Candida parapsilosis, Candida metapsilosis and Candida orthopsilosis are emerging as relevant causes of candidemia. Moreover, they show differences in their antifungal susceptibility and virulence. The echinocandins are different in terms of in vitro antifungal activity against Candida. Time-kill (TK) curves represent an excellent approach to evaluate the fungicidal activity of antifungal drugs.
AimsTo compare the fungicidal activities of anidulafungin, caspofungin and micafungin against C. parapsilosis species complex by TK curves.
MethodsAntifungal activities of three echinocandins against C. parapsilosis, C. metapsilosis and C. orthopsilosis were studied by TK curves. Drug concentrations assayed were 0.25, 2 and 8μg/ml. CFU/ml were determined at 0, 2, 4, 6, 24 and 48h.
ResultsKilling activities of echinocandins were species-, isolates- and concentration-dependent. Anidulafungin reached the fungicidad endpoint for 6 out of 7 isolates (86%); it required between 13.34 and 29.67h to reach this endpoint for the three species studied, but more than 48h were needed against one isolate of C. orthopsilosis (8μg/ml). Caspofungin fungicidal endpoint was only achieved with 8μg/ml against one isolate of C. metapsilosis after 30.12h (1 out of 7 isolates; 14%). Micafungin fungicidal endpoint was reached in 12.74–28.38h (8μg/ml) against one isolate each of C. parapsilosis and C. orthopsilosis, and against both C. metapsilosis isolates (4 out of 7 isolates; 57%).
ConclusionsC. metapsilosis was the most susceptible species to echinocandins, followed by C. orthopsilosis and C. parapsilosis. Anidulafungin was the most active echinocandin against C. parapsilosis complex.
Candida parapsilosis, Candida metapsilosis y Candida orthopsilosis son causas relevantes de candidemia. Además, muestran diferencias en la sensibilidad a los fármacos antifúngicos. Las equinocandinas muestran diferente actividad antifúngica in vitro frente a Candida. Las curvas de tiempo-letalidad (TK) representan una excelente aproximación para evaluar la actividad fungicida de los fármacos antifúngicos.
ObjetivosComparar la actividad fungicida de la anidulafungina, la caspofungina y la micafungina frente al complejo C. parapsilosis mediante las curvas de TK.
MétodosSe estudió la actividad de tres equinocandinas frente a C. parapsilosis, C. metapsilosis y C. orthopsilosis mediante las curvas de TK. Las concentraciones ensayadas fueron 0,25, 2 y 8μg/ml. Se determinaron las UFC/ml a las 0, 2, 4, 6, 24 y 48h.
ResultadosLa actividad de las equinocandinas fue especie-, aislamiento- y concentración-dependiente. La anidulafungina alcanzó el límite fungicida frente a 6 de 7 aislamientos (86%), y necesitó 13,34-29,67h para alcanzar este límite en las tres especies estudiadas; para un aislamiento de C. orthopsilosis, requirió más de 48h (8μg/ml). El límite fungicida de la caspofungina solo se alcanzó con 8μg/ml frente a un aislamiento de C. metapsilosis después de 30,12h (1 de 7 aislamientos; 14%). La micafungina alcanzó este límite en 12,74-28,38h (8μg/ml) frente a un aislamiento de C. parapsilosis y C. orthopsilosis y frente a ambos aislamientos de C. metapsilosis (4 de 7 aislamientos; 57%).
ConclusionesC. metapsilosis fue la especie más sensible a las equinocandinas, seguida de C. orthopsilosis y C. parapsilosis. La anidulafungina fue la equinocandina más activa frente al complejo C. parapsilosis.
