An overview of current trends in Latin American Experimental Medical Mycological research since the beginning of the 21st century is done (search from January 2000 to December 2009). Using the PubMed and LILACS databases, the authors have chosen publications on medically important fungi which, according to our opinion, are the most relevant because of their novelty, interest, and international impact, based on research made entirely in the Latin American region or as part of collaborative efforts with laboratories elsewhere. In this way, the following areas are discussed: 1) molecular identification of fungal pathogens; 2) molecular and clinical epidemiology on fungal pathogens of prevalence in the region; 3) cell biology; 4) transcriptome, genome, molecular taxonomy and phylogeny; 5) immunology; 6) vaccines; 7) new and experimental antifungals.
Se presenta una revisión de las más importantes líneas de investigación en micología médica experimental en América Latina desde el inicio del siglo XXI (búsqueda bibliográfica desde enero de 2000 a diciembre de 2009). Usando las bases de datos PubMed y LILACS, los autores hemos escogido publicaciones en hongos patógenos de importancia clínica que, de acuerdo a nuestra opinión, son las más relevantes por su novedad, interés e impacto internacional, basadas en investigaciones realizadas totalmente en la región latinoamericana o como parte de esfuerzos colaborativos con laboratorios de otras partes del mundo. De esta forma, discutimos las siguientes áreas: 1) identificación molecular de patógenos fúngicos; 2) epidemiología clínica y molecular de hongos patógenos prevalentes en la región; 3) biología celular; 4) transcriptoma, genoma, taxonomía y filogenia moleculares; 5) inmunología; 6) vacunas; 7) antifúngicos nuevos o experimentales.
Recent data208 indicate that fungal diseases in Brazil do carry a high toll on fatal outcome of systemic mycoses. Although Prado et al's figures are limited to that country, it is reasonable to assume that given the similarities in regional and local health services and other social factors, their results may be representative of events in other Latin American countries. So, according to the authors, death tolls in Brazil within the period 2005-2006, amount to 44.6% (paracoccidioidomycosis, PCM), 26.8% (cryptococcosis), 16.3% (candidiasis), 5.6% (histoplasmosis), 5.0% (aspergillosis), 0.9% (zygomycosis), and 0.8% (coccidioidomycosis) of total patients treated for these diseases.208 It is obvious, then, that Latin American countries suffer from a significant burden of systemic mycoses, which need to be addressed not only in terms of public health policies but also, and equally important, with an aggressive program on academic, experimental and clinical research aimed to understand the phenomena underlying this serious and ever growing health problem.
This review highlights publications chosen by the authors as representative of the most relevant research in experimental medical mycology carried out in Latin America in the period January 2000-December 2009. Seven areas are covered and discussed within the framework of international research: 1) molecular identification of fungal pathogens; 2) molecular and clinical epidemiology on fungal pathogens of prevalence in the region; 3) cell biology; 4) transcriptome, genome, molecular taxonomy; 5) immunology; 6) vaccines; 7) new and experimental antifungals.
Searching in PubMed and the Latin American LILACS data bases (key words mycosis+
A major obstacle to the successful treatment of invasive fungal infections is the paucity of rapid, sensitive and specific methods that would help in the early diagnosis of fungal infections. PCR assays for diagnostic purposes are being extensively used though the method still lacks standardization and cannot be used as the sole test for early detection or for the purpose of defining invasive fungal infection [for a recent review, see 229].
In Latin America, PCR methodology for identification and diagnostic purposes has been applied ever since it appeared in the scientific literature as a promising technique. The Histoplasma capsulatum H or M antigens, pluripotent glycoproteins that elicit both humoral and T cell-mediated immune responses, are proteins whose genes have been used for the design of primers aimed at molecular diagnosis.32,111 Bracca et al32 developed a highly specific and sensitive semi-nested PCR assay in which three oligonucleotides, placed at the fifth exon of the gene encoding the H antigen, were chosen for their ability to differentiate H. capsulatum sequences from sequences of other fungal ß-glucosidases in the databases. Meanwhile, Guedes et al111 used the M-antigen gene (highly homologous to catalases) to design four oligonucleotide sequences in the less homologous regions, for application in a one-step PCR detection and identification of H. capsulatum var. capsulatum. De Aguirre et al72 used PCR technology in an enzyme immunoassay format for the rapid differentiation of Aspergillus species from other medically important opportunistic molds and yeasts. With oligonucleotide probes, directed to the ITS2 region of ribosomal DNA from several Aspergilli, they were able to differentiate 41 isolates; a single DNA probe to detect all seven of the most medically important Aspergillus species (Aspergillus flavus, Aspergillus fumigatus, Aspergillus nidulans, Aspergillus niger, Aspergillus terreus, Aspergillus ustus, and Aspergillus versicolor) was also designed.
In a prospective study in a high-risk population of candidemia, Moreira-Oliveira et al171 used ITS5 and ITS4 primers for PCR, followed by sequencing of products for Candida spp. identification. They reported 72.1 and 91.2% sensitivity and specificity, respectively. A combination of PCR and RFLP helped in the identification of Candida species in immunocompromised and seriously diseased patients.206Candida albicans was the species most frequently observed, except for the group of newborns who were infected preferentially by Candida parapsilosis.206 RAPD in combination with the OPE-18 primer was reported to be a very specific and sensitive method for the identification of Candida glabrata, Candida guilliermondii, Candida tropicalis, Candida pelliculosa, C. albicans, Candida krusei, and Candida lusitaniae.14 RAPD was also used for the correct identification of C. albicans and Candida dubliniensis.20 In 2006, the latter species was detected for the first time in Venezuela by means of a PCR approach.34 A different methodological approach for the identification of candidemia by C. albicans, C. tropicalis, and C. parapsilosis was used by Berzaghi et al.24 With the help of an inhibition enzyme-linked immunosorbent assay (ELISA) and anti-65-kDa monoclonal antibody, they were able to detect a 65-kDa antigen that presented three different patterns of antigenemia when tested against sera from patients: i) total clearance of antigenemia, ii) initial clearance and relapse of antigenemia, and iii) partial clearance of antigenemia. These results suggest that detection of the 65-kDa protein may be a valuable tool for the differential diagnosis of candidemia caused by either of these Candida species.
The antigen 2/proline-rich antigen has been used for the PCR detection of Coccidioides posadasii.26,71 This antigen is common to C. posadasii and Coccidioides immitis.
Diagnostic primers in P. brasiliensis have been designed primarily from the nucleotide sequence of its reference antigen, gp43.25,103 Some of the gp43 sequences were taken from regions where later studies166 revealed the presence of informative and non informative nucleotide substitutions in this highly polymorphic gene,166 a result that may influence the use of gp43 as a universal reference antigen.231 A second set of diagnostic primers tested in clinical samples is that of San-Blas et al.230 They were designed from two specific DNA fragments (Mw 0.72 and 0.83 Kb) common to and specific for all P. brasiliensis samples, generated when using the arbitrary primer OPG18 (Operon Biotechnology).37 Such primers were capable of rapidly identifying P. brasiliensis DNA from sputum and cerebrospinal fluid of PCM patients.230
A duplex polymerase chain reaction (PCR) targeting the ITS1-5.8S-ITS2 region of the ribosomal DNA was designed for rapid and specific identification of 69 Fonsecaea pedrosoi isolates; 4 Fonsecaea compacta samples and several other dematiaceous isolates did not produced identification bands.73 The frequency of Fonsecaea-positive results was similar between duplex PCR (68.0%) and morphology (67.0%). However, 4% isolates were positive by duplex PCR but negative by morphology, indicating that PCR method may be the test of choice when dealing with samples unable to produce conidia. On the other hand, 3% samples were positive by morphology and negative by Fonsecaea-specific PCR. These isolates have high similarity to the genus Phialophora when DNA sequencing analyses were performed.
Epidemiology of prevalent fungal pathogens in the regionClinical epidemiologyAntimicrobial resistance surveillance serves for the detection and tracking of resistance trends and emerging new resistance threats, and also as a means to monitor the prevalent pathogens causing serious infections. In order to address effectively any of these objectives, the availability of a geographically diverse collection of isolates from clinically important sites of infection is essential.203 Very few programs provide information on fungal infections and antifungal resistance, among them, the ARTEMIS Global Antifungal Susceptibility Program (ARTEMIS Program) and the Regional Laboratory Network for Surveillance of Invasive Fungal Infections and Antifungal Susceptibility in Latin America, both mainly focused on candidemia from several Candida spp. Of recent formation and consequently, few reports to date, the Regional Network is coordinated by the Essential Medicines, Vaccines, and Health Technologies Unit of the Pan American Health Organization, with the technical and financial support of the National Center for Microbiology of the Carlos III Health Institute (Spain), and the technical support of the Microbiology Department of the Dr. C. Malbrán National Institute on Infectious Diseases (Argentina) and the Microbiology Unit of the Parasitology Service of the Adolfo Lutz Institute (Brazil).67 The Network's main objectives are epidemiological surveillance of invasive fungal infections through detection of antifungal resistance and identification of emergent, invasive fungal infections; establishment of norms and common protocols for early diagnosis of mycoses; strengthening coordination, communications, and transference mechanisms among participant countries.60 The older ARTEMIS program was initiated in 2001 to provide focused surveillance of the activities of fluconazole and voriconazole against Candida spp. causing invasive infections, and to provide continuous development and validation of various broth- and agar-based antifungal susceptibility test systems. The ARTEMIS Program has provided a massive amount of data; it uses a central reference laboratory and an international network of 105 participating centers as sources of clinical isolates203; several Latin American laboratories provide essential information within this program. Their results indicate that more than 90% of invasive infections due to Candida spp. are attributed to five species, C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei. Although C. albicans remains the dominant species causing bloodstream infections (BSI), the frequency of occurrence varies throughout the world from 37% in Latin America to 70% in Norway. Most notable is the very low frequency of C. glabrata as a cause of BSI in Latin America, where only 4% to 7% of Candida BSIs are attributed to this species.104,204 The frequency of isolation of C. glabrata from blood cultures follows a trend toward decreased frequency in Latin America (7.4% to 4.7% of BSIs), Europe (10.5% to 8.8%), and the Asia-Pacific region (12.1% to 7.2%) between 2002 and 2004.171 The frequency of invasive candidiasis due to C. parapsilosis has increased in recent years, most notably in Latin America. Whereas C. tropicalis is only the fourth most common species of Candida causing BSI in North America (7% of BSIs), it ranks second in Latin America (20%) and is more common than C. glabrata in the Asia-Pacific region (14-21% vs. 10-12%, respectively). C. guilliermondii and Candida rugosa are relatively uncommon species of Candida that appear to be increasing in frequency as agents of invasive candidiasis. These two species can be found in Latin America, where they each account for 3% to 5% of all candidemias and may be more common than either C. glabrata or C. krusei.60,78,104,109
The above mentioned global figures hide the documented geographic differences in rates and epidemiology of candidemia in different countries and cities within them (Table 1). Prospective candidemia surveillance in 11 medical centers located in 9 major Brazilian cities (March 2003 to December 2004) indicated that C. albicans was the most common species (40.9%), followed by C. tropicalis (20.9%) and C. parapsilosis (20.5%).60 Further candidaemia surveillance studies in Brazil104 indicated that in the genetically heterogeneous taxon C. parapsilosis (C. parapsilosis, Candida orthopsilosis and Candida metapsilosis), the prevalence of each species among 141 bloodstream isolates was 88%, 9% and 3%, respectively. All isolates but three 5-fluorocytosine-resistant C. orthopsilosis were susceptible to polyenes, triazoles and caspofungin.
Similar studies78 in six health care centers of Caracas, Venezuela, from January 2003 through August 2005, indicated that C. albicans was the most frequently isolated yeast (46.7%), followed by C. tropicalis (19.0%), C. glabrata (9.2%) and C. parapsilosis (6.0%). During the first year of an ongoing surveillance program of invasive fungal infections in 13 hospitals in Chile, Silva et al236 found that C. albicans (40.8%), C. parapsilosis (13.1%), C. tropicalis (10%) and Cryptococus neoformans (10%) were the most common yeast species. A multicenter study performed to determine the species distribution associated to candidemias in Argentina214 also brought about similar results, that is, C. albicans (40.75%), C. parapsilosis (28.67%), C. tropicalis (15.84%), Candida famata (3.77%), C. neoformans (3.77%), C. glabrata (2.64%), and others (4.53%). Interestingly, C. parapsilosis (37.9%) was the most frequent species found by González et al109 in a 3-year surveillance program (2004 to 2007) in Monterrey, Mexico, followed by C. albicans (31.9%), C. tropicalis (14.8%), C. glabrata (8.0%), among the most frequent. The species distribution differed with the age of the patients, a result also documented by Pfaller and Diekema.203
C. dubliniensis is associated with oral candidiasis in immunodepressed individuals. Using classical phenotypic methods combined with PCR techniques, Jewtuchowicz et al114 found that this species is present in 4.4% of periodontal pockets of immunodepressed Argentinean patients. C. albicans was the most frequent species, corresponding to 24.4% (44/180). Other non-C. albicans species were found, among them C. parapsilosis, C. tropicalis, and C. guilliermondii.
These studies were always accompanied by screening of resistance to antifungals such as azoles and echinocandins, indicating a wide range of variability in the susceptibility of strains. Together, these data point to the importance of local and regional surveillance studies to guide physicians towards the most effective treatment of candidiasis and other fungal diseases.
Retrospective studies characterizing acute/subacute PCM incidence in the Botucatu area, São Paulo State, Brazil, from 1969 to 1999 and their relationship with climate variables (antecedent precipitation, air temperature, soil water storage, absolute and relative air humidity, and Southern Oscillation Index) have been done by Barrozo et al.17 They concluded that correlations may reflect enhanced fungal growth after increase in soil water storage in the longer term and greater spore release with increase in absolute air humidity in the short term.
Molecular epidemiologyThe basidiomycetous yeasts C. neoformans and Cryptococcus gattii are closely related sibling species that cause respiratory and neurological disease in humans and animals. Within these two recognized species, phylogenetic analysis reveals cryptic species or molecular types within the pathogenic Cryptococcus species complex, corresponding to serotypes A (C. neoformans var. grubii; VNI, VNII), D (C. neoformans var. neoformans; VNIV), AD (Hybrid; VNIII), B and C (C. gattii; VGI-VGIV).92,119,163 To acquire basic knowledge of C. neoformans in IberoAmerican countries, 266 clinical, 7 veterinary, and 67 environmental isolates from Argentina, Brazil, Chile, Colombia, Mexico, Peru, Venezuela, Guatemala, and Spain were typed by means of the M13 polymerase chain reaction-fingerprinting and orotidine monophosphate pyrophosphorylase (URA5) gene restriction fragment length polymorphism (RFLP) analysis with HhaI and Sau96I in a double digest.163 The majority of the isolates (68.2%) were VNI (C. neoformans var. grubii, serotype A), which agrees with the fact of this variety being the cause of most human cryptococcal infections worldwide, particularly in HIV-positive patients. Of the remaining, 5.6% were VNII; 4.1% VNIII; 1.8%, VNIV; 3.5% VGI; 6.2% VGII; 9.1% VGIII, and 1.5% VGIV. Chile and Spain shared similar molecular types, with a large number (15.8% and 42.1%, respectively) of molecular type VNIII isolates (AD hybrids). VNIV serotype D isolates were present only in Chile (26.3%). Patients with no known risk factors had C. gattii (VGI-VGIV) as the main fungal agent (fig. 1).163
Geographic distribution of the molecular types obtained from IberoAmerican Cryptococcus neoformans isolates by polymerase chain reaction fingerprinting and URA5 gene restriction fragment length polymorphis analysis (total numbers studied per country given in parentheses).163 Reproduced by permission.
