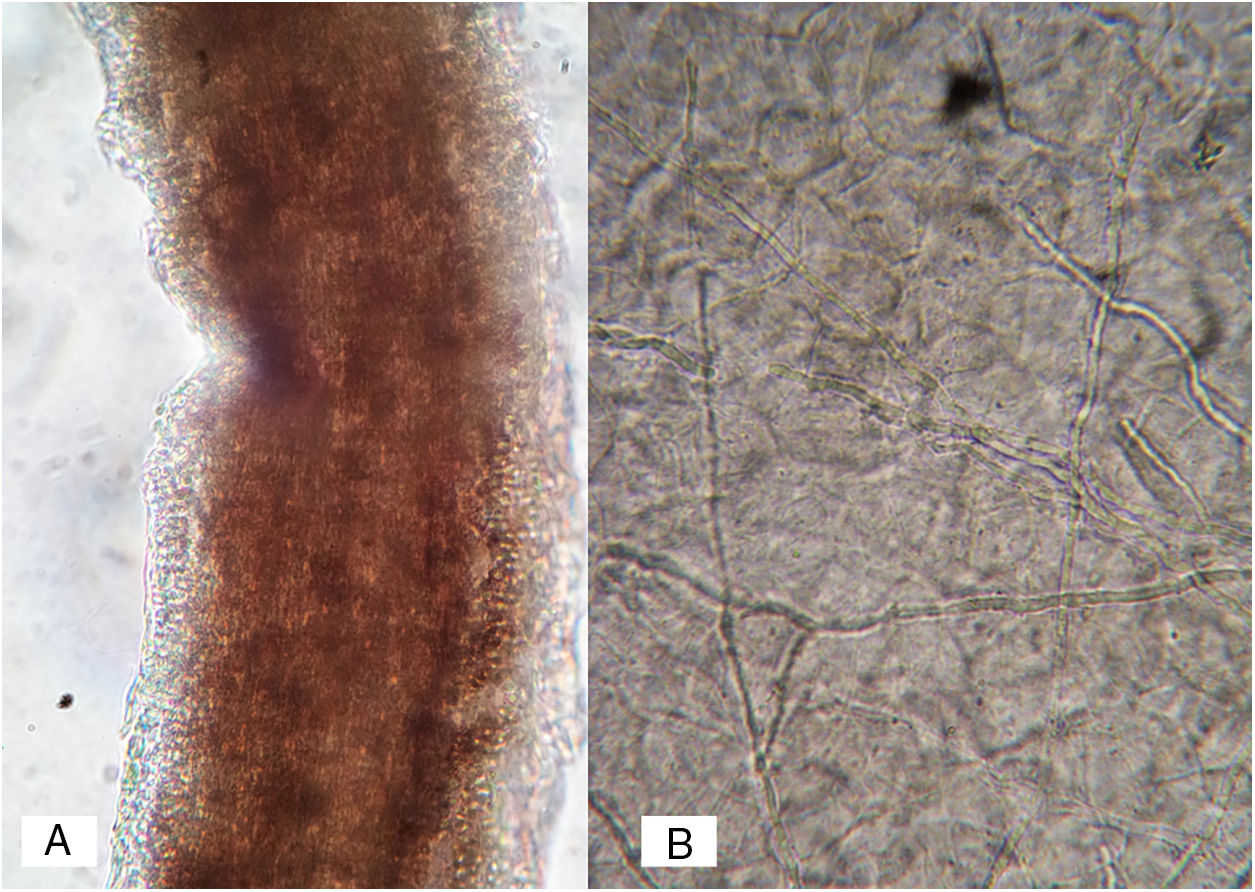
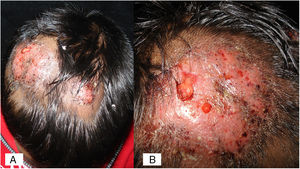
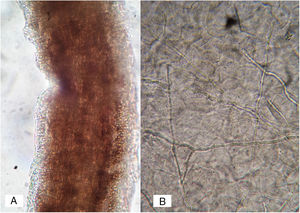
Case 1A previously healthy 8 year-old boy, who lived in Yurécuaro (Michoacán, México), a pig-farm area, presented to our consultation with a 1-month history of a tumoral lesion with hair loss, the presence of scales, multiple pustules and abscesses that drained seropurulent material, and pseudoalopecic areas with few short hairs, accompanied by intense pain (Fig. 1). The patient had worked in a pig farm. We made the diagnosis of kerion Celsi and took samples for direct examination and culture. On direct examination with KOH (10%) we observed ectothrix infection (Fig. 3); on Sabouraud dextrose agar medium with antibiotic, incubated at 28°C, we obtained beige-colored and brown pigmented colonies on the reverse side. In the direct examination of the culture, multiple small egg-shaped macroconidia of 12–15μm with 1–3 septa and few sessile microconidia, corresponding to N. nana, were observed.