Invasive candidiasis is an important cause of morbidity and mortality worldwide. Incidence of infections due to non-Candida albicans species is increasing, although C. albicans remains the most common etiology in most countries.37Candida parapsilosis is an emerging and relevant cause of candidemia in both adults and children, being the second or third most frequent species depending on the geographical area.21 Moreover, Candida orthopsilosis and Candida metapsilosis are newly recognized members of C. parapsilosis complex. These three species show substantial differences in their antifungal susceptibility and virulence, and their epidemiology is a matter of increasing interest.26,33,44
The echinocandin drugs, anidulafungin, caspofungin and micafungin, inhibit 1,3-β-d-glucan synthesis of fungal cell wall, causing fungistatic as well as fungicidal effects. Echinocandins are considered front-line antifungal agents for the therapy of candidemia and invasive candidiasis.30,31 Despite the mechanism of action and chemical structure of these echinocandins are very similar, there are subtle differences among them in terms of in vitro antifungal activity against many Candida species.20,29 Although traditionally dose and drug selection in antimicrobial therapy is based on a single static in vitro parameter, such as the minimum inhibitory concentration (MIC), the in vivo antimicrobial effect is the result of a dynamic exposure of the infective agent to the antifungal drug. Time-kill (TK) curves describe the drug-microorganism interactions in a more dimensional way by a dynamic integration of concentration and time, being a more rational approach than the classical MIC value.28 The aims of our study were to compare and to widen the knowledge on the TK curves of anidulafungin, caspofungin and micafungin against C. parapsilosis, C. metapsilosis and C. orthopsilosis.
Materials and methodsMicroorganismsSeven clinical isolates and strains from the C. parapsilosis complex were studied, including 3 C. parapsilosissensu stricto (1 blood isolate -UPV/EHU 09-378- and 2 reference strains -ATCC 22019 and ATCC 90018-); 2 C. metapsilosis (1 blood isolate -UPV/EHU 07-045- and ATCC 96143 strain) and 2 C. orthopsilosis (1 blood isolate -UPV/EHU 07-035- and ATCC 96139 strain). Blood isolates were obtained from the fungal culture collection of the Laboratorio de Micología Médica, Universidad del País Vasco/Euskal Herriko Unibertsitatea (UPV/EHU), Bilbao, Spain. Isolates were identified by metabolic properties (ATB ID 32C, bioMérieux, France) and by molecular methods, as previously described.27,32
Antifungal agentsCaspofungin (Merck Sharp & Dohme, Spain), micafungin (Astellas Pharma, Spain) and anidulafungin (Pfizer SLU, Spain) were dissolved in dimethyl sulfoxide to obtain a stock solution of 5120μg/ml. Further dilutions were prepared in RPMI 1640 medium with l-glutamine and without NaHCO2 buffered to pH 7 with 0.165M morpholinepropanesulfonic acid (MOPS) (Sigma-Aldrich, Spain). Stock solutions were stored at −80°C until use.
In vitro susceptibility testingMICs, defined as minimum concentrations that produce ≥50% growth reduction, were determined and interpreted following the CLSI M27-A3, M27-A3 S4 and M60 documents.7–9 Results were read after 24h of incubation.
Time-kill proceduresBefore TK studies were performed, the antifungal carryover effect was determined as described by Cantón et al.6 Studies were carried out as previously described on microtiter plates for a computer-controlled microbiological incubator (BioScreen C MBR, LabSystems, Finland) in RPMI by using an inoculum size of 1–5×105CFU/ml and a final volume of 200μl.2,5,17,18 Antifungal drug concentrations assayed were 0.25, 2 and 8μg/ml. Echinocandins concentrations were chosen taking into account their MICs for the studied isolates. Plates were incubated during 48h at 36±1°C without agitation. At 0, 2, 4, 6, 24 and 48h, aliquots were removed from each culture, including control and test solution wells. These aliquots were serially diluted in phosphate buffered saline to determine the number of CFU/ml. Volumes of 5, 10, 50 or 100μl, depending on the dilution and concentration of the drug, were plated onto Sabouraud dextrose agar and incubated at 36±1°C for 24 to 48h. The lower limit of accurate and detectable colony counts was 20CFU/ml. TK studies were conducted in duplicate on two different days.