Similar studies in Colombia84 indicated a prevalence of serotype A (91.1%) followed by serotypes B (8.4%) and C (0.5%) in clinical samples, figures that moved to 44.2, 42.6 and 13.2%, respectively, in environmental isolates. No serotype D or AD samples were isolated. With the same technique used by Meyer et al,163 the majority of clinical serotype A and environmental serotype B isolates were grouped into the molecular types VNI (98.1%) and VGII (100%), respectively. Molecular type VGII was the predominant genotype (77.7%) in both clinical and environmental Colombian C. gattii isolates. This contrasts with previous reports in which VGII was only found occasionally in tropical and subtropical regions.163
The most common molecular type found in Brazil was VNI (64%), followed by VGII (21%), VNII (5%), VGIII (4%), VGI and VNIV (3% each), and VNIII (< 1%).270 Primary cryptococcosis caused by C. gattii, molecular type VGII, prevailed in immunocompetent hosts, mainly young people and children, in the North and Northeast regions where C. gattii is endemic. On the other hand, in the Brazilian Southern region, sporadic infections by C. gattii were recorded. Overall, the most common molecular types were VNI (64%) and VGII (21%), followed by VNII (5%), VGIII (4%), VGI and VNIV (3% each), and VNIII (< 1%). Molecular type VGIV was not identified among the Brazilian isolates.270
Out of 72 Mexican clinical isolates (PCR-fingerprinting with the primer M13), 55 VNI, five VNII, three VNIII, one VNIV, two VGI, two VGII, two VGIII and two VGIV isolates were reported.191 The results show that most cryptococcosis cases in Mexico are AIDS-related and are caused by C. neoformans var. grubii, genotypes VNI and VNII. In addition, this study revealed for the first time the presence of genotypes VNIV and VGII among Mexican clinical isolates. Hence, all genotypes that have been described for the Cryptococcus species complex are found in Mexico, indicating a much wider geographic distribution of genotypes than previously reported.
Using a different molecular approach, Díaz et al77 employed sequence analysis of the intergenic spacer regions, IGSI and IGSII, the most rapidly evolving regions of the rDNA families. The IGSI region displays the higher genetic variability, represented by nucleotide base substitutions and the presence of long insertions/deletions (indels). In contrast, the IGSII region exhibits less heterogeneity and less extensive indels than the IGSI region. Both intergenic spacers contain short, interspersed repeat motifs, which can be related to length polymorphisms observed between sequences. Phylogenetic analyses, undertaken in the IGSI, IGSII and IGSI +5S rRNA+IGSII regions, revealed the presence of six major phylogenetic lineages, some of which segregated into subgroups. The major lineages are represented by genotypes 1 (C. neoformans var. grubii), genotype 2 (C. neoformans var. neoformans), and genotypes 3 to 6 represented by C. gattii, not always coincident with the molecular types found in the previously reviewed data.72,163,270
H. capsulatum is a dimorphic fungus that has been recognized as an important worldwide pathogen, agent of histoplasmosis. The disease, which in some Latin American regions is a public health threat, presents a wide diversity of clinical manifestations. Studies on chromosomal band profiles of clinical isolates might shed light on the role of fungal genetic diversity in the evolution of different clinical forms of the disease. Using pulsed-field gel electrophoresis, Canteros et al42 analyzed intact chromosomes of 19 clinical isolates of H. capsulatum isolated in Argentina, Mexico and Guatemala and the laboratory reference strain G186B from Panama. Chromosomal banding patterns, grouped in 13 different electrokaryotypes, ranged between 5 and 7 bands, 1.3 to 10 Mbp in size. Strain G186B produced five bands of approximately 1.1, 2.8, 3.3, 5.4 and 9.7 Mbp. Such chromosomal variability did not correlate with geographical or clinical source. In spite of the apparently high chromosome-length polymorphism, three clusters of identical patterns were identified. The largest group, karyotype I, included only Argentinean isolates of clinical origin, although this was not the only karyotype in harbouring isolates from this country. All Mexican H. capsulatum isolates were polymorphic among them.
Clinical observations in some Latin American countries indicate that the lymphocutaneous form of sporothricosis is prevalent in Mexico and Guatemala, whereas the fixed cutaneous form prevails in Colombia. Mesa-Arango et al162 aimed to determine the genotypic and phenotypic relatedness among Sporothrix schenckii isolates in these countries. Clinical and environmental isolates of S. schenckii were subjected to RAPD analysis-PCR with 10-mer primers OPBG-01, OPBG-14, and OPBG-19. The 44 S. schenckii isolates fell into four major groups by hierarchical cluster analysis. Group I cluster together 25 out of 27 Mexican isolates, into two subgroups, Ia with 10 environmental isolates and Ib with 14 clinical isolates. Group II also split into two subgroups: IIa, Colombian isolates, and IIb, Guatemalan isolates. Groups III and IV each had only one clinical Mexican isolate. The low thermotolerance at 35 and 37 oC of the Colombian isolates could be associated with superficial skin lesions in patients with fixed clinical forms of sporotrichosis, the most frequent form of the disease in Colombia. Even though the isolates were grouped by geographical origins, a high degree of genotypic variability was observed among the isolates. Reporting a sporothrichosis epidemic in Rio de Janeiro, Brazil, Reis et al210 demonstrated its zoonotic character using molecular methodology. For this, the RAPD technique with three different primers and DNA fingerprinting using the minisatellite derived from the wild-type phage M13 core-sequence allowed the authors to cluster 19 human and 25 cat S. schenckii isolates into 5-10 genotypes. The RAPD profiles of epidemic S. schenckii isolates could be distinguished from that of the United States-reference isolate, displaying 20% similarity to each primer and 60% when amplified with the M13 primer. DNA fingerprinting of S. schenckii isolated from the nails (42.8%) and the oral cavities (66%) of cats were identical to related human samples, suggesting that a common infection source for animals and humans in this epidemic, cats serving as a vehicle for dissemination of S. schenckii.
By cladistic analysis of partial sequences of the calmodulin gene using the maximum parsimony and neighbor-joining methods, Madrid et al133 determined that one out of 25 isolates from Mexico (4%), one out of three isolates from Guatemala (33.3%), and two out of four isolates from Colombia (50%) belonged to Sporothrix globosa, while all other isolates belonged to S. schenckii sensu stricto, this being the first record of S. globosa from Mexico, Central and South America.
Cell biologyMetabolic and regulatory processes in growth and morphogenesisCell cycle and interaction between DNA replication, nuclei segregation and budding in P. brasiliensis have been poorly studied. Almeida et al3 focused on the characteristics of the cell cycle profile of P. brasiliensis yeast cells during batch culturing and under the effects of benomyl, an antifungal drug known to promote a cell cycle arrest in the G2/M phases of Saccharomyces cerevisiae. Their results suggested that even though benomyl progressively blocks nuclear division of P. brasiliensis yeast form, treated cells retained their capacity for DNA replication.
Cells possess rapidly responding, highly complex signaling pathways to allow them to quickly adapt to a changing environment. Most prominent among them are the mitogen-activated protein kinase cascades. Some aspects of such complex systems are under study in Latin America. The cAMP-dependent protein kinase (PKA) from C. albicans is a tetramer composed of two catalytic subunits and two type II regulatory subunits encoded by TPK1 and TPK2, respectively, whose autophosphorylation site in Ser180 possibly conforms a modulatory mechanism for C. albicans PKA activity in vivo.289,290TPK1 is a positive regulator of the morphogenetic transition of C. albicans in the absence of the TPK2 gene.58 The loss of one catalytic isoform is not compensated by overexpression of the other.247 During Y-M transition, a sharp increase in TPK1 mRNA levels and in PKA-specific activity correlated with the onset of germ-tube formation in strain tpk2Δ, reinforcing the idea that Tpk1p is important for faster germ-tube appearance.
Bcy1p is a regulatory subunit of the PKA catalytic subunits TPK1 and TPK2.100BCY1-C. albicans yeast cells were used to generate a double bcy1 tpk2 mutant,47 with which it was proven that its constitutive PKA activity was cAMP independent, indicating that the cells harbored an unregulated phosphotransferase activity. Strains with one BCY1 allele displayed pseudohyphae and true hyphae, while hyphal morphology was almost exclusive in strains having both BCY1 alleles, suggesting a tight regulation of PKA activity for hyphal growth.100 Further work101 with mutants having heterozygous or homozygous deletions of TPK1 and/or TPK2 indicated that tpk1Δ/tpk1Δ strains developed a lower tolerance to saline exposure, heat shock and oxidative stress as well as defects in glycogen storage, whereas wild-type and tpk2Δ/tpk2Δ mutants were resistant to these stresses and accumulated higher levels of the polysaccharide, indicating that both isoforms play different roles in the stress response pathway and carbohydrate metabolism. In Yarrowia lipolytica, instead, an active PKA pathway promotes yeast-like growth and opposes mycelial development.52
The Y-M transition in S. schenckii responds to protein kinase C (PKC) effectors, indicating the involvement of PKC in this regulation. The presence of two pkc genes, pkcSs-1 and pkcSs-2, were confirmed by Southern blot.8 The latter has an ORF of 3942 nucleotides interrupted by five introns, to encode a protein of 1194 amino acids and 132.84 kDa. pkcSs-2 is expressed at all intervals during the Y-M transition.8 Also in S. schenckii, a Gαi subunit was found in a study aimed to search the role of G proteins in signal transduction, the first time such subunit was reported in a pathogenic fungus.76 The cDNA sequence revealed a 1059bp ORF encoding a 353 amino acid Gαi subunit of 41 kDa.
A homolog of the Pho85 cyclin-dependent kinase (Cdk) was found in S. schenckii.75 Pho85 has been identified as a regulator of phosphate metabolism and modulator of the transcriptional response to nutritional signals. The phoSs gene consists of 990bp, contains one intron, and encodes a protein of 306 amino acids. Expression of the phoSs gene decreased 30-fold during the Y-M transition. The addition of extracellular calcium accelerated the dimorphic transition and restored phoSs expression, suggesting that PhoSs may participate in the control of the Y-M transition in S. schenckii.75 As an initial step to understand the PHO pathway in A. fumigatus, de Gouvea et al.74 characterized the PHO80 homologue, PhoBPHO80 and showed that the ΔphoBPHO80 mutant has a delayed germ tube emergence; by phenotypic and phosphate uptake analyses, the authors were able to establish a link between PhoBPHO80, calcineurin and calcium metabolism. Several genes of the Pho complex, namely, phoDPHO84, phoEPHO89, phoCPHO81, and vacuolar transporter Vtc4 were more expressed both in the ΔphoBPHO80 mutant background and under phosphate-limiting conditions of 0.1 mM Pi. ΔphoBPHO80 and ΔphoDPHO84 mutant strains were fully virulent in a murine low dose model for invasive aspergillosis.
The glyoxylate cycle, apparently involved in fungal pathogenicity (for a review, see Dunn et al83), allows for the use of lipids in the synthesis of glucose via acetate → citrate → isocitrate. Its two initial steps are identical to those in the citric acid cycle. After cleavage into succinate and glyoxylate and further condensation with acetyl-CoA, malate is produced. Malate synthase is present in P. brasiliensis; with a calculated 539 amino acids and a molecular mass of 60 kDa, the gene that encodes it (Pbmls) has 1617bp.287 The enzyme is located on the fungal cell surface and possibly plays a role in the binding of fungal cells to the host, behaving as an anchorless adhesion system.178
During the infective process, pathogenic fungi are subjected to a significant environmental stress, including exposure to reactive oxygen and nitrogen species produced by host cells. Mitochondria are the main source of reactive oxygen species which need to be controlled by detoxification mechanisms. Tudella et al271 analyzed an alternative oxidase and an uncoupling protein in the respiratory chain of A. fumigatus. A functional respiratory chain (complex I-V) was demonstrated: adenosine 5’-diphosphate (ADP) induced an oligomycin-sensitive transition from resting to phosphorylating respiration, in the presence of the oxidizable substrates malate, glutamate, alpha-ketoglutarate, pyruvate, dihydroorotate, succinate, N,N,N’,N’-tetramethyl-p-phenylenediamine and exogenous NADH. They were also able to demonstrate the presence of an alternative NADH-ubiquinone oxidoreductase, an alternative oxidase and an uncoupling protein in the respiratory chain of A. fumigatus. Cloning and functional expression of the mitochondrial alternative oxidase of A. fumigatus indicated that its gene (Afaox) is 1173bp long, and encodes a 40 kDa protein.135 In P. brasiliensis mitochondria, a complete (Complex I-V) functional respiratory chain was also demonstrated.146 An alternative NADH-ubiquinone oxidoreductase, malate/NAD(+)-supported respiration, and alternative oxidase mechanism in the yeast form of the fungus suggested the existence of alternative respiratory chain pathways in addition to Complex I in P. brasiliensis.146 Similar results were found in C. albicans mitochondria51,226 and C. parapsilosis.164 Because such pathways are absent in animal cells, they may be exceptional targets for the design of new chemotherapeutic agents. Other mitochondrial genes coding for enzymes involved in the respiratory electron-transport chain, namely, proline oxidase, riboflavin kinase, and cytochrome c oxidase, have been reported in the dermatophyte Trichophyton rubrum.235
Flavoprotein monooxygenases constitute a family of enzymes involved in a remarkably wide variety of oxidative reactions and are, therefore, oxidoreductases; they are mainly related to anti-oxidative stress in fungi and participate in several metabolic pathways. One such protein has been identified in P. brasiliensis.140 It is the glycoprotein gp70, a concanavalin A-binding component recognized by about 96% of sera from untreated patients with PCM. Its gene encodes for a 79 kDa protein 718 aminoacids long. An increased PbGP70 transcript accumulation is observed under H2O2-induced oxidative stress, during fungal growth, and in macrophage phagocyted/bound yeasts. In this way, GP70 may work as a protector against oxidative stress and as elicitor of an immune response.140 Also related to oxidative stress are catalases, whose main function is to prevent the oxidative damage triggered by the reactive oxygen species of the host. Three catalases have been reported in P. brasiliensis, two of which (CatA and PbCatC) are monofunctional catalases and the third one (CatP), a catalase peroxidase54; additionally, P. brasiliensis has both cytosolic and peroxisomal catalase isoenzymes and a single cytochrome-c peroxidase.70 PbCatA manifested higher activity in the mycelial phase, during M-Y transition or endogenous oxidative stress. PbCatP showed higher activity in yeast cells since it is putatively involved in the control of exogenous reactive oxygen species. In C. glabrata, a high resistance to oxidative stress is mediated by a single catalase, Cta1p, controlled by the transcription factors Yap1p, Skn7p, Msn2p, and Msn4p.66C. dubliniensis, on the other hand, mounts an adaptive response to stress that leads to an increased survival against lethal doses of H2O2–like oxidants,268 characterized by the induction of enzymes with known antioxidant function (glucose-6-phosphate dehydrogenase, superoxide dismutase and catalase). C. dubliniensis is less resistant to oxidants than C. albicans, displaying higher susceptibility to their toxic effects.268
Enzymes whose encoding genes are differentially expressed through the morphogenetic process have been reported. Ornithine decarboxylase (ODC) is associated to the metabolism of polyamines.212 Early work indicated that in C. albicans, Mucor rouxii, and Y. lipolytica, the activity of ODC was higher in the mycelial phase; in P. brasiliensis, instead, it is the yeast phase that shows a higher activity of the enzyme, either at the extreme phases or through mycelial to yeast transition.232 However, PbrODC expression remained constant at all stages of the fungal growth, a result that suggests a post-transcriptional regulation of the PbrODC product.184C. immitis ODC, cloned in the pETCiODC plasmid under control of T7lac promoter, was produced in transformant strains Escherichia coli BL21(DE3), BL21(DE3)pLysS and BLR(DE3) (λ DE3 lysogen), and EWH319 (odc- null mutant).196E. coli BL21(DE3)pLysS-pETCiODC expressed the highest specific ODC activity, suggesting that this strain could be successfully used for protein structure and drug testing studies.