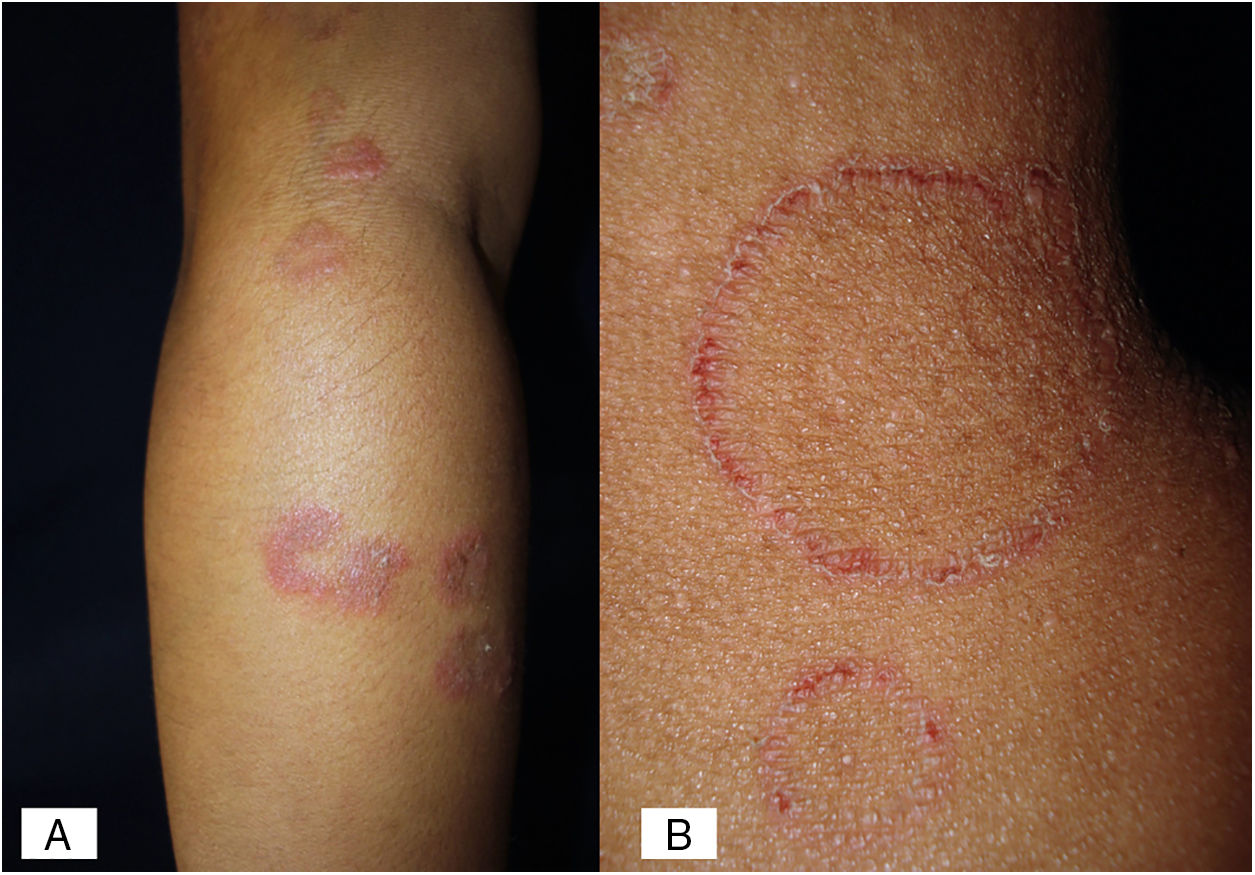
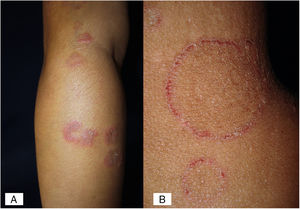
Case 2A previously healthy 6 year-old girl, sister of the boy of case 1, came to our clinic due to a 20-day disseminated dermatosis on the left cheek, trunk and upper limbs, with low to moderate pruritus; several 2–3cm erythematous, scaly, round plaques with raised borders and scarce crusts were observed. We made a diagnosis of microsporic tinea (Fig. 2). Upon direct examination with KOH (10%), multiple, thin, and septate hyphae were present; culture showed the same characteristics of those previously seen in case 1. We made a diagnosis of tinea corporis by N. nana (Fig. 4).