Data analysisThe killing kinetics of the fungicidal activity were analyzed by fitting the TK data at each time point to the exponential equation Nt=N0×ekt, which was transformed into a line by applying logarithms [logNt=logN0+Kt]. Nt is the number of viable yeast cells at time t, N0 is the starting inoculum, K is the killing or growing rate, and t is the incubation time. Thus, the six time points on each killing curve were reduced to one value, K. The goodness of fit for each isolate/drug was assessed by the r2 value (>0.8). The mean time to achieve reductions in viable cells of 99.9%, and the time to reach the fungicidal endpoint (t99.9=3/K) were calculated from the K value. Fungicidal activities were compared by using K values: positive values indicate growth and negative values show killing.4 The analysis of variance was performed to determine significant differences in killing kinetics among species, echinocandins and concentrations. A p value <0.05 was considered significant.
ResultsIn vitro susceptibility testingAnidulafungin, caspofungin and micafungin showed similar activity against C. parapsilosis (geometric mean -GM- MIC: 1.58, 1.25 and 1.25μg/ml, respectively), C. metapsilosis (GM MIC: 1, 1.41 and 2μg/ml, respectively) and C. orthopsilosis (GM MIC: 1, 1.41 and 0.7μg/ml, respectively).
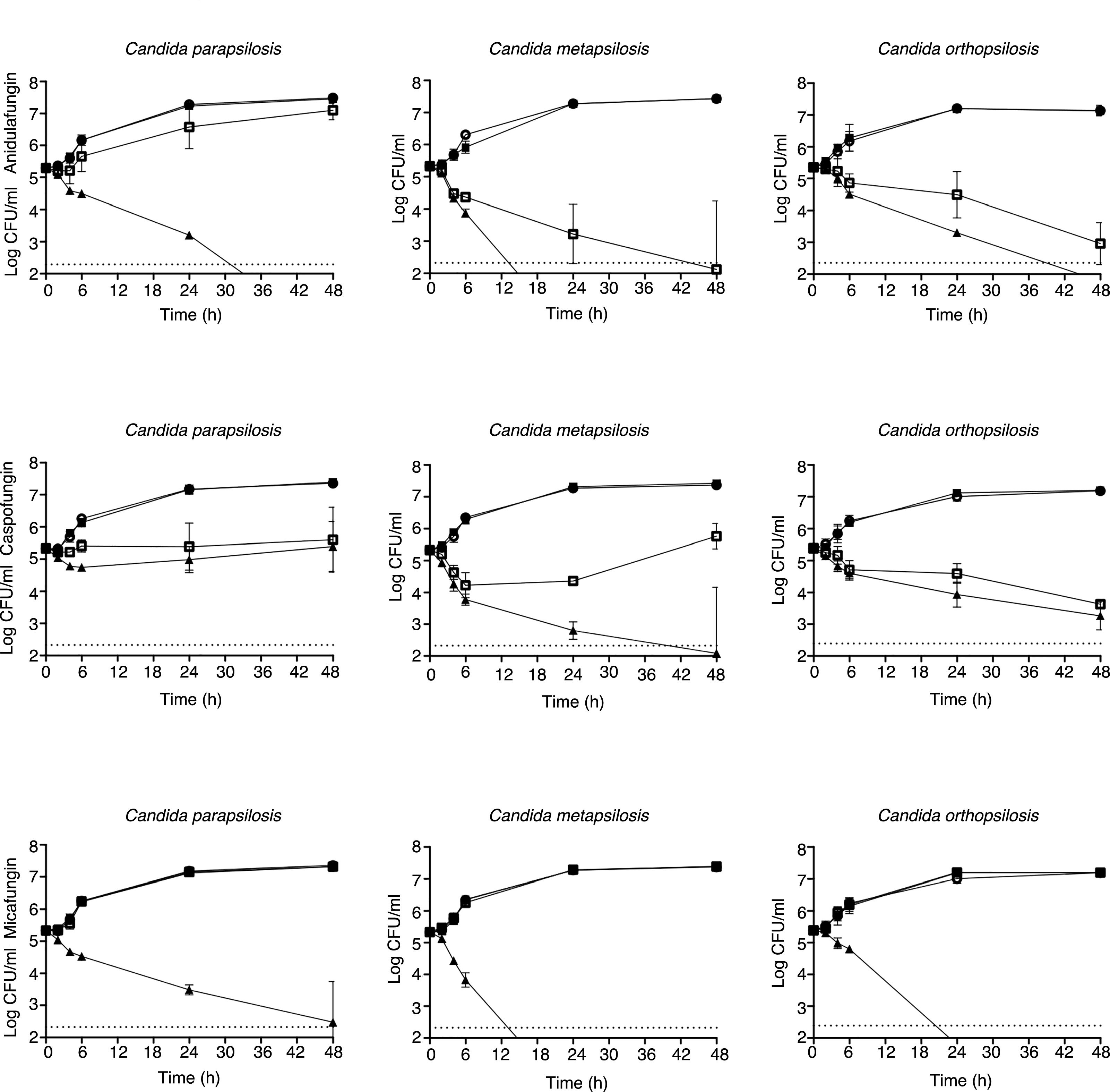
Time-kill assaysNo antifungal carryover effect was detected in TK studies. The mean TK curves and standard deviations of the three echinocandins against 3 C. parapsilosis, 2 C. metapsilosis and 2 C. orthopsilosis are depicted in Fig. 1. Killing activities of anidulafungin, caspofungin and micafungin were species- and isolate-dependent and increased with time. All echinocandins were more active against C. metapsilosis than against C. orthopsilosis or C. parapsilosis.