Calcineurin is a Ca2+/calmodulin-dependent, serine/threonine-specific phosphatase essential for adaptation to environmental stress, growth, morphogenesis, and pathogenesis in many fungal species. Calcineurin controls hyphal and yeast morphology, M-Y dimorphism, growth, and Ca2+ homeostasis in P. brasiliensis.40 In fungi calcineurin acts largely through regulating Crz1p-like transcription factors. The A. fumigatus CRZ1 homologue CrzA was characterized242; it is involved in mediation of cellular tolerance to increased concentrations of calcium and manganese, also affecting conidiation. Additionally, crzA- mutants suffer altered expression of calcium transporter mRNAs under high concentrations of calcium, and loss of virulence when compared with the corresponding complemented and wild-type strains. The actual calcineurin A gene (calA), coding for the catalytic subunit, is involved in hyphal morphology related to apical extension and branching growth, as shown by the defective and drastically decreased filamentation in calA-A. fumigatus mutants.136 Such mutants also showed an increased alternative oxidase (aoxA) mRNA accumulation and activity. The authors also identified four transcription factors (zfpA, htfA, nosA, and ctfA) that have increased mRNA expression in the absence of calcineurin, suggesting a negative regulation by this phosphatase. The deletion of the genes encoding these transcription factors yielded disturbed mRNA accumulation of pmcA and pmcB encoding calcium transporters. These deletion strains were also less susceptible to itraconazole, caspofungin, and SDS.
TOR (target of rapamycin) is a pathway by which a regulation is exerted on the translation of ribosomal proteins and, in yeast, of ribosome biogenesis. In C. albicans, morphogenesis towards hyphal development is impaired by the addition of rapamycin, an inhibitor of TOR, in the culture medium. Additionally, lithium suppressed hyphal outgrowth in C. albicans in a way that also suggested inhibition of the TOR pathway.145,146
Proteinases occur naturally in all organisms, and are involved in a multitude of physiological reactions. They are divided into four major groups: serine-, cysteine (thiol)-, aspartic-, and metallo-proteinases. Aspartyl- and serine-proteinases have been reported in several fungal species by Latin American research groups. Of the former, a 66 kDa, N-glycosylated secreted aspartyl protease (PbSAP) of P. brasiliensis was identified in the yeast cell wall. The expression of putative genes CdSAP1, CdSAP2, CdSAP3, and CdSAP4 coding for secreted aspartyl proteases of C. dubliniensis were reported.126,198 In addition, CdSAP7, 8, 9, and 10, orthologous genes of C. albicans, were recognized in C. dubliniensis genome. The expression of CdSAP1 and 2 was independent of the morphological stage of C. dubliniensis.198CdSAP3 expression, instead, was related to the infective process of keratinocytes. Expression of CdSAP4 predominated during the mycelial phase and the initial stage of keratinocyte infection. These results suggest a role of C. dubliniensis Saps as virulence factors, similar to those from C. albicans.126 Genome mining and phylogenetic analyses revealed the presence of new members of the Sap superfamily in C. tropicalis (8), C. guilliermondii (8), C. parapsilosis (11) and Candida lusitaniae (3).198 An extracellular aspartyl-related proteolytic activity was also detected in mycelial and conidial forms of F. pedrosoi. Pepstatin A was able to inhibit the growth of conidium and its transformation into mycelium, suggesting a possible participation of aspartyl peptidases in growth and differentiation.193,194
Exocellular serine-proteinases have been reported in P. brasiliensis286 and C. immitis.127 In the latter, the mycelial 25 kDa peptidase was able to degrade keratin while an additional 18 kDa serine peptidase activity was evidenced solely when casein was used as the substrate. In P. brasiliensis, Venancio et al276 reported a kexin-like gene (Pbkex2) codifying for a kexin protein that belongs to the subtilase family of serine-proteinases. It is conformed by an open reading frame (ORF) of 2622bp interrupted by one single 93bp intron. The deduced protein sequence consists of 842 amino acid residues.266,276 Also serine proteinases are the Lon proteins, with roles in the maintenance of mitochondrial DNA integrity and mitochondrial homeostasis. A LON gene homologue from P. brasiliensis (PbLON) was identified by Barros and Puccia.16PbLON ORF is within a 3,369-bp fragment interrupted by two introns located in the 3’ segment; an MDJ1-like gene was partially sequenced in the opposite direction, sharing with PbLON a common 5’ untranslated region.19 The authors propose that this chromosomal organization might be functionally relevant, since Mdj1p is a type I DnaJ molecule located in the yeast mitochondrial matrix and is essential for substrate degradation by Lon and other stress-inducible ATP-dependent proteinases. An exocellular serine-thiol proteinase (PbST) activity was reported by Matsuo et al147,148 in the yeast phase of P. brasiliensis. It was capable of cleaving proteins associated with the basal membrane, such as human laminin and fibronectin, type IV collagen and proteoglycans.147,148 A 50-kDa serine peptidase was identified in C. albicans that was active over a broad pH range (5.0-7.2) and was able to hydrolyze some soluble human serum proteins and extracellular matrix components.79 Conversely, when this isolate was grown in yeast carbon base supplemented with bovine serum albumin, a secretory aspartyl peptidase activity was measured, instead of metallo- and serine peptidases, suggesting that distinct medium composition induces different expression of released peptidases in C. albicans. Also in C. albicans, the STE13ca gene encodes for a dipeptidyl aminopeptidase A involved in the maturation of α-factor mating pheromone. This 2793 pb gene is homozygotic and encodes for a predicted protein of 930 amino acids with a molecular weight of 107 kDa. STE13ca increases its levels of expression in conditions of nutritional stress (proline as nitrogen source) and during formation of the germinal tube.21
Selective degradation of intracellular proteins in eukaryotic cells is carried out by a 26S proteasome/polyubiquitin system, in which polyubiquitin-labeled proteins are marked for destruction by the proteasome (for a review, see Sorais et al240). The basic unit of the 26S proteasome is the 20S proteasome, which in C. albicans yeast cells has a MW 640 kDa, distributed within 14 polypeptides.88 The enzyme shows chymotrypsin-like, trypsin-like, and peptidylglutamyl-peptide-hydrolyzing activities. The regulation of its activity may be mediated, in part, by phosphorylation, as suggested by experiments in vivo, using homologous protein kinase CK2 as the substrate.89
Ecto-ATPases have been reported in F. pedrosoi59 and C. neoformans.116 In the presence of 1mM EDTA, F. pedrosoi fungal cells hydrolyzed adenosine-5’-triphosphate (ATP) at a rate of 84.6 +/- 11.3 nmol Pi/ h/ mg mycelial dry weight, while a value of 29.36+/-3.36nmol Pi/h/108 cells was reported for C. neoformans. MgCl2 (0.05mM) was able to increase such activities 5 and 70 times, respectively. Based on their differential expression in the different morphological stages of F. pedrosoi, a possible role in this process was suggested. Since inhibition of ectophosphatase activity in cryptococci results in smaller rates of association of fungi with animal epithelial cells, it was proposed that ectophosphatase in C. neoformans may contribute to fungal colonization of the animal host. A cell wall-associated phosphatase has been detected in F. pedrosoi cell walls.118 It was strongly inhibited by exogenous inorganic phosphate (Pi); on the other hand, removal of Pi resulted in a 130-fold increase of ectophosphatase activity. Conidia with high ectophosphatase activity showed greater adherence to mammalian cells than did fungi cultivated in the presence of Pi, suggesting a role in adhesion to host cells.
F. pedrosoi, F. compacta, Phialophora verrucosa, Cladosphialophora carrionii, Cladophialophora bantiana and Exophiala jeanselmei have urease, gelatinase and lipase activity.246 Instead, only phospholipase was detected in F. pedrosoi, a result that prompted the authors to suggest phospholipase detection as a tool to differentiate this species from other agents of chromomycosis.246 On the other hand, keratinases (but not elastase, lipase or DNase) produced by Microspoum canis have been proposed as a virulence factor277 due to the strong correlation between high keratinase activity and the development of symptoms in samples isolated from symptomatic or asymptomatic dogs and cats.
Exocellular enzymes depend on a secretory system to carry them out of the cellular environment. Bernardo et al22 studied the pre-vacuolar branch of exocytosis in C. albicans, and were able to identify structural homologs of several S. cerevisiae pre-vacuolar secretory genes, including the late-Golgi vacuolar protein sorting gene VPS1. C. albicans VPS1 contains a 2082 bp intronless open reading frame whose deduced protein product is 73.3% similar to S. cerevisiae Vps1p and includes GTP-binding regions that are conserved in members of the dynamin-like GTPase family of proteins. VPS1- mutants lost their ability to secrete extracellular proteinases, and were incapable of producing filaments. Both facts are related to C. albicans virulence and therefore, the vacuolar system becomes an important element in the pathogenic process.
Genes involved in fungal cell wall synthesisThe fungal cell wall structure and its involvement in the dimorphic process has been a constant subject of research. In P. brasiliensis, several papers report the cloning, characterization and expression of genes such as β-1,3-glucan synthase,201 α-1,3-glucan synthase and the regulatory small GTPase Rho2,239 the chitin synthase multigene family182–185,225,228,231 and β-1,3-glucanosyl-transferase.50
β-1,3-Glucan is a fungal cell wall polymer synthesized by the multi-subunit enzyme β-1,3-glucan synthase (FKS). The only FKS gene in P. brasiliensis (FKS1) has an open reading frame of 5942bp, interrupted by two putative introns, and a deduced sequence of 1926 amino acids. P. brasiliensis Fks1p is a transmembrane protein.201 Activation of β-1,3-glucan synthase in P. brasiliensis requires the participation of the PbrRHO1 product as the GTPase regulatory subunit.239
The α-1,3-glucan synthase gene (PbrAGS1) presents six exons accounting for a putative coding region of 7293bp, separated by five introns.239 It encodes a predicted protein of 2431 amino acids, with a calculated mass of 274 kDa. It is expressed in the Y phase, where the polysaccharide is solely found. Comparison of the levels of expression of P. brasiliensis AGS1 and RHO2 in the M and Y stages of the fungus shows a direct correlation, suggesting a post-transcriptional regulation of P. brasiliensis AGS1, through the product of RHO2.239 Also in this family is Cdc42, a pivotal molecule in establishing and maintaining polarized growth for diverse cell types, as well as during pathogenesis of certain fungi. Almeida et al2 evaluated its role during cell growth and virulence of the yeast form of P. brasiliensis and found that the expression of PbCDC42 in yeast cells promoted a decrease in cell size and more homogenous cell growth, altering the typical polymorphism of wild-type cells. Reduced expression levels also led to increased phagocytosis and decreased virulence in a mouse model of infection. Hence, Pbcdc42p seems to be an important protein during host-pathogen interaction, with special relevance to the polymorphic nature and cell size in the pathogenesis of P. brasiliensis.
The third important polysaccharide component of P. brasiliensis cell wall is chitin. It serves functions in strengthening the fungal cell wall and protection of the cell against lysis provoked by the internal turgor pressure; it also participates in the connection of capsular polysaccharides to the cryptococcal cell wall, forming soluble complexes with glucuronoxylomannan (GXM).96 Cultivation of C. neoformans in the presence of an inhibitor of glucosamine 6-phosphate synthase resulted in altered expression of cell wall chitin. These cells formed capsules that were loosely connected to the cryptococcal wall and contained fibers with decreased diameters and altered monosaccharide composition. GXM, the major capsular component, is synthesized in cytoplasmic compartments and transported to the extracellular space in vesicles. Cytoplasmic structures associated to vesicular compartments and reticular membranes are in close proximity to the polysaccharide. GXM was generally found in association with the membrane of intracellular compartments and within different layers of the cell wall.192 Analysis of extracellular fractions from cryptococcal supernatants by transmission electron microscopy in combination with serologic, chromatographic and spectroscopic methods revealed fractions containing GXM and lipids. These results indicate an intimate association of GXM and lipids in both intracellular and extracellular spaces consistent with polysaccharide synthesis and transport in membrane-associated structures.