The two patients were otherwise healthy without any immunosuppressive disorder or therapy and had no significant medical history. Both cases received treatment with griseofulvin at 15mg/kg/day. In case 1 the duration of the treatment was 40 days, and the patient had also oral prednisone at 0.5mg/kg/day; in case 2, the duration of the treatment was 20 days and ketoconazole 2% cream (applied twice daily) was added. There were clinical and mycological cure in both cases.
DiscussionN. nana, reported as M. nanum, was first described in 1954 by Fuentes et al.6 in Cuba as the etiological agent of tinea capitis in a child, first described as a type of Microsporum gypseum. The initial fungal description was a dwarf form.2,6 Its natural habitat is swine; there's and epidemic reported in sows in Spain.9 Infections have been reported in exceptional cases in other animals such as dogs, cats and mice.7,9,16,21 Its distribution is worldwide, but few cases have been described in humans.1,2,6,7,9,13–15,18,20 It is considered a low virulence agent. The pigs may have this species as part of their microbiota.21 The majority of patients have a history of contact with these animals in pig farms; this is in accordance with our two cases, where the coexistence with these animals was direct, daily and long-lasting.6,15,17,18
In humans, N. nana is related to tinea corporis (with its characteristic ring shape as an erythematous, scaly, circinate plaque, with peripheral induration, similar to that in M. canis and N. gypsea cases), as well as tinea capitis (both dry and inflammatory types), in a similar fashion among children and adults. N. nana does not invade hair shafts; it only grows on keratinized layers of the epidermis (ecthotrix) and rarely into the dermis.7 There are also exceptional reports of tinea of the feet and onychomycosis.3,12,13 In the inflammatory type (kerion Celsi), N. nana produces several metabolic products that diffuse to the surrounding cells, developing inflammatory (suppurative granuloma and even tuberculoid type) and hypersensitivity reactions. It is an entity that tends to cure but leaves permanent alopecia.3,10 According to the aforementioned it is important to prescribe prednisone to decrease the inflammatory response and, thus, avoid scarring alopecia.