Mean time-kill plots for anidulafungin, caspofungin and micafungin against 3 C. parapsilosis, 2 C. metapsilosis and 2 C. orthopsilosis isolates. Each data point represents the mean result±standard deviation (error bars) for the indicated number of strains. White circles (○): control; black squares (■): 0.25μg/ml; white squares (□): 2μg/ml; black triangles (▴): 8μg/ml. The broken lines represent ≥99.9% growth reduction compared with that of the initial inoculum (fungicidal effect).
Anidulafungin reached the mean maximum log decreases of CFU (>3.5log) with 8μg/ml against the three species of the C. parapsilosis complex, except for one isolate each of C. metapsilosis and C. orthopsilosis, against which this decrease was achieved at 2μg/ml (6 out of 7 isolates; 86%). It must be noted that against the isolate C. orthopsilosis UPV/EHU 07-035 no fungicidal endpoint was attained.
With caspofungin, the fungicidal endpoint (99.9% killing) was only achieved against one isolate of C. metapsilosis (1 out of 7 isolates; 14%), with 8μg/ml, being ≥4log the maximum log decrease in CFU/ml. Caspofungin did not display fungicidal activity against the rest of strains and isolates studied.
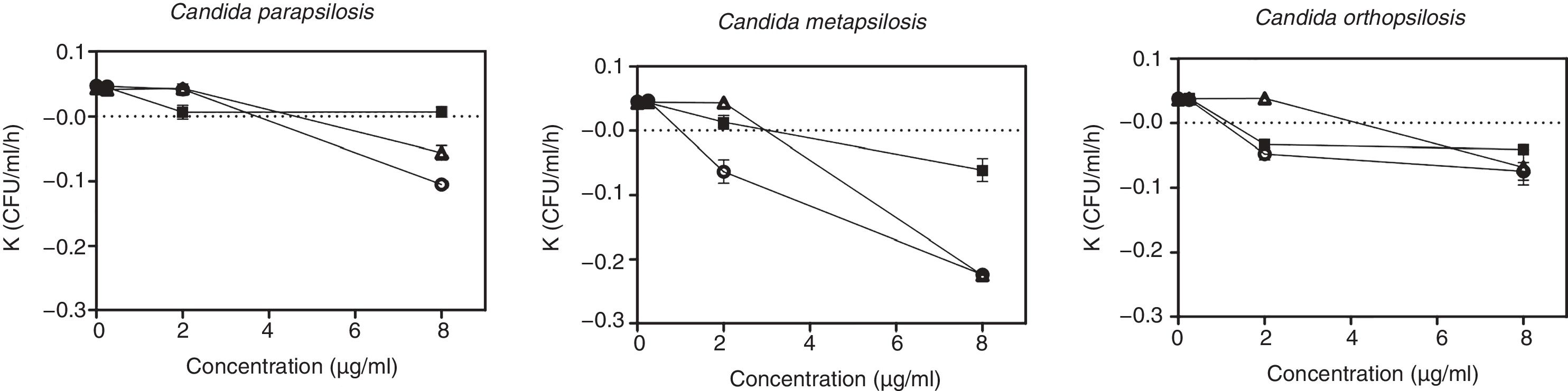
A concentration of 8μg/ml of micafungin was fungicidal for one isolate of C. orthopsilosis, the two isolates of C. metapsilosis and one isolate of C. parapsilosis (4 out of 7 isolates; 57%), although the log decreases of C. metapsilosis were higher than those reached for C. parapsilosis and C. orthopsilosis (≥4log, 2.9±2.18log, and 3.6±2.52log, respectively). Fig. 2 shows the effect of echinocandin concentrations on the killing rates for C. parapsilosis, C. metapsilosis and C. orthopsilosis.