GXM is also produced by species of the Trichosporon genus, i.e., Trichosporum asahii.95 Trichosporal and cryptococcal GXM share antigenic reactivity, but Trichosporum polysaccharide has smaller effective diameter and negative charge. GXM anchoring to the cell wall was perturbed by dimethylsulfoxide and required interactions of chitin-derived oligomers with the polysaccharide. GXM from T. asahii supernatants are incorporated by acapsular mutants of C. neoformans, which renders these cells more resistant to phagocytosis by mouse macrophages. Despite similarities in cell wall anchoring, antigenic and antiphagocytic properties, trichosporal and cryptococcal GXMs manifested major structural differences that may affect polysaccharide assembly at the fungal surface.95
Chitin synthesis is controlled by a multigene family, some of them redundant. Based on differences in regions of high sequence conservation, chitin synthases have been organized according to their amino acid sequences into two domains and seven classes.224 In P. brasiliensis six different chitin synthase genes have been identified.182,183,265PbrCHS5 has a 5583 bp-long ORF, interrupted by three introns of 82, 87 and 97bp. The deduced PbrCHS5 protein contains 1861 amino acids with a predicted molecular weight of 206.9 kDa.183,185 Two domains are identified, one towards the N-terminal end of the protein (aa 16 to 786), with partial identity to myosin motor-like domains, and a second one towards the C-terminal end (aa 1221 to 1752) with homology to fungal chitin synthases. PbrChs4, while being a protein as large as PbrChs5, lacks sequences characteristic of myosin motors in its N-terminal region.182,185 5’UTR sequencing overlaps with a previously reported sequence containing the CHS4 gene,185 arranged in a head-to-head configuration with CHS5, in a similar fashion as MDJ1 and LON,19 mentioned in a previous section. P. brasiliensis CHS3 is the only one to have a higher expression in the yeast phase and at the end of the mycelium-yeast transition15; it contains a single ORF 3817bp long with two introns (71 and 86bp) encoding a 1220 amino acid polypeptide with high similarity to other fungal chitin synthases.
chs2 was chosen by Matute et al152 to study background selection at the locus in P. brasiliensis species complex. For this, the DNA sequence for the chs2 locus was determined in 67 samples. Of the 16 nucleotide substitutions located in the coding regions, 5 of them were synonymous and 11 non-synonymous. Because of the very limited levels of polymorphism within each one of the P. brasiliensis species and the low recombination levels observed in this region, the observed data could be more likely explained by the selective forces that affect loci over most of the chromosome, but at a considerable distance from chs2.
In other fungal species, efforts have been addressed mainly to cell wall-associated proteins and glycoproteins. In C. albicans, S. cerevisiae and Y. lipolytica, cell wall proteins were either labeled with biotin or radiolabeled with amino acids, and chased for a period of time representing several generations. No significant turnover took place during the chase period, and in fact radioactive proteins were accumulated in the wall during the period, indicating that proteins bound to the cell wall are stable and that there is no precursor-product relationship among those linked by non-covalent bonds and the covalently bound ones.224 The composition, structure and synthesis of the cell wall of C. albicans display both subtle and important differences with the wall of different saprophytic fungi, of utmost importance for its pathogenic behavior (for a review, see Ruiz-Herrera et al223).
Important cell wall proteins are adhesins that help in host-pathogen interactions, inasmuch as adherence to target cells is a prerequisite for fungal dissemination and systemic complications. Adherence to extracellular matrix (ECM) proteins has been extensively studied in S. schenckii.121,122,258 Early experiments with immobilized fibronectin122 indicated that yeast cells and conidia adhered equally to the glycoprotein, in a dose-dependent manner; however, when the experiment was carried out with soluble fibronectin, conidia displayed a very low binding capacity compared to the yeast cells. This contradictory result may be the consequence of tridimensional modification of the protein structure, once this is subjected to an immobilization procedure that leads to modifications on the exposure of adhesive domains, as reported for other microorganisms.121S. schenckii binding to fibronectin may be associated to the classical tripeptide arginine-glycine-aspartic acid (RGD) adherence region of ECM molecules, a conclusion derived from the fact that while S. schenckii binds to the RGD-containing 120 kDa fibronectin fragment, inhibition assays with RGDS and GRGDESP peptides did reduce adherence by 50% to soluble fibronectin,121 while no reduction was observed when immobilized fibronectin was used.122 Further research258 aimed to correlate S. schenckii virulence with protein pattern of cell wall proteins and capacity to bind fibronectin indicated that no direct relationship between virulence and clinical or environmental clinical isolates. The lowest virulence was found in an isolate recovered from a patient with meningeal sporothrichosis. This isolate (IPEC 17943) exhibited the lowest capacity to interact with fibronectin, and showed only one fibronectin-binding protein, a 67 kDa variant of gp70 reported as a cell wall protein involved in fungal adherence to dermal extracellular matrix.222 The most virulent isolates (IPEC 15383 and 1099-8, from disseminated cutaneous and osteoventricular, and subcutaneous sporothrichosis, respectively) showed a higher adhesive capacity, and expressed at least four fibrinogen-binding proteins (92, 55, 44, and 37 kDa) besides the 70 kDa band characteristic of gp70.258
H. capsulatum yeast-cell binding to glycosylated surface molecules of murine peritoneal or alveolar macrophages was studied using attachment inhibition assays with different carbohydrate-treated yeast cells.81,256 Galactose (mainly as the β-anomer) and its derivatives were the most efficient sugar inhibitors. These results suggested the presence of a lectin-like component in H. capsulatum yeast cells and revealed involvement of galactosylated surface molecules of murine macrophages as specific-sugar (ligand) residues recognized by the fungal lectin. H. capsulatum yeast cells are also able to bind to erythrocytes irrespective of blood groups, an effect that could be inhibited not only by galactose but also by galactose-containing disaccharides and glycosaminoglycans, mainly chondroitin sulfate C, suggesting a possible association of the inhibitory effect with the presence of negative charges on the cell surface.256
In a histochemical study designed to evaluate the correlation between the adherence of C. albicans and C. parapsilosis to human buccal epithelial cells and the expression of fungal cell surface carbohydrates, Lima-Neto et al123 found that adherence was higher in C. albicans than C. parapsilosis, and that individual strain differences correlated with a high content of α-L-fucose residues in cell surface glycoconjugates, suggesting that this monosaccharide may represent recognition molecules for interactions between the yeast and the host. In C. glabrata, host-pathogen interaction in vitro depends mainly on the adhesin Epa1, one of a large family of cell wall proteins. Most of the EPA genes are located in subtelomeric regions, where they are transcriptionally repressed by silencing. In order to better characterize the transcriptional regulation of the EPA family, Rosas-Hernández et al221 assessed the importance of C. glabrata orthologues of known regulators of subtelomeric silencing (SIR2, SIR3, SIR4, HDF1 (yKu70), HDF2 (yKu80), and RIF1) in S. cerevisiae. They found that, whereas the SIR proteins are absolutely required for silencing of the reporter genes and the native subtelomeric EPA genes, the Rif1 and the Ku proteins regulate silencing at only a subset of the analyzed telomeres. A cis element adjacent to the EPA3 locus can silence a reporter gene when placed at a distance of 31kb from the telomere.221
The cell surface of C. albicans and other ascomycetous yeasts is enriched in highly glycosylated mannoproteins that play roles in the interaction with the host tissues. As with other biological systems, C. albicans protein glycosylation occurs mainly through two distinct pathways, either O- or N-glycosylation. Examples of the former are the enzymes dolichol phosphate glucose synthase that catalyzes the transfer of sugar moieties from either UDP-Glc to dolichol phosphate glucose,9 the corresponding mannose synthase, activated by cAMP-mediated protein phosphorylation,10 and the mannosyl transferase.11 Example of the latter is the N-glycosylation helped by α-1,2-mannosidase (MNS1), an enzyme involved in the hydrolysis of Man9GlcNAc2 and Man8GlcNAc2 oligosaccharides.168 This goes by means of a post-translational modification initiated in the endoplasmic reticulum, where the Glc(3)Man(9)GlcNAc(2) N-glycan is processed by alpha-glucosidases I and II and alpha1,2-mannosidase to generate Man(8)GlcNAc(2), enzymes codified by CWH41, ROT2, and MNS1, respectively.168 The N-oligosaccharide is then elaborated in the Golgi to form N-glycans with highly branched outer chains rich in mannose. Disruption of C. albicans CWH41, ROT2, and MNS1 leads to mutants that tend to aggregate, have a lower content of cell wall phosphomannan and other changes in cell wall composition, and have a constitutively activated PKC-Mkc1 cell wall integrity pathway.168 MNS1 is localized to the endoplasmic reticulum169 and is converted into a cytosolic soluble enzyme with the help of the Kex2 protease.167
The extraction of isolated cell walls from the yeast phase of S. schenckii with SDS and separation of proteins by SDS–PAGE led to the identification of a periodic acid-Schiff (PAS)-reacting 70 kDa glycoprotein (Gp70) that was purified by elution from electrophoresis gels. The purified glycopeptide exhibited a pI of 4.1 and about 5.7% of its molecular mass was contributed by N-linked glycans with no evidence for O-linked oligosaccharides. It is uniformly distributed at the cell surface. Gp70 seems specific for S. schenckii as no immunoreaction was observed in SDS-extracts from other pathogenic and non-pathogenic fungi. Yeast cells of the fungus abundantly adhered to the dermis of mouse tails and the anti-Gp70 serum reduced this process in a concentration-dependent manner, suggesting Gp70 involvement in S. schenckii pathogenesis.222
Sialic acids have also been described as components of the fungal cell wall in several species, where they contribute to the negative charge of fungal cells, playing a role in their specific interaction with the host tissue. Back in 1998, Soares et al.237 reported that sialic acid residues are major anionogenic groups exposed on the P. brasiliensis surface, joined to galactose by means of α-2,6- and α-2,3- links. Similar results were later reported when studying the cell-surface expression of sialic acids in two isolates of C. albicans.238 Sialic acid reduces the binding of laminin and increases the binding of fibronectin to S. schenckii yeast cells.121
Melanin has been proposed as a virulence factor in fungi. Although not strictly a constituent element of the cell wall structure, when it is produced, it accumulates mainly within the cell wall mesh, giving cells and colonies a characteristic brown to black pigmentation. Melanin is synthesized by laccase enzymes, a group of multifunctional enzymes, in medium containing substrates such as L-dopa. To evaluate and compare laccase enzymes from clinical and environmental strains of C. neoformans, 30 Brazilian strains (15 clinical and 15 environmental isolates), belonging to serotypes A and B, were analysed.200 All strains showed laccase enzyme activity; over half of the clinical strains of C. neoformans (56.2%) produced the lowest melanin intensities, suggesting that melanin production may not be the main virulence factor against host defence. Furthermore, virulence could not be associated with the origin of the sample, either clinical or environmental.
Fungal sphingolipidsSeveral glycosphingolipids (GSL) from different human pathogens have been characterized, and frequently involved in host-pathogen interaction. Fungi also present unique glycolipids which may have an important role for the fungal development and/or disease establishment. The different biological roles for GSL of different pathogens as infectivity factors and potential targets for development of new therapeutic strategies have been reviewed by Suzuki et al.250
Following studies on GSL in several dimorphic fungi, Takahashi's group262–264 analysed their structure, composition, and dimorphic expression in S. schenckii. In lipids extracted from the mycelial phase, a single cerebroside (Cer) component (glucosyl-Cer) was observed, while in the yeast phase a galactosyl-Cer was also detected. It is worth noting that glucosyl-Cer and its corresponding synthase have been reported as a virulence factor in C. neoformans.250 The major long chain core in all three cerebrosides was found to be (4E,8E)-9-methyl-4,8-sphingadienine, as reported for the majority of fungi.263
Glycosylinositol phosphorylceramides (GIPCs) are a class of GSL that appear to be essential for fungal survival. In S. schenckii, GIPC structures were determined to be Manα1→6Ins1-P-1Cer and Manα1→3Manα1→6Ins1-P-1Cer (where Ins=myoinosytol, P=phosphodiester) in the mycelial and the yeast phases.263 An additional GIPC with the structure Manα1→3Manα1→6GlcNH2α1→2Ins1-P-1Cer was reported in both phases.264
Acidic GSL components were extracted from A. fumigatus and identified as inositol phosphorylceramide and glycosylinositol phosphorylceramides.261 The structures of six major components were elucidated as Ins-P-Cer, Manα1→3Manα1→2Ins1-P-1Cer, Manα1→2Manα1→3Manα1→2Ins1-P-1Cer, Manα1→3[Galβ1→6]Manα1→2Ins1-P-1Cer, and Manα1→3Manα1→6GluNα1→2Ins1-P-1Cer.262 Similar glucosylceramide and galactosylceramide are present in A. nidulans, playing roles in germination and hyphal growth, as demonstrated by their inhibition when the fungus was treated with D-threo-1-phenyl-2-palmitoyl-3-pyrrolidinopropanol (P4) and D-threo-3’,4’-ethylenedioxy-P4, belonging to a family of compounds known to inhibit GlcCer synthase in mammals.120Pseudallescheria boydii, a fungal pathogen that causes disease in immunocompromised patients, also synthesizes glucosylceramides as major neutral glycosphingolipids. Ceramide monohexosides are detectable on the surface of mycelial and pseudohyphal but not conidial forms of P. boydii, suggesting a differential expression of glucosylceramides according with the morphological phase. Addition of antiglucosylceramide antibodies to cultures of C. albicans clearly inhibited the generation of germ tubes, suggesting an involvement of ceramide monohexosides in differentiation and infectivity.205 In F. pedrosoi, the main cerebroside species found in mycelia and conidial forms is N-2’-hydroxyhexadecanoyl-1-beta-d-glucopyranosyl-9-methyl-4,8-sphingadienine, while the major cerebroside species purified from sclerotic cells carries an additional hydroxyl group, bound to its long-chain base. The structural difference between cerebrosides from mycelial and sclerotic cells was apparently not relevant for their antigenicity, since they were both recognized at similar levels by sera from individuals with chromoblastomycosis and a monoclonal antibody to a conserved cerebroside structure.180
An interesting application of lipid biology to the clinics of PCM comes from the work of Bertini et al.23 By enzyme-linked immunosorbent assay of sera from 31 PCM patients, these authors analyzed immunoglobulin classes and isotypes of antibodies directed to acidic glycosphingolipids (GSLs) and glucosylceramide of P. brasiliensis. Only the GSL Pb-1 antigen, which presents the carbohydrate structure Galf-β1-6(Man-α1-3)Man-β1, was reactive with the PCM patient sera. The Galf residue is essential for antibody reactivity, as shown by the lack of reactivity of Pb-2, the biosynthetic precursor of Pb-1, in which that sugar moiety is absent. The Pb-1 glycolipid from nontreated patients elicited a primary immune response with immunoglobulin M (IgM) production and subsequent switching to IgG1 production. The IgG1 titer increased after the start of antifungal treatment, and general decreases in the anti-Pb-1 antibody titers were observed after 5 months of treatment. These results suggested that the Pb-1 antigen has potential application as an elicitor of the host immune response in PCM patients.