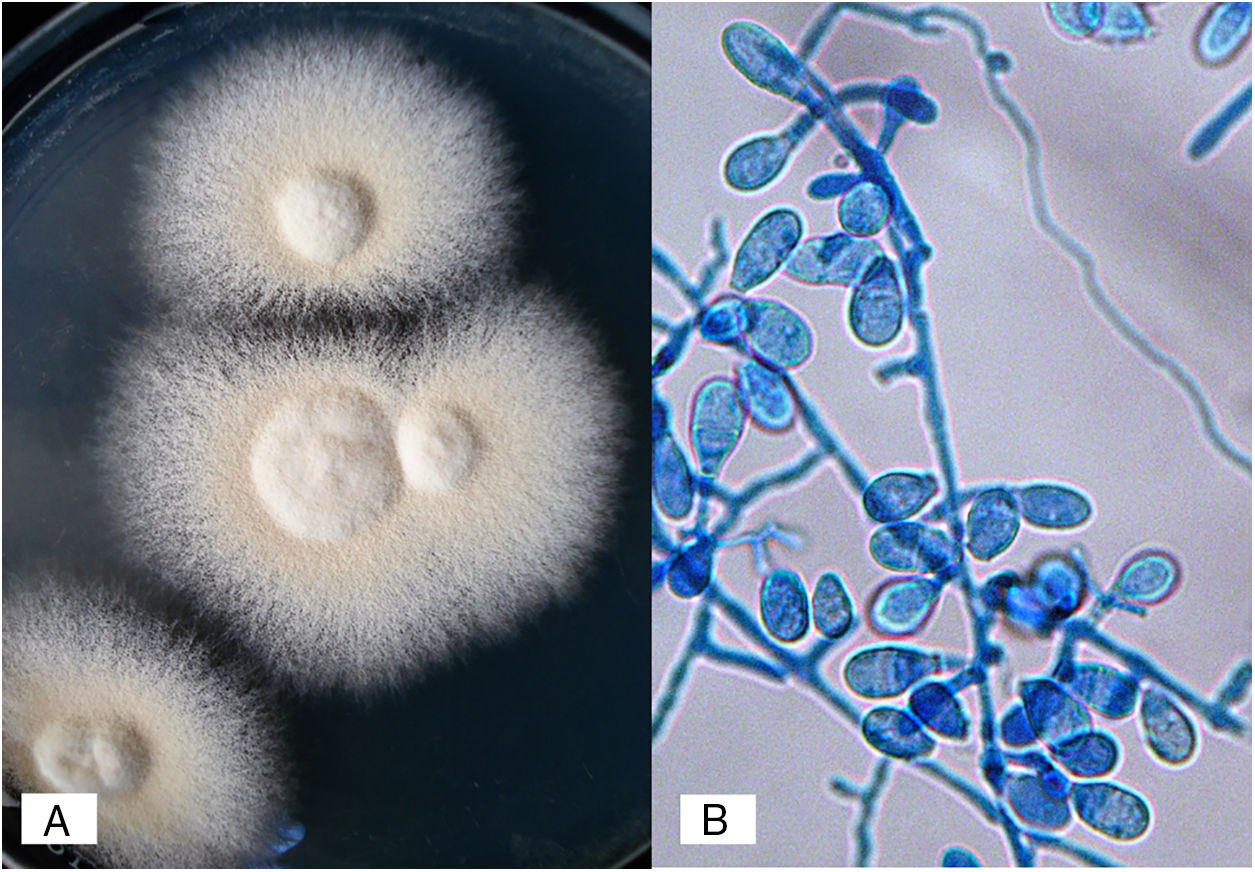
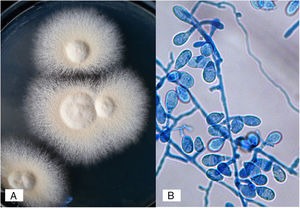
N. nana develops flat, cream to buff colonies, with a suede-like to powdery texture; a reddish-brown pigmentation was present in the deep of the agar. Micromorphological examination of the colonies revealed rough, thick-walled macroconidia ranging from 1–3 cells, accompanied with a large number of short egg-shaped macroconidia (also referred to as “dwarf”) with 2–3 septa and few sessile microconidia.13,21
The in vitro susceptibility to the main antifungals indicates that N. nana is sensitive to miconazole, clotrimazole and ketoconazole. There are few reports of minimum inhibitory concentration (MIC) of this species; Wildfeuer et al. found the following MICs: griseofulvin 3.1μg/ml; voriconazole and itraconazole 0.78μg/ml, and ketoconazole 0.2μg/ml.19 There are no precise data about terbinafine sensitivity. It is important to note that these studies include only with one strain. Although in the report of Wildfeuer et al. griseofulvin had the highest MIC, our two cases had a good response to that antifungal, although the case of tinea corporis took also topical ketoconazole, that had the lowest MIC.19 The antifungal therapy was chosen based on the response observed when treating other dermatomycoses caused by fungus of the genus Microsporum.4,8,10
We provide two rare cases; the rarity of the infections by N. nana may be due to the infrequent infection by this fungal agent, or to an underdiagnosis. In our clinical cases, the diagnosis of N. nana infection was based on the habitat of both patients, an extensive swine production area in Mexico (La Piedad, Michoacán region). The different clinical presentation in both patients may be due to the low antigenic recognition in case 1, but both children had a good response to therapy.
FundingThis work did not have any funding.
Conflict of interestAll the authors declare that they do not have any conflict of interest.