Effect of anidulafungin, caspofungin and micafungin concentrations on the killing rates . White circles (○): anidulafungin; black squares (■): caspofungin; white triangles (▵): micafungin. Values above the broken lines indicate growth, and values below the broken lines indicate killing.
The highest killing rates in C. parapsilosis and C. metapsilosis were obtained with 8μg/ml and 2μg/ml of anidulafungin, respectively. These killing rates were significantly higher than those of micafungin and caspofungin (p<0.05). Concerning C. orthopsilosis, anidulafungin and caspofungin showed similar killing rates with 2μg/ml and were higher than that of micafungin. However, with 8μg/ml similar killing rates were obtained with anidulafungin and micafungin, in both cases higher than that observed with caspofungin. The ranges of killing rates with the echinocandins against C. parapsilosis species complex were the following: 0.02–0.07 and 0.03 with 2μg/ml (anidulafungin and caspofungin, respectively) and 0.07–0.22, 0.04–0.06 and 0.06–0.22 with 8μg/ml (anidulafungin, caspofungin and micafungin, respectively) (Fig. 2).
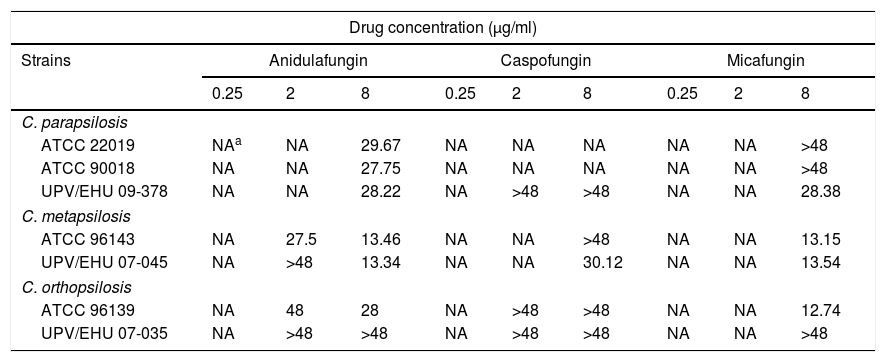
Table 1 shows the mean times needed to reach the fungicidal endpoint for each isolate and echinocandin concentration tested. Anidulafungin required 8μg/ml and between 13.34 and 29.67h to reach the fungicidal endpoint against 6 out of 7 isolates (86%) of C. parapsilosis complex, however, the killing of the clinical isolate of C. orthopsilosis (UPV/EHU 07-035) required more than 48h. By contrast, the caspofungin fungicidal endpoint was only reached in one isolate of C. metapsilosis after 30.12h with 8μg/ml (1 out of 7 isolates; 14%). Micafungin achieved the fungicidal endpoint with 8μg/ml after 12.74–28.38h against one isolate each of C. parapsilosis (UPV/EHU 09-378) and C. orthopsilosis (ATCC 96139) and against both isolates of C. metapsilosis (4 out of 7 isolates; 57%).