Sphingolipids and cholesterol, as important components of the cell membrane, may be organized in membrane rafts that play an essential role in different cellular functions, including host cell-pathogen interaction. In P. brasiliensis, the involvement of epithelial cell membrane rafts in the adhesion process of the pathogen and activation of cell signaling molecules was demonstrated once the ganglioside GM1, a membrane raft marker, was localized at P. brasiliensis-epithelial cell contact sites; the inhibition of fungal adhesion to host cells pre-treated with cholesterol-extractor (methyl-beta-cyclodextrin) or cholesterol-binding (nystatin) agents was additional proof of the interaction.36,153
Lipid rafts may also be involved in the trafficking of polysaccharide macromolecules from the cytoplasm to their final destination in the outer cell wall, without breaking apart membranes.1,216,217 Recent reports indicate that extracellular vesicles, physiologically secreted across the cell wall, help in the export process not only of the major C. neoformans capsular polysaccharide glucuronoxylomannan (average mass, 1.7 x 106 to 7 x 106 daltons),216 but also of a variety of virulence factors (e.g., glucosylceramides, laccase, urease).216,217 Additionally, 76 vesicle-located proteins were identified by proteomic analysis, of which 27 had already been reported as vesicular proteins in mammalian exosomes.217 Such vesicles are built with bilayered membranes containing key fungal lipids, such as GlcCer, and ergosterol, supporting the idea that they are enriched in lipid rafts, and conforming a sophisticated trans-cell wall vesicular transport secretory mechanism that is not available in prokaryotes; it may also indicate that extracellular vesicles function as “virulence bags” that deliver a concentrated payload of fungal products to host effector cells and tissues.213,216,217 Analogous findings have been reported in H. capsulatum,1 suggesting a general mechanism in fungi for the transport of virulence-related macromolecules through vesicular secretion. Additionally, the fact that similar vesicles have been found in species belonging in ascomycetes (H. capsulatum) and basidiomycetes (C. neoformans) may suggest that the shuttle system is ancient, predating the divergence of these branches 0.5-1.0 billion years ago.189
Transcriptome, genome, molecular taxonomyStudies on the transcriptome of P. brasiliensis carried out by Felipe et al.86 and Goldman et al102 have revealed expressed sequence tags (EST) that could be organized in functional categories such as cellular metabolism, information storage and processing, cellular processes-cell division, posttranslational modifications, morphogenetically-linked genes, among others. Molecular techniques such as microarrays and substraction hybridization have allowed the identification of genes involved in basic and cell wall metabolism, sulfur metabolism, amino acid catabolism, signal transduction, growth and morphogenesis, protein synthesis, genome structure, oxidative stress response, and development genes that are preferentially expressed in the yeast phase,18,143,190 or differentially expressed in host-fungus interaction.13,65,254 Since conidia are more likely to be the infecting propagules, García et al.98 studied this process and found sequences not previously described, which could represent novel exclusive conidia-yeast transition genes.
Two fungal species, C. immitis and P. brasiliensis, both strongly related to the Latin American region, have been the subject of extraordinary advances in molecular taxonomy and phylogeny. C. immitis, the etiologic agent of coccidioidomycosis, is endemic to arid soils of the American continent, principally the lower Sonoran life zone and desertic areas of Argentina and Venezuela.93 Fisher et al,93,94 in a continental joint effort led by John Taylor at the University of California, Berkeley, were able to collect 161 clinical and two environmental isolates, covering the known geographical range of C. immitis. In them, allele distributions at the nine microsatellite loci were sampled from eight geographical populations. The resulting tree showed that isolates occur within one of two major clades, known as the Californian and non-Californian phylogenetic species; the latter was renamed Coccidioides posadasii to honour Alejandro Posadas, the Argentinean researcher who in 1892 reported the fungus for the first time. It may represent a divergent, genetically recombining monophyletic clade.93,94C. posadasii is the most frequent species in the northern-central region of Mexico.49
P. brasiliensis is confined to the Latin American region.231 This fungus is considered clonal according to mycological criteria; at the same time, it shows extensive genetic variability when analyzed by molecular tools. RAPD analyses,37 RFLP,181 and partial sequences of some genes112,166 from a high number of P. brasiliensis isolates, revealed genetic variability and clusters correlated with geography37,181 or virulence.45,165 Matute et al149 analyzed P. brasiliensis phylogenetically in search of cryptic species and found that this fungus consists of at least three distinct, previously unrecognized phylogenetic species: S1 (species 1 with 38 isolates of assorted geographical origin), PS2 (phylogenetic species 2 with six isolates, five Brazilian and one Venezuelan), and PS3 (phylogenetic species 3, with 21 Colombian isolates). S1 and PS2 were sympatric across their range, suggesting barriers to gene flow other than geographic isolation. Variations in virulence and gene expression of antigenic proteins have been found between P. brasiliensis isolates now known to belong to species S1 and PS2.112 Despite their differences, all three species are capable of inducing disease in both humans and armadillos.45 Matute et al151 also developed a marker system for DNA-based recognition of phylogenetic species S1 and PS2 in P. brasiliensis, based on microsatellites. Searching for positive selection in putative virulence factors, Matute et al150 reported on the selection of 12 such genes involved in different cellular processes, either antigenic or involved in pathogenesis. Only two genes (p27 and gp43) have unknown functions. All other genes were classified in four different categories: metabolically related (fas2, his1), cell wall related (fks, mnn5, ags1), heat shock proteins, detoxification related (tsa1, sod1, hsp88) and signal transduction (cdc42, cst20). Several replacement mutations in gp43 were under positive balancing selection. The other three genes (fks, cdc42 and p27) showed very little variation among the P. brasiliensis lineages and appeared to be under positive directional selection.
Following phylogenetic studies, Carrero et al44 reported coding and non-coding regions from various genes and the ITS region in 21 isolates of P. brasiliensis, seven of them new. This study showed that the majority of the sequences used by Matute et al149 and those used in this study, grouped within two (S1 and PS3) of the three clades proposed by these investigators. However, one P. brasiliensis isolate, Pb01, was placed at the base of, and quite distant from, the three species reported by Matute et al,149 clustering together with strain IFM 54648, an atypical strain isolated from a patient in the southern Brazilian region of Paraná.253 This finding suggested the possibility of more than three phylogenetic species in P. brasiliensis.44 Further work257 gave strength to this hypothesis, once the identification of 17 isolates, out of 88 samples, genotypically similar to strain Pb01, allowed their grouping as Pb01-like isolates. They are considered a new phylogenetic species distinct from the S1, PS2 and PS3 clades previously reported by Matute et al,149 since it is strongly supported by all independent and concatenated genealogies, with highly significant values of posterior probability (1.0) and bootstrap agreement (100%). The speciation event that defined this new phylogenetic group is sympatric relative to S1 and PS2. The two separate groups that include S1, PS2, PS3 on one side and Pb01-like on the other, were highly divergent (fig. 2).257 Based on molecular phylogenetic data, distinctive morphological characters and a long period of genetic isolation (> 30 million years) that set the two groups apart, the Pb01-like clade may be considered a new phylogenetic species, and we proposed the binomial name Paracoccidioides lutzii,257 whose specific descriptor means to honour Adolpho Lutz, the Brazilian researcher who first reported P. brasiliensis in 1908.
Bayesian unrooted phylogram showing the relationship between the isolates from the three phylogenetic species S1, PS2, PS3 and the isolates from the “Pb01-like” cluster. Eight concatenated loci, comprising 3,565 nucleotides, from dataset 1 (fks-exon2, fks-exon3, chs2-exon1, chs2-exon2-4, gp43-promoter-exon1, gp43-exon2, arf and α-tubulin). This is a consensus unrooted tree highlighting the distance that separate the three phylogenetic species of P. brasiliensis from the “Pb01-like” (Paracoccidioides lutzii) group. The scale means the numbers of substitutions per site analysed [modified from 255]. Reproduced by permission.
Electrophoretic karyotypes of 12 clinical and environmental P. brasiliensis isolates from different geographic areas indicated the possible existence of haploid and diploid (or aneuploid) isolates of the fungus.85 Further studies by flow cytometry and comparison with previous electrophoretic data3 revealed a genome size ranging from 26.3±0.1 Mb (26.9±0.1 fg) to 35.5±0.2 Mb (36.3±0.2 fg) per uninucleated yeast cell in 10 P. brasiliensis isolates. The analysis of intra-individual variability of the highly polymorphic P. brasiliensis gp43 gene166 indicated that only one allele was present; therefore, all isolates presented a haploid, or at least aneuploid, DNA content; no association was detected between genome size/ploidy and the clinical-epidemiological features of the isolates.3
One extraordinary step forward in the field has been the recent public release of the genome of three P. brasiliensis strains, among them, the above mentioned Pb01 isolate, in an effort led by the Broad Institute, MIT, Boston, that included all Latin American laboratories involved in molecular biological research of the fungus (Brazil, Colombia and Venezuela), under the Paracoccidioides Comparative Genome Analysis Project. Data can be found at http://www.broad.mit.edu/annotation/genome/paracoccidioides_brasiliensis.2/MultiHome.html. Preliminary data-mining analyses indicate that the Pb01 strain does have important differences with the other two isolates, Pb18 and Pb03, particularly with regards to the genome size (32.94, 29.06 and 29.95 Mb, respectively) and number of genes (9132, 7875 and 8741 genes, respectively) [manuscript in preparation], a result that provides additional arguments in favour of the proposed classification of Pb01-like isolates as P. lutzii.
Despite their telomeric (sexual) stages or mating system being unknown, C. immitis, C. posadasii, P. brasiliensis and P. lutzii have been classified by molecular criteria as belonging to the phylum Ascomycota, Order Onygenales.231 Recent work267 aimed to determine the presence of the mating type locus in 71 P. brasiliensis isolates from various sources. Two heterothallic groups (MAT1-1 or MAT1-2) were recognized and, in some isolates, gene expression was confirmed, indicating the existence of a basal gene expression. The distribution of two mating type loci in the studied population suggested that sexual reproduction might occur in P. brasiliensis. This finding points towards the possibility of applying a more precise definition of the concept of biological species to P. brasiliensis.
Beginning with the first reported human case of Jorge Lobo's disease, its etiologic agent, Lacazia loboi, has been at the center of a taxonomic dispute. The fungus was described as Loboa loboi but subsequent morphological, serological and molecular studies argued that L. loboi was a Paracoccidioides species.279 To investigate the phylogenetic position of this species, Vilela et al279 conducted a phylogenetic analysis using 20 Lacazia loboi isolates (as the species was renamed). To this effect, they used L. loboi DNA sequences from ITS rRNA, and partial coding sequences of chitin synthase 4, ADP-ribosylation factor, and gp43 and compared them to those from 17 P. brasiliensis strains that represented the known variation in this species,44,149,279 and outgroup taxa in the Onygenales (Ajellomyces and Coccidioides species). Nucleotide variation among strains of L. loboi was minor but numerous nucleotide mismatches and multiple gaps were found for these gene regions among members in the Ajellomycetaceae, including P. brasiliensis. Phylogenies inferred using neighbor-joining, maximum parsimony and Bayesian analyses depicted L. loboi as a well-supported, monophyletic group that was sister to the Paracoccidioides clade. The authors concluded that L. loboi should be maintained as a taxon independent from Paracoccidioides within the Ajellomycetaceae.278
ImmunologyInnate immunity effector mechanisms in mycosesOne major development in the field of immunology is the acknowledgment that innate immunity, although not being highly specific, is able to efficiently detect microbial infections through the recognition of pathogen-associated molecular patterns (PAMPs) by specialized pattern recognition receptors (PRRs); membrane-bound Toll-like receptors (TLR), cytoplasmic nucleotide oligomerization domain-like receptors (NOD), dectin C, and others. These evolutionarily conserved structures are mainly present in monocytes, macrophages, dendritic cells, T and B lymphocytes, mediating the recognition of microbial pathogens and the subsequent inflammatory and immune responses.158 In fungi, dectin-1, mannose receptor, TLR4, TLR2, and galectin-3 that recognize β-(1,3)-glucans, mannans, mannoproteins, phospholipomannan and β-mannosides, respectively, have been identified (reviewed by Gow et al110).
The simultaneous activation of multiple PRRs by a fungal pathogen directs the immune system to mount an ample and effective specific immune response against the fungus. Thus, the importance of PRRs and TLRs resides not only in directing the innate immunity but also in orchestrating the adaptive immunity developed in sequence. Many fungal wall components are recognized by host PPRs. González et al108 showed that MyD88, an adaptor protein of TLR, is dispensable for resistance to P. brasiliensis and that TLR2, TLR4 and dectin-1 do not play a significant role in the recognition of P. brasiliensis yeast cells. However, further research90 implicated TLR2 expression in susceptibility to this fungus. The group of Calich in Brazil has extensively studied the role of MyD88 and also of TLR2 and TLR4 in experimental paracoccidioidomycosis.130 They demonstrated that TLR2 deficiency resulted in the development of milder infection, decreased nitric oxide synthesis and increased production of KC (murine analogous of IL-8), TGF-β, IL-6, IL-23, and IL-17.38 Dectin-1, CD18, and TLR2 receptors are also involved in the lipid body formation induced by the cell wall β-glucan of H. capsulatum, a phenomenon linked to leukotriene B4 generation.241 Studies with C. albicans showed that the interaction of this fungal agent with intrahepatic lymphocytes resulted in the up-regulation of TLR-2 expression in this cell population.211 The involvement of TLR4 in the recognition of S. schenckii was suggested by studies that compared TLR4 deficient and sufficient mice and which showed that both pro-inflammatory and anti-inflammatory mediators were reduced in the TLR4-deficient group.234 Therefore, the involvement of TLR4 in S. schenckii recognition by the host was first described by Latin American authors.
A critical point at the initial stage of antifungal defense is the production of chemotactic factors (cytokines, chemokines and leukotriens) at the site of the infection, for the effective recruitment of phagocytes (neutrophils, monocytes and macrophages), dendritic and natural killer cells. For instance, the cytokine IFN-γ modulates the chemokine production and leukocyte recruitment to the lungs of P. brasiliensis-infected mice.248 On the other hand, H. capsulatum induced generation of high levels of MIP-1-α, and of low levels of eotaxin and its β-glucan cell wall component induced a little MIP-1-α but considerably higher concentrations of eotaxin, suggesting that chemokines and leukotrienes may play key roles in the inflammatory cell influx to H. capsulatum infection.156
The production of pro-and anti-inflammatory cytokines and chemokines during the early stages of mycotic infections decisively influences not only the inflammatory response that is mounted shortly after the infection but also enhances or impairs the subsequent development of an effective protective immune response, being therefore decisive to the outcome of the subsequent disease developed. For this reason, the effect of each fungal agent on the production of different types of these mediators was the object of study of many Latin American authors.
Some of these results strongly suggest that an imbalance in the production of pro-inflammatory and anti-inflammatory cytokines may be associated with the pathogenesis of some mycoses. Peraçoli et al199 have characterized the cytokines produced by monocytes from PCM patients and were able to demonstrate that endogenous levels of TNF-α, IL-1β, IL-6, IL-8, IL-10 and TGF-β, detected in monocytes from patients, were significantly higher than those produced by healthy controls. IL-18, a recently described cytokine, important in the regulation of both innate and acquired immune response, was also studied in PCM. The results showed that IL-18 knockout (IL-18 -/-) BALB/c mice were more resistant to P. brasiliensis than their wild type controls.195 Mamoni and Blotta138 showed that gene expresión, kinetics of cytokines and chemokines distinguishes P. brasiliensis infection from disease, as deduced from the earlier and higher levels of TNF-α, IFN-γ and chemokines mRNAs in PCM-infected patients as compared with patients suffering from the juvenile form of the disease. Preferential induction of pro-inflammatory cytokines was also demonstrated at the onset of experimental P. brasiliensis infection.107 Patients with chromoblastomycosis produce high levels of IL-10 and low levels of IFN-γ, resulting in the development of a somehow impaired immune response.244
Phagocytes, (neutrophils and macrophages), which constitute the first cell population to confront the fungus after infection, have an important role, not only in the events directly related with fungal lysis but also with the activation of acquired immunity. Macrophages are activated by different mechanisms in order to perform their function with increased efficiency. Receptors for complement system components, mannans and β-glucan (like dectin 1) activate different pathways to make sure that fungi are engulfed by the phagocytes. These aspects were studied by Jiménez et al.115 in relation with complement and mannose receptors. Employing congenic murine bone-marrow-derived macrophage lines infected with P. brasiliensis conidia, the authors suggested the participation of mannose receptor in phagocytosis and a major activating effect on the antifungal activity of these cells by cytokines, mainly IFN-γ.
Human phagocytes were also studied in this mycosis. Monocytes obtained from PCM patients, were preactivated with recombinant IFN-γ and evaluated for their fungicidal activity against P. brasiliensis. Cells from healthy subjects failed to present such activity while those from PCM patients showed significant fungicidal activity against virulent Pb18; in contrast, both patient and control cells were significantly fungicidal against avirulent Pb265.39 Some factors can also inhibit phagocytosis: monocytes treated with indomethacin exhibited an effective killing P. brasiliensis, suggesting a role of prostaglandin E2 (PGE2) in the inhibitory process. Human monocytes challenged with the fungus produced high PGE2 levels, which in turn repressed the fungicidal activity by reducing H2O2 and TNF-α production.31 Some fungi are able to secrete products that alter the fungicidal properties of macrophages. Indeed, a lipid component of the S. schenckii cell wall was shown to inhibit phagocytosis by macrophages.43
Apoptosis induction may lead to the impairment of some cells that play essential roles in the establishment of protective immunity against fungal infections. Peritoneal macrophages co-incubated with C. albicans strain CR1 in vitro show early signs of apoptosis, but evolve to necrosis after 2h. At the same time, an increase in IL-10 production is observed. Treatment of CR1 with pepstatin (a proteinase inhibitor) prevented the process of apoptosis and significantly reduced IL-10 production, suggesting that the increased production of this cytokyne was caused by processes occurring during the initial phase of infection, such as apoptosis, necrosis and uptake of death cells.99 The liver constitutes the first barrier in the control of hematogenous dissemination of C. albicans of intestinal origin. Renna et al.211 studied the involvement of apoptosis and pro-apoptotic signals in the hepatic injury during the acute phase of C. albicans infection and concluded that in the scenario of early liver injury, the recruited intrahepatic lymphocytes and the modulated expression of TNF-α, Fas-L and TLR-2 molecules could act coordinately in delivering death signals.
Neutrophils are also deeply affected by cytokines, which can enhance or impair their phagocyitic activity. IFN-γ, as well as the more recently studied cytokine IL-15 facilitate P. brasiliensis killing by human neutrophils, in contrast to the effect of IL-10.12,64,255 Using an experimental model of candidosis, it was shown that neutrophils, at a first phase and later, macrophages, are involved in clearing an experimental infection by C. albicans.131F. pedrosoi experimental inoculation elicited marked neutrophils migration to the inflammatory site followed by microbicidal activity, particularly against hyphae, suggesting that host resistance to this fungus is primarily mediated by neutrophils. As a high number of destroyed conidia was found intracellularly in macrophages, the further participation of these cells is suggested.132
The destruction of phagocytes through induction of apoptosis has been described in this review as a mechanism of fungal escape from immune response. H. capsulatum is a facultative intracellular parasite, found in neutrophils and mononuclear cells, suggesting that it is capable of evading damage and surviving inside these cells. The work of Medeiros et al155 shows that H. capsulatum-infected leukocytes presented less apoptosis than controls, suggesting that this fungus induces an antiapoptotic state on neutrophils and monocytes. This phenomenon may represent an extraordinary escape mechanism, by delaying cell death and allowing H. capsulatum to survive inside phagocytes.
Macrophages and dendritic cells (DCs) are antigen-presenting cells with a fundamental role in connecting the innate and the acquired immune responses. DCs in particular have been recently recognized as initiators and modulators of immune responses and their role in mycotic infections constitutes a major area of research. After fungal infection, immature DCs are recruited to the inflammation site and transformed into mature DCs. They recognize fungal cells by their TLR receptors, and are induced to produce proinflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8 and IL-15. Cytokines, as well as co-stimulatory molecules are needed to render lymphocytes fully activated and so trigger acquired immune response.
The role of DCs is essential in subcutaneous mycoses, such as chromoblastomycosis, because its causative agent, the fungus F. pedrosoi and DCs have plentiful opportunity to interact at the onset of the infection. DCs from patients exhibited an up-regulated expression of human leucocyte antigen D-related (HLA-DR) and of co-stimulatory molecules (CD86). In the presence of conidia, the expression of HLA-DR and CD86 was up-regulated by DCs from patients and also from controls.245 In one of the rare immunological studies in dermatophytosis, it was shown that T. rubrum-infected macrophages have down-regulated expression of co-stimulatory molecules (CD80 and CD54) and that the ingested conidia grow and differentiate into hyphae inside macrophages, leading to rupture of the cell membrane, suggesting the existence of mechanisms that evade or suppress protective immunity.41 The expression of co-stimulatory molecules was also studied on T-cells and monocytes of active PCM patients and healthy individuals cured of past PCM. CD28 expression, critical for optimal T-cell activation, was comparable between patients and controls, whereas CD152, PD-1 and ICOS, which preferentially deliver negative signaling, were over expressed on stimulated and unstimulated T-cells from patients. CD80 and CD86 were equally expressed on monocytes from patients and controls, but over expressed in T-cells from patients.36
The recognition of fungal cells by the innate immunity leads to the immediate mobilization of effector and regulatory mechanisms that have as a consequence the formation of an inflammatory environment for the recognition of the pathogen, the mounting of the first line of defense that controls the fungi during the establishment of the acquired immune response, leading to the activation of either cellular or humoral immune response.
Mechanisms of humoral immunity–Role of specific antibodies in mycosesDuring the last decade it has been demonstrated that, although not as determinant as cellular immunity, humoral immune response is also important in the mounting of an adequate immune protection against fungal infections, provided that protective antibodies be present in adequate concentration.46,207 Although the protective role of specific antibodies against P. brasiliensis is still controversial, the contribution of Taborda's group was essential for the understanding of the humoral arm of anti-fungal immunity. In fact, Buissa-Filho et al35 studied the effects of monoclonal antibodies to the major P. brasiliensis antigen (gp43) using in vitro and in vivo infection models. As reported by the authors, the passive administration of monoclonal antibodies against gp43 before and after infection led to a reduced fungal burden and decreased pulmonary inflammation, associated with enhanced phagocytosis of P. brasiliensis by macrophages, and increase in nitric oxide production by these cells.
In a series of studies on mycotic patients, several Latin American authors detected patterns of preferential isotypes production according to the clinical manifestations of each mycosis. For instance, Biselli et al27 found that 100% of patients with the severe acute form of PCM produced high levels of anti-gp43 antibodies of IgE, an isotype which is preferentially synthesized in a Th2 cytokines environment, whereas only 27% of the patients with the chronic form of the disease produced this isotype at high levels. These results support the notion that the relatively more severe impairment of cellular immunity in the acute form of PCM is probably related to a Th2 pattern of immune response.
Patients with other mycoses were also the object of such studies. The IgG, IgM, and IgA immunoglobulins in sera of patients with sporotrichosis before antifungal treatment and also from patients with sporotrichosis during itraconazole treatment were quantified. More than 95% of patients had detectable IgA antibodies, and more than 85% had IgM and IgG antibodies before treatment. The number of patients with IgG antibodies increased to 91% during treatment. Conversely, significantly fewer samples from treated patients were positive for IgM (71%) and IgA (89%). Overall, 78% of patients had detectable levels of all isotypes tested at diagnosis, and this percentage dropped to 62.9% in patients that were receiving itraconazole.5
The majority o f substances that elicit good immune responses are proteins. They need to be processed and presented to T lymphocytes by antigen presenting cells (APCs) and are called T-dependent antigens. There are, however, some carbohydrate antigens that can be recognized as native molecules by B lymphocytes; these are the T-independent antigens. The fungus C. neoformans has a polysaccharide capsule composed of glucuronoxylomannan (GXM), which is such an antigen. Parra et al197 quantified the production of IgG subclasses specific for GXM intervals after C. neoformans infection in moderately resistant (Balb/c), highly resistant (CBA/j) and susceptible (C57BL/6) mouse strains. Early production of IgG1, described as protector antibodies, coincided with a decrease of the number of C. neoformans colony forming units in the lungs.
Some fungi are able to produce and secrete products that interfere with components of the immune response, constituting, eventually, fungal virulence factors. Antigenic preparations from S. schenckii yielded proteases that are were able to cleave different subclasses of human IgG.68
The knowledge that antibodies against fungi could be protective led to the development of a very original approach by a group of Latin American and Italian researchers. They tested the in vitro fungicidal and the in vivo therapeutic activity of an engineered synthetic decapeptide was derived from the sequence of a recombinant anti-idiotypic antibody, that represents the internal image of a Pichia anomala killer toxin. This compound markedly reduced the fungal load in organs (liver, lung, spleen) of mice infected with P. brasiliensis, opening a new field in the induction of protective immunity against fungi.269
Mechanisms of cellular immunity–Role of lymphocytes and cytokines in mycosesThere is evidence of efficient activation of cell-mediated immunity after exposure to fungi. Lymphocytes from healthy subjects show strong proliferative responses against fungal antigens, producing numerous cytokines. In many mycotic diseases, the efficient tissue response against fungal invasion is a granulomatous inflammatory response, characteristic of cellular immunity. Resistance against fungi is based on a triple response, i.e., induction of a strong cellular immune response mediated by T helper lymphocytes with CD4 phenotype, production of cytokines and action of effector phagocytes (fig. 3).
Histological analysis of granulomatous lesions in B10.A mice, susceptible to P. brasiliensis. The organ was collected 15 days after IP infection with the highly virulent Pb18 isolate. This figure illustrates the close interaction of different cell populations with the immune response in the course of experimental infection. A.Compact granulomatous lesion with presence of yeast cells, inflammatory cellular influx and extracellular matrix components. Magnification x200; HE dye. B.Marked influx of phagocytic cells of different lineages, some showing ingested P. brasiliensis cells. Magnification ×400; HE dye. C. Concentric collagen fibers circumscribing the granuloma and eventually containing fungal dissemination. Magnification x200; Sirius red dye observed under Polarized light. D. Marked deposition of thick collagen fibers delimiting the lesion. Magnification x400; Sirius red dye. E. P. brasiliensis at the center of the lesion. Magnification x200; Groccot dye. F. P. brasiliensis with preserved or altered morphology. Magnification x400; Groccot dye. Extracellular matrix components (blue arrows), neutrophils (green arrows), multinuclear giant cells (red arrows), fungi with preserved (violet arrows) or altered (brown arrows) morphology. Figure authors: RFS Molina and E. Burger. Original figure for this manuscript.
The necessity of a strong, functional cellular immune response is illustrated by chronic mucocutaneous candidosis patients, who present defects in the cellular immunity. Peripheral blood mononuclear cells from these patients produced lower levels of IFN-γ and IL-2 than controls in response to Candida antigens, but did not produce higher levels of IL-4 and IL-10, suggesting that, even though Th1 cytokines are decreased, the Th2 response is not increased in this severe form of candidosis.36 In cryptococcosis, however, the presence of capsular polysaccharidic components induce a dominant Th2 pattern, with high levels of IL-4 and IL-10 production and undetectable inflammatory cytokines, such as TNF-α and IFN-γ, constituting a powerful virulence factor.4
Corbellini et al61 studied the delayed-type hypersensitivity response developed against F. pedrosoi exoantigens in 16 male guinea pigs, all but one showing positive response 48h after inoculation, results that show that a specific T cell response, develops after exposure to chromoblastomycosis. The combined effects of both CD4+ and CD8+ T lymphocytes and Th1 and Th2 cytokines are required for the induction of resistance to various fungi. Chiarella et al57 demonstrated in a murine model that CD8+ T cells were the major elements involved in the control of P. brasiliensis loads, whereas CD4+ T cells were responsible for delayed type hypersensitivity responses and antibody production. Teixeira de Souza et al,259 infecting mice deficient in CD4 and CD8 T cells with F. pedrosoi showed that absence of CD4(+) cells induces a more severe disease in chromoblastomycosis.
Th1 lymphocytes produce predominantly IFN-γ and elicit phagocyte activation leading to respiratory burst. In contrast, Th2 lymphocytes synthesize predominantly IL-4 and IL-10 and promote the synthesis of antibodies, resulting in susceptibility to fungal infections and allergic reactions. Some fungi subvert the Th1/Th2 dicothomy in their favor. C. neoformans GXM profoundly alters the immune response, being responsible for many immunomodulation phenomena. It was shown that it suppresses lymphoproliferation in response to either concanavalin A or heat-killed C. neoformans, modulates cytokine production, determining high production of IL-10, and low secretion of IL-2, IFN-γ and TNF-α and also triggers macrophage apoptosis through NO generation.55,56 The capsular polysaccharides, galactoxylomannan (GalXM) and glucuronoxylomannan (GXM) induced different cytokines profiles in macrophages. GalXM induced production of TNF-α, NO and iNOS expression, while GXM induced predominantly TGF-β secretion, but both induced macrophages apoptosis mediated by Fas/FasL interaction. All these phenomena constitute mechanisms by which capsular polysaccharides from C. neoformans might compromise host immune responses.280
High levels of IL-10 and TNF-α in the sera of chronic PCM patients have been reported.97 Marques-Mello et al.144 associated IL-4 and IL-5 production with a Th2 immune response to P. brasiliensis infection. Romano et al. showed that PCM-related Th1 immunosuppression was associated with down-modulation of the IL-12 pathway, and that patients cured from PCM may not fully recover their immune responsiveness.220 The group of Blotta characterized the immune response of PCM patients suffering from clinically different manifestations of the disease: in the adult forms, the immune response is heterogeneous, with balanced Th1 and Th2 responses, preferential production of antibody isotype IgG1 in unifocal, milder cases, or IgE and IgG4 in multifocal severe cases; in the juvenile form, instead, Th2 response dominates, with production of IgA, IgE and IgG4 antibodies.139 They also associated IL-18 and TNF receptor 2 with the severity of the disease.62 In experimental models, it was demonstrated that the absence of functional IL-12 determines severe PCM in mice125 and that this cytokine protects mice against disseminated infection but enhances pulmonary inflammation,12 suggesting that resistance to P. brasiliensis infection correlates with preferential Th1 immune response.117
This may also be the case in other mycoses. Severe forms of chromoblastomycosis are characterized by the production of high levels of IL-10 and TNF-α, associated to low levels of IFN-γ and lymphocyte proliferation, in contrast with the mild cases, in which low levels of IL-10, high levels of IFN-γ and good lymphocyte proliferation were observed.154 It has been described that immune modulation with recombinant IL-12 or anti-IL-10 can restore the antigen-specific Th1-type immune response in chromoblastomycosis patients by up-regulating HLA-DR and co stimulatory molecules in monocytes.244 Zaga-Clavellina et al285 reported that C. albicans induced differential synthesis and secretion of IL-1β, IL-6 and prostaglandin-E.