Time (h) to achieve 99.9% (t99.9) growth reduction from the initial inoculum.
| Drug concentration (μg/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strains | Anidulafungin | Caspofungin | Micafungin | ||||||
| 0.25 | 2 | 8 | 0.25 | 2 | 8 | 0.25 | 2 | 8 | |
| C. parapsilosis | |||||||||
| ATCC 22019 | NAa | NA | 29.67 | NA | NA | NA | NA | NA | >48 |
| ATCC 90018 | NA | NA | 27.75 | NA | NA | NA | NA | NA | >48 |
| UPV/EHU 09-378 | NA | NA | 28.22 | NA | >48 | >48 | NA | NA | 28.38 |
| C. metapsilosis | |||||||||
| ATCC 96143 | NA | 27.5 | 13.46 | NA | NA | >48 | NA | NA | 13.15 |
| UPV/EHU 07-045 | NA | >48 | 13.34 | NA | NA | 30.12 | NA | NA | 13.54 |
| C. orthopsilosis | |||||||||
| ATCC 96139 | NA | 48 | 28 | NA | >48 | >48 | NA | NA | 12.74 |
| UPV/EHU 07-035 | NA | >48 | >48 | NA | >48 | >48 | NA | NA | >48 |
C. parapsilosis is frequently involved in invasive infections worldwide, especially in neonates. In addition, recent findings suggest that C. metapsilosis and C. orthopsilosis are clinically relevant species. Lockhart et al.26 reported that among 1929 bloodstream infections presumed to be caused by C. parapsilosis, 91.3% were caused by C. parapsilosis, 6.1% by C. orthopsilosis, and 1.8% by C. metapsilosis. Despite of this clinical importance of C. parapsilosis, C. metapsilosis and C. orthopsilosis, there are few studies on the fungicidal activities of anidulafungin, caspofungin and micafungin by TK methodology2,15,43,47 and only two studies have head-to-head compared the activity of these echinocandins by TK curves.2,41 Besides, echinocandins play a central role in the current prevention and treatment of invasive candidiasis. The methodology of our study and the number of isolates evaluated is based on previous TK studies. This number of isolates studied in the TK tests is significantly lower than that used in the MIC studies.2,3,11,14,23,25,38,40,41,43,45 The greater complexity of the TK analysis, its heavier laboriousness and the complex handling of the much broader information obtained with the TK curves are some of the main reasons that justifies the lower number of isolates of our study. In the current study, anidulafungin and micafungin MIC values for C. parapsilosis were similar to those recently reported by Pfaller et al.34–36 These authors observed that anidulafungin and micafungin MIC values for C. parapsilosis isolates ranged between 0.03–8μg/ml and 0.03–4μg/ml, respectively. The MIC results in our study are close to those previously reported by García-Effron et al.,16 who obtained MIC values for anidulafungin of 0.5–2μg/ml and 0.5–2μg/ml for micafungin against the majority of the studied isolates of C. parapsilosis, as well as Pfaller et al. In this sense, recently, Valentin et al.46 evaluated the in vitro activity of anidulafungin against two isolates of C. parapsilosis and they found MIC values of 2μg/ml for both isolates. The methodology used in all these articles was the same as the methodology described in our manuscript, and all tested isolates in the mentioned articles were obtained from blood cultures, as were all our isolates. Moreover, MIC values of anidulafungin, caspofungin and micafungin for C. parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were within the MIC limits recommended by CLSI. Despite this result, caspofungin MICs should be cautiously interpreted because an important variability in modal MICs has been reported.12
We have compared the killing activity of the anidulafungin, caspofungin and micafungin against C. parapsilosis species complex up to 48h, providing new results about killing patterns and expanding the knowledge on the activities of echinocandins against the C. parapsilosis species complex. Previously, the fungicidal activities of echinocandins against C. parapsilosis, C. metapsilosis and C. orthopsilosis, resulted in highest killing rates for C. metapsilosis followed by C. orthopsilosis.2 Micafungin and anidulafungin showed similar killing rates, both being greater than those of caspofungin. None of the drugs reached the fungicidal endpoint, not even using a higher concentrations than in the present study, against C. parapsilosis.2 However, in the current