Da Silva et al69 demonstrated that Langerhans cells were able to phagocytose F. pedrosoi conidia but not sclerotic cells, inhibiting hyphae formation. The development of local immune responses at the site of the granulomatous lesions and the interaction of fungi with extracellular matrix components and cytokines was studied in P. brasiliensis106,187,188 and S. schenckii models.91
The role of nitric oxide (NO) in the fungicidal effect of macrophages has been a recurrent subject of research. Macrophages represent the major cell defense against fungi; when activated with IFN-γ, they are fungicidal by an oxygen-independent mechanism via the enhanced production of NO. However, the role of NO in mycosis is much more complex and the data obtained with studies of various fungi, albeit numerous, are mostly inconclusive.
One example is the persistence of Cryptococcus in the central nervous system in spite of marked local expression of mRNAs of nitric oxide synthase (iNOS) and stimulatory cytokines: increased levels of transcripts corresponding to IL-1, TNF-α and iNOS were detected as early as day 1 post infection, with TNF-α rising by approximately 30-fold and iNOS increasing by approximately 5-fold by day 7.134
In PCM an inverse correlation between NO concentration and transformation of P. brasiliensis conidia was observed.105 Nishikaku et al186 showed that NO has an important role in granuloma modulation, by controlling OPN and MMP production, as well as by inducing loose granulomas formation and fungal dissemination, resulting, at later phases, in PCM progression, thus confirming earlier results by Nascimento et al,177 who described the dual role of this gas in experimental PCM. Moreira et al,170 in turn, were able to show an association of IFN-γ or TNF-α-activated macrophages with higher levels of H2O2 and NO when compared to non-activated cells, an effect reversed with the addition of inhibitors. The role of nitric oxide synthase (iNOS) in PCM has also been studied by Livonesi et al124 who suggest that iNOS is a resistance factor in PCM by controlling fungal proliferation, influencing cytokines production, and appeasing the development of a high inflammatory response and concurrent necrosis.
NO was found to be fungicidal against S. schenckii. Mice defective in the production of reactive nitrogen intermediates (iNOS-/- mutants) or wild-type mice treated with an iNOS inhibitor were used to investigate the role of endogenous NO during systemic sporotrichosis. The results suggest that although NO was an essential mediator to the in vitro killing of S. schenckii by macrophages, the activation of NO system in vivo contributes to the immunosuppression and cytokine balance during early phases of infection with this fungus.87 Using in vivo and in vitro models of chromoblastomycosis, Bocca et al30 demonstrated that, during the infection, F. pedrosoi peritoneal macrophages show an increased phagocytic capacity and H2O2 production, but also a reduced ability to produce NO, suggesting that inhibition of macrophages NO synthesis by the fungus-produced melanin could be partially responsible for the host's inability to eliminate F. pedrosoi, leading to the development of chronic disease.
The overall contribution of these and other works, led to the following concepts regarding cellular immunity in fungal infections: The production of IFN-γ is regulated by cytokine IL-12, considered to be the primary inducer of the inflammatory response. Deficiency in IL-12 and IFN-γ leads to extremely severe fungal diseases due to the inability of the host to liberate activation signals to effector phagocytes. On the other hand, the cytokine IL-4 is the most potent autocrine signal for commitment to Th2 reactivity, negatively modulating the protective Th1 responses, although higher susceptibility to mycotic infections is not always associated with increased production of IL-4. Many clinical observations suggest an inverse correlation between IFN-γ and IL-10 production in mycotic patients.
VaccinesAmong viral, bacterial, and fungal diseases, the latter are the only branch of infectious diseases without a vaccine for any of their causative agents. This is at odds with a disease burden that remains unabated by conventional chemotherapy and infection control measures.48
Using cell-free antigens (CFAgs), potential candidates to be developed as vaccines against H. capsulatum have been tested in murine models.227 CFAgs not only induced a more potent delayed-type hypersensitivity response in H. capsulatum-infected mice than did histoplasmin, but also stimulated spleen cells from immune mice to produce higher amounts of IFN-γ in vitro, and protected against a lethal inoculum of H. capsulatum. In fact, yeast cells of H. capsulatum led to death in 83% of non-immunized mice after 45 days of I.V. infection, contrasting with 100% survival of CFAg-immunized mice; furthermore, intratracheal infection (the natural route of infection in humans) induced death of non-immunized mice after 18 days, whereas 72% of those immunized with CFAgs survived until the end of the 60-day postinfection observation period. Such induced protective immunity was reflected in a reducing fungal burden in lung and spleen. Protection of wild-type mice immunized with cell-free Ags from H. capsulatum against histoplasmosis was associated with increased leukotriene B4 and IFN-γ production as well as recruitment of memory T cells into the lungs.157 In contrast, CFAg-immunized mice lacking 5-lipoxygenase(-/-), a critical enzyme involved in leukotriene synthesis, displayed a marked decrease on recruitment of memory T cells to the lungs associated with increased synthesis of the anti-inflammatory cytokine TGF-β and the Th1-related cytokine IL-10. These effects were associated with increased mortality to 5-lipoxygenase(-/-)-infected mice. In this way, an important immunomodulatory role of leukotrienes is established, in both the primary and secondary immune responses to histoplasmosis.157 To improve on the method of vaccination, CFAgs have been encapsulated into biodegradable PLGA (poly(d,l-lactide-co-glycolide) microspheres (MS) that could allow the controlled and/or sustained release of the encapsulated antigens from H. capsulatum.80
The use of peptides as therapeutic vaccine adjuvants to chemotherapy in P. brasiliensis is an approach used by Luiz Travassos's group142 in São Paulo, Brazil. For this, they used the main Paracoccidioides diagnostic antigen, gp43, secreted exocellularly by the infective yeast phase, as their source of potential vaccines.251 The T-cell epitope of this antigen was mapped to a 15-amino-acid peptide (P10), in which 12-mer or longer sequences were active, confirming presentation by major histocompatibility complex ii. The HTLAIR P10 inner core was the essential domain of the epitope. Immunization of mice with both gp43 and P10 led to vigorous protection against intratracheal challenge by virulent P. brasiliensis, with a >200-fold decrease in lung colony forming units (CFU) and no dissemination to spleen and liver. The protective effect of P10 was mainly attributed to an IFN-γ-mediated cellular immune response and not to the humoral (Th-1 and Th-2 activation) response seen with gp43.251 To improve P10 delivery, these researchers synthesized a multiple antigen peptide with the protective T-cell epitope expressed in a tetravalent 13-mer analog of P10 (M10). M10 significantly protected intratracheally infected mice.141,252 This research group also evaluated new anti-Paracoccidioides vaccine formulations based on the intranasal administration of P. brasiliensis gp43 or the P10 peptide in combination with the Salmonella enterica FliC flagellin, an innate immunity agonist binding specifically to the Toll-like receptor 5 (TRL5), in a murine model.33 Mice immunized with recombinant purified flagellins genetically fused with P10 at the central hypervariable domain, or the synthetic P10 peptide admixed with purified FliC, elicited a prevailing Th1-type immune response based on lung cell secreted type-1 cytokines, and reduced P. brasiliensis growth and lung damage, suggesting a modulation of S. enterica FliC flagellin in the immune response to P. brasiliensis P10 antigen (fig. 3).
Using another approach, Reis et al209 cloned a cDNA coding for an antigenic protein (Pb27) from P. brasiliensis. Mice immunized with purified recombinant Pb27 (rPb27) were able to develop high levels of IgG2b, moderate levels of IgG1 and low levels of IgG2a. At the same time the levels of TGF-β and IFN-γ were high while a very low production of IL-10 was verified. Using confocal microscopy with anti-rPb27 mouse serum against P. brasiliensis yeast forms, surface and cytosolic staining pattern were observed. Immunization of mice with this antigen induced a significant degree of protection in the lungs (93%), liver (93%) and spleen (100%) at 60 days after challenge with infection. Thus, the granulomatous lesions revealed a greater degree of compaction and organization, with few lesions in the lungs and no dissemination of the fungus to other organs. These results showed that rPb27 promoted acquired protection against infection with P. brasiliensis yeast forms, suggesting the use of this protein for future development as a prophylactic vaccine against PCM.
Glycosylceramides (GlcCer; see section 3.3) are immunologically active components inducing the production of antifungal antibodies. Nimrichter et al179 purified and characterized a major GlcCer from mycelial forms of F. pedrosoi, the most frequent causative agent of chromoblastomycosis. By fast atom bombardment mass spectrometry (FAB-MS) analysis, the purified molecule was identified as N-2’-hydroxyhexadecanoyl-1-beta-D-glucopyranosyl-9-methyl-4,8-sphingadienine. A monoclonal antibody against this structure was used in indirect immunofluorescence with the different morphological stages of F. pedrosoi. Only the surface of young dividing cells was recognized by the antibody. Treatment of F. pedrosoi conidia with the monoclonal antibody against GlcCer resulted in a clear reduction in fungal growth. In addition, the monoclonal antibody also enhanced phagocytosis and killing of F. pedrosoi by murine cells, results that point to a possible use of monoclonal antibodies to GlcCer as potential tools in antifungal immunotherapy. Using C. neoformans GlcCer, Rodrigues et al218 proved that passive immunization with a monoclonal antibody to GlcCer significantly reduced host lung inflammation and prolonged the survival of mice lethally infected with C. neoformans, revealing a potential therapeutic strategy to control cryptococcosis.218 In the presence of the antibodies to GlcCer, inflammatory responses were better controlled by the host, resulting in reduced damage to host tissues and more effective killing of C. neoformans by host effector cells. In fact, the reduced inflammation in mice treated with the MAb to GlcCer corresponded to the increased lung concentrations of IL-4 on day 1 postinfection and anti-inflammatory cytokines, such as IL-4 and IL-6, on day 7.
Search for new antifungalsThe rational design of new experimental antibiotics becomes an important tool to approach the search for new and more effective chemotherapeutic agents against fungal pathogenic species. Latin American researchers have been particularly active in this area. Many years ago, ajoene [(E,Z)-4,5,9-trithiadodeca-1,6,11-triene 9-oxide], a platelet aggregation inhibitor chemically derived from garlic,7 was reported to have antifungal effects against P. brasiliensis,233A. niger and C. albicans,284Cladophialophora carrionii and F. pedrosoi.202 The antiproliferative effects of ajoene in P. brasiliensis were associated with a marked reduction in the content of phosphatidylcholine, with a concomitant increase in the levels of its precursor phosphatidylethanolamine, and a large increase in the amounts of unsaturated fatty acids in the Y phase.233 A recent report137 revealed that ajoene is capable of controlling the evolution of intraperitoneally induced PCM in Swiss mice, significantly reducing the levels of antibodies from the 10th week of treatment onwards. Ajoene therapy is also effective in association with sulfametoxazol/trimethoprim, showing a positive additive effect in mice intratracheally infected with P. brasiliensis,260 expressed through the development of Th1-type cytokine responses producing higher levels of IFN-γ and IL-12 when compared to the infected but untreated members of the control group. Therefore, the antifungal activity of ajoene involves not only a direct effect on fungi but also a protective pro-inflammatory immune response.
The metabolic pathway to membrane sterols has been an all-time favourite in the search for selective antifungals. The pathway is blocked by allilamines (by inhibition of the squalene epoxidase) or azoles (by inhibition of the cytochrome P-450 enzyme 14α-sterol demethylase). Both mechanisms are common to the fungal pathogen and the host and consequently, drugs that interfere with them affect selectivity towards the pathogen, hence their undesirable side effects. However, the metabolic pathway to the synthesis of sterols also involves differentiated steps in both fungal and mammal organisms, that may be used for blocking growth of the former without affecting the latter. One such step refers to the sterol C-methylations catalyzed by the enzyme (S)-adenosyl-L-methionine: Δ24_ sterol methyl transferase (SMT).273,291 SMTs are a common occurrence in Nature, though are absent in animal systems, suggesting an interesting alternative in the search for selective antifungals affecting this particular step.272,273,281 AZA-1 (fig. 4; 22,26-azasterol, 0.1 to 5μM) inhibited P. brasiliensis growth in a dose-dependent fashion, reaching 100% growth arrest at the latter concentration and above,281 a result that is similar to those previously reported for parasites (T. cruzi, L. donovani)273 and fungi (P. carinii).272 AZA-2 (22-piperidin-2-il-pregnan-22(S),3β-diol), instead, was only able to inhibit 60% growth at the highest concentration used in these experiments (10μM), while AZA-3 (22-piperidin-3-il-pregnan-22(S),3β-diol) was the most powerful drug, since a concentration of 0.5μM was able to completely inhibit fungal growth in a fungistatic manner.281,282 A detailed lipid analysis indicated that on exposure to AZA-1, ergosta-5,7,24(28)-trien-3β-ol (17.1%) and lanosterol (11%) accumulated, while AZA-2 led to an important accumulation of ergosta-5,7,22,24(28)-tetraen-3β-ol (50.5%), a result that suggested a significant inhibition of the Δ24(28) sterol methyl reductase (SMR), an enzyme that catalyzes the saturation of the Δ24(28) double bond in the biosynthesis of brassicasterol. With AZA-3, instead, an important accumulation of lanosterol (34.5%) was observed, indicative that a specific blockage of SMT activity was in effect.273,274 Sterol hydrazones derivatives, such as 20-hydrazone-imidazolin-2-yl-5α-pregnan-3β-ol (H1), 20-hydrazone-pyridin-2-yl-5α-pregnan-3β-ol (H2), 22-hydrazone-imidazolin-2-yl-chol-5-ene-3β-ol (H3) and 22-hydrazone-pyridin-2-yl-chol-5-ene-3β-ol (H4) also show similar inhibitory properties against P. brasiliensis SMT [Visbal et al., submitted].
The azasterols 20-piperidin-2-yl-5α-pregnan-3β-20(R)-diol (AZA), and 24(R,S),25-epiminolanosterol (EIL), were tested against 70 clinical isolates of the genus Candida.113 All strains were susceptible to amphotericin B; however, some isolates, mostly Candida non-albicans such as C. guilliermondii, Candida zeylanoides, and Candida lipolytica, were fluconazole- and itraconazole-resistant, but susceptible to both AZA and EIL. Reference strain C. krusei (ATCC 6258, fluconazole-resistant) was consistently susceptible to AZA, although not to EIL. The fungicidal action of these compounds was more prominent against Candida non-albicans species than against C. albicans isolates. A concentration of 4.0μg/ml of either compound was enough to kill 100% of C. lusitanae, C. zeylanoides, and Candida rugosa, and 50% of C. glabrata. In contrast, this same concentration killed only 4.7% (AZA) and 9.5% (EIL) of C. albicans isolates, a result that opens new roads for the eventual use of such drugs as alternative treatments for candidiasis provoked by fluconazole- and itraconazole-resistant Candida spp. Treatment with sub-inhibitory concentrations of AZA and EIL induced ultrastructural alterations, such as changes in the cell-wall shape and thickness, a pronounced disconnection between the cell wall and cytoplasm with an electron-lucent zone between them, mitochondrial swelling, and the presence of electron-dense vacuoles. Fluorescence microscopy analyses indicated an accumulation of lipid bodies and alterations in the cell cycle of the yeasts.113
The above mentioned research on azasterols and sterol hydrazones has been assisted by the use of methods for the rational design of antifungals173–175,282 to find the most probable suited molecules for a given action, prior to experiments. QSAR (quantitative structure-activity relationship) investigation was applied to find a correlation between the different physicochemical parameters of a new series of furan-3-carboxamides and their biological activity.288 QSAR analysis of heterocyclic antifungals was also applied by Duchowicz et al.82 to 1202 numerical descriptors that encode the various aspects of the topological, geometrical and electronic molecular structure with the aim of achieving the best QSAR relationship between the antifungal potencies against C. albicans and the structure of 96 heterocyclic ring derivatives. From the model arisen of such search the authors predicted the biological activity for 60 non-yet measured compounds. Bi- and multilinear PLS (partial least squares) coupled to MIA-QSAR (multivariate image analysis applied to quantitative structure-activity relationship) were used in the prediction of antifungal activities of some benzothiazole derivatives that act as C. albicans N-myristoyltransferase (Nmt) inhibitors.28 Two different regression methods were used: N-PLS, applied to the three-way array, and PLS, applied to the unfolded array. Both models demonstrated excellent predictive ability, with results comparable to those obtained through 3D approaches. In order to compare the results obtained through MIA descriptors with the predictions of a classical 2D QSAR, some representative physicochemical descriptors were calculated and regressed against the experimental pIC50 values through multiple linear regression, demonstrating that MIA-QSAR was superior for this series of compounds.28
Contrary to allylamines and azoles, that act by blocking the synthesis of membrane sterols, amphotericin B, the golden standard of antifungals, acts by physically impairing the cell membrane, be it from fungal or mammalian cells. Consequently, while being highly effective as a fungicidal agent, it is also a highly nephrotoxic drug. To alleviate this condition, lipid preparations such as Ambisome are currently available. However, their high cost makes them prohibitive as the drug of choice in poor populations, such as those frequently found in Latin America. Hence, some research is being devoted to the preparation of alternative lipid derivatives of amphotericin B. Amaral et al6 reported on the preparation and testing of a desoxycholate amphotericin B (D-AMB) sustained delivery system based on poly(lactic-co-glycolic acid) (PLGA) and dimercaptosuccinic acid (DMSA) polymeric blends (Nano-D-AMB) aimed at reducing the number of AMB administrations required to treat mycosis. To this effect, mice were infected with P. brasiliensis, Y phase, and treated with Nano-D-AMB (6mg/kg/every 3 days) or D-AMB (2mg/kg/daily). Efficacy was comparable in both treatments, although Nano-D-AMB-treated group presented lower loss of body weight and absence of stress sign (piloerection and hypotrichosis) than D-AMB-treated group. No renal (blood urea nitrogen, creatinine) or hepatic (pyruvic and oxalacetic glutamic transaminases) biochemical abnormalities were found. Genotoxic or cytotoxic effects were absent. It was concluded that the D-AMB-coated PLGA-DMSA nanoparticle showed antifungal efficacy, fewer undesirable effects and a favourable extended dosing interval, results that hold promise for improved methods of treatment against systemic fungal infections.
Natural products or chemically derived compounds are being tested for antifungal properties. One preferred target has been the fungal cell wall, a structure absent in mammalian cells, required for the survival of the fungal cells inasmuch as it confers a physical barrier against the internal turgor pressure of the cytoplasm. Argentinean Zacchino and collaborators in several other Latin American countries, among other research groups, have devoted many years to the subject. 4-Aryl- or 4-alkyl-N-arylamine-1-butenes were transformed into 2-substituted 4-methyl-tetrahydroquinolines and 4-pyridyl quinolines that displayed a range of antifungal properties in particular against Epidermophyton floccosum and M. canis, by way of β-(1-3) glucan-synthase and chitin-synthase inhibition.159,275 Similar effects were reported for N-phenyl-, N-aryl-, N-phenylalkyl- maleimide and 3,4-dichloromaleimide derivatives128 when tested against a panel of standardized yeasts and filamentous fungi as well as clinical isolates of C. albicans. The activities of N-phenylalkyl-3,4-dichloromaleimide derivatives but not those of N-phenylalkyl-maleimide derivatives showed to be dependent on the length of the alkyl chain, exerting a fungicidal, not fungistatic activity. Some of them possessed strong antifungal activities against all the tested Candida strains with MICs between 0.48 and 3.90μg/ml, values that are similar to those of amphotericin B (0.12–1.56 μg/ml) and in some cases better that those of ketoconazole (0.12–6.25μg/ml).243 N-Phenylpropyl-3,4-dichloromaleimide showed the broadest spectrum of action and lower minimal inhibitory concentrations (MIC) in all of the fungi tested. Later on, López et al129 produced a semisynthetic mixture of compounds by diversification of a natural product extract through the chemical transformation of common chemical functionalities, mainly molecules with a high frequency of carbonyl groups in natural products, into chemical functionalities rarely found in nature, e.g. pyrazoles. The resulting mixture showed antifungal activity against C. albicans, whereas the starting extract did not show such activity.
Of the β-1,3-glucan synthase inhibitors so far studied, echinocandins (namely, caspofungin, anidulafungin and micafungin) are the only ones that have made their way into clinics. A recent study219 indicates that the inhibitory effect of caspofungin on the yeast phase of 5 P. brasiliensis strains ranged between 20 and 65%, depending on the strain. The mycelial phase was more susceptible to caspofungin, inhibition varying between 74 and 81%, in agreement with the 3-times higher amount of β-1,3-glucan present in the mycelial cell wall as compared with the yeastlike phase. The variable sensitivity of each strain towards caspofungin, in a given morphological phase, was independent from the different amounts of α- and β-1,3-glucan present in each strain. The drug induced physical changes in the cell walls of both fungal phases, as well as cytoplasmic deterioration. Caspofungin has been reported to have a paradoxical behaviour when used in high concentrations.249 A recent report by Melo et al160 points to the paradoxical growth of biofilms of Candida sp. clinical isolates (4C. albicans, 6C. tropicalis, 7C. parapsilosis, 8C. orthopsilosis, and 5C. metapsilosis) in the presence of high caspofungin concentrations. With the exception of C. tropicalis, all isolates displayed paradoxical growth more frequently when they were grown as biofilms than when grown as planktonic cells.
The characteristics of C. tropicalis biofilm formation in vitro were described by Bizerra et al.29 By an XTT-reduction assay, an increase in metabolic activity was observed up to 24h of biofilm formation, and this activity showed a linear relationship with sessile cell density. Mature biofilms consisted of a dense network of yeast cells and filamentous forms of C. tropicalis. Increased resistance of sessile cells against fluconazole and amphotericin B was also demonstrated. Real-time reverse transcription-PCR quantification showed that sessile cells overexpressed ERG11 (coding for lanosterol 14 alpha-demethylase) and MDR1 (coding for an efflux protein belonging to the major facilitator superfamily). These mechanisms may contribute to the fluconazole resistance of the C. tropicalis biofilm. C. albicans secretory aspartyl proteinase, a virulence factor, has been reported to be enhanced in biofilms.161,172
Multiple resistance mechanisms among A. fumigatus mutants with high-level resistance to itraconazole were found to reside in point at Gly54 (G54E, -K, or -R) in the azole target gene CYP51A,176 in agreement with information from other sources.283 Additionally, two genes, AfuMDR3 and AfuMDR4, showed prominent changes in expression levels in many highly resistant mutants. Analysis of the deduced amino acid sequence encoded by AfuMDR3 revealed high similarity to major facilitator superfamily transporters, while AfuMDR4 was a typical member of the ATP-binding cassette superfamily. By real-time quantitative PCR it was shown that overexpression of one or both of these newly identified drug efflux pump genes of A. fumigatus and/or selection of drug target site mutations are linked to high-level itraconazole resistance and are mechanistic considerations for the emergence of clinical resistance to itraconazole. Of the five sequential C. neoformans isolates recovered from an AIDS patient with recurrent meningitis, four isolates were fluconazole susceptible, while the fifth isolate developed fluconazole resistance, due to a point mutation (G484S) in the 14-alpha lanosterol demethylase gene (ERG11).215
To provide insights in drug resistance, transporter in P. brasiliensis were deduced by data mining in its transcriptome.63 Twenty two groups with good similarity with other fungal ATP binding cassette transporters, and four P. brasiliensis sequence tags that probably code for major facilitator superfamily proteins were found, among them, homologs to C. albicans CDR1, CDR2, and MDR1, S. cerevisiae PDR5 and Aspergillus AtrF genes, all of them related to azole resistance. Also in T. rubrum, a gene encoding an ABC transporter, TruMDR1, was cloned. The open reading frame of TruMDR1 was 4838bp long and the deduced amino acid sequence showed high homology with ABC transporters involved in drug efflux of other fungi. An increase in expression level was observed when the fungus was exposed to ethidium bromide, ketoconazole, cycloheximide, fluconazole, griseofulvin, imazalil and itraconazole, suggesting the participation of this gene in drug efflux in this dermatophyte.53
ConclusionsThis account of highlighted experimental medical mycological research in Latin America in the initial years of the 21st Century provides us with the notion that despite chronic difficulties in our region, there are groups working hard to advance local research at international levels of quality, frequently within programs of international cooperation. The potentiality exists to keep and improve such level of excellent performance, and to provide more opportunities for the training of young researchers. For this to be achieved, good will, strong governmental policies and funds are required in order that society, as a whole, profits from advances in scientific knowledge.
EB acknowledges the finantial support of FAPESP and CNPq 304630/2009-8 through grants No. 06-60091-6 and 304630/2009-8, respectively.
Conflicts of interestThere are no conflicts of interest in either author.






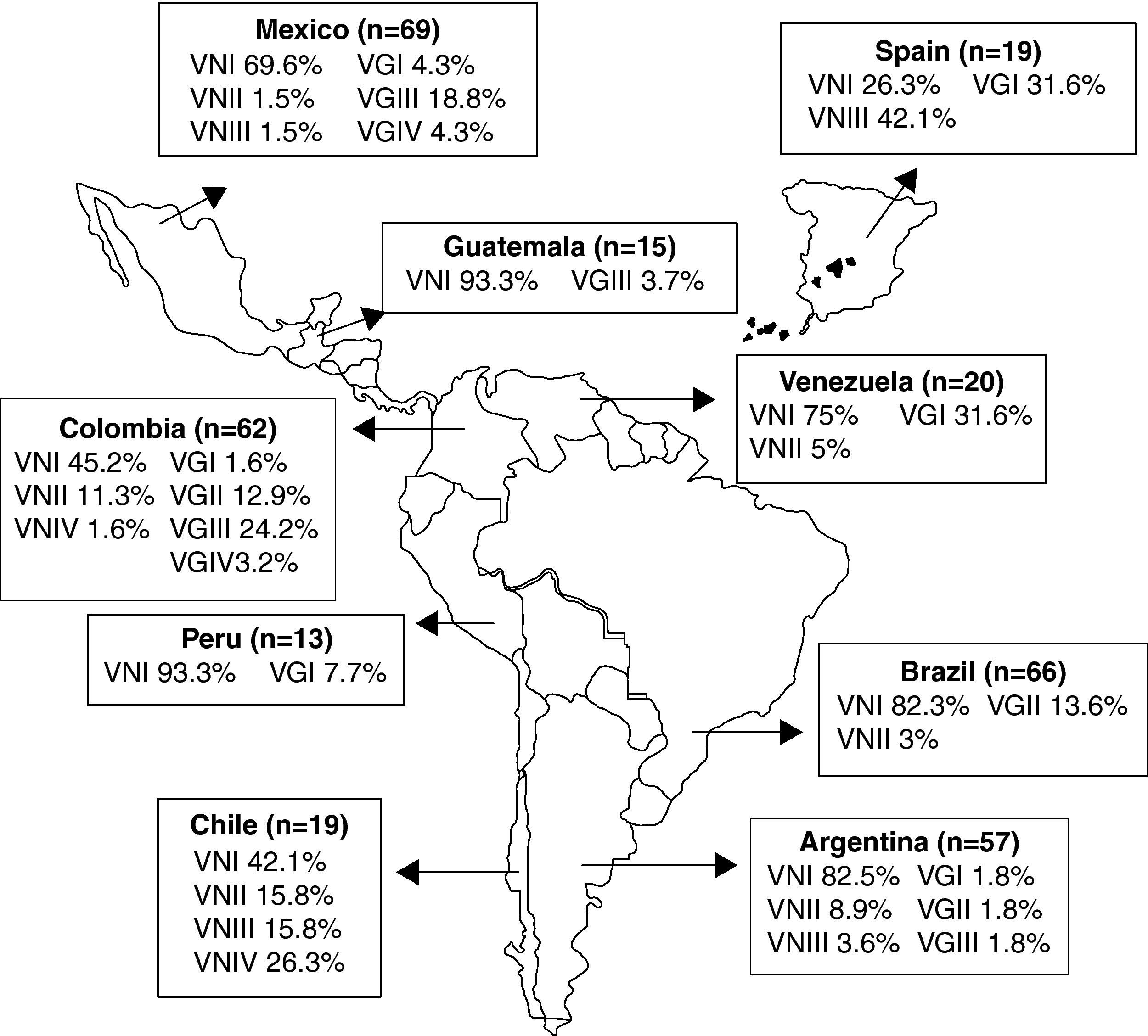