report, fungicidal activities were observed with anidulafungin and micafungin at 8μg/ml, despite C. parapsilosis was the least susceptible of the three species. This discrepancy could be related to the inter-strain variability, as other authors reported differences in azole antifungal activities against these species. In this sense, Szabo et al.42 reported that fluconazole and voriconazole, but not posaconazole, seemed to be less active in vitro against C. orthopsilosis and C. metapsilosis, than against C. parapsilosis. Similar to our findings, Spreghini et al.41 did not observe a fungicidal effect of caspofungin against C. parapsilosis at concentrations of 2 and 32μg/ml. In this sense, Cantón et al.2 reported that none of the echinocandins had a fungicidal activity against C. parapsilosis, not even with a concentration of 32μg/ml. Foldi et al.15 also demonstrated the fungicidal activity of micafungin against C. parapsilosis, C. orthopsilosis and C. metapsilosis at ≥2–8 MIC concentrations after 48h. However, the fungicidal activity of micafungin in our study was reached earlier against several isolates. Similarly, our results are consistent with the reported by Szilagyi et al.,43 who did not observe a fungicidal effect of caspofungin against C. parapsilosis, not even using 16μg/ml of this drug. In a recent study, Hall et al.19 showed the fungicidal activity of 8μg/ml of anidulafungin and rezafungin (formerly CD101, a novel echinocandin) against the 2 isolates studied of C. parapsilosis. These results are similar to ours concerning the anidulafungin.
We conclude that anidulafungin was the most active antifungal drug against C. parapsilosis, C. metapsilosis and behaved similar to micafungin against C. orthopsilosis. We have also previously reported this higher anidulafungin activity against C. albicans and the Candida glabrata clades, although caspofungin and micafungin also showed optimal in vitro activities.17,18 Caspofungin demonstrated favorable efficacy and safety profiles in the treatment of invasive candidiasis caused by non-C. albicansCandida species, even in those regions or countries where C. parapsilosis is highly prevalent.10 With respect to the studied species, C. metapsilosis was the most susceptible to echinocandins, followed by C. orthopsilosis and C. parapsilosis. Despite the presumed reduced susceptibility of C. parapsilosis to echinocandins, these antifungal drugs are currently effective in invasive candidiasis.1,13 While the MIC values of echinocandins were similar for the three species, there were differences in the fungicidal activities against different isolates and species. These discrepant results found between MIC and TK curves may be attributed to the different methodologies used in MIC and TK studies. Information obtained from TK curves is broader than that obtained from MIC methodology. These different results between MIC methodology and TK curves methodology have also been previously reported2,17,18,39 and for this reason it would be advisable to perform in vitro TK assays and consider the results obtained from these TK curves. Other authors that have used the TK methodology have also suggested that clinicians should consider these TK results as a tool for therapeutic decision-making.22,24 This fact highlights the importance of a correct identification and acquaintance of the antifungal susceptibility patterns of these cryptic Candida species for an adequate therapeutic approach of the invasive infections caused by the C. parapsilosis species complex.
Conflict of interestWe have no specific conflicts of interest related to the current manuscript but declare the following: GQ has received research grants from Astellas Pharma, Pfizer, Merck Sharp & Dohme, and Scynexis. GQ has served on advisory/consultant boards for Merck, Sharp & Dohme, and Scynexis, and he has received speaker honoraria from Abbvie, Astellas Pharma, Merck Sharp & Dohme, Pfizer, and Scynexis.
SG-A has a postdoctoral grant from the Universidad del País Vasco-Euskal Herriko Unibertsitatea (UPV/EHU) (ESPDOC17/109). This work was supported by Consejería de Educación, Universidades e Investigación (GIC15/78 IT990-16) and the UPV/EHU (UFI 11/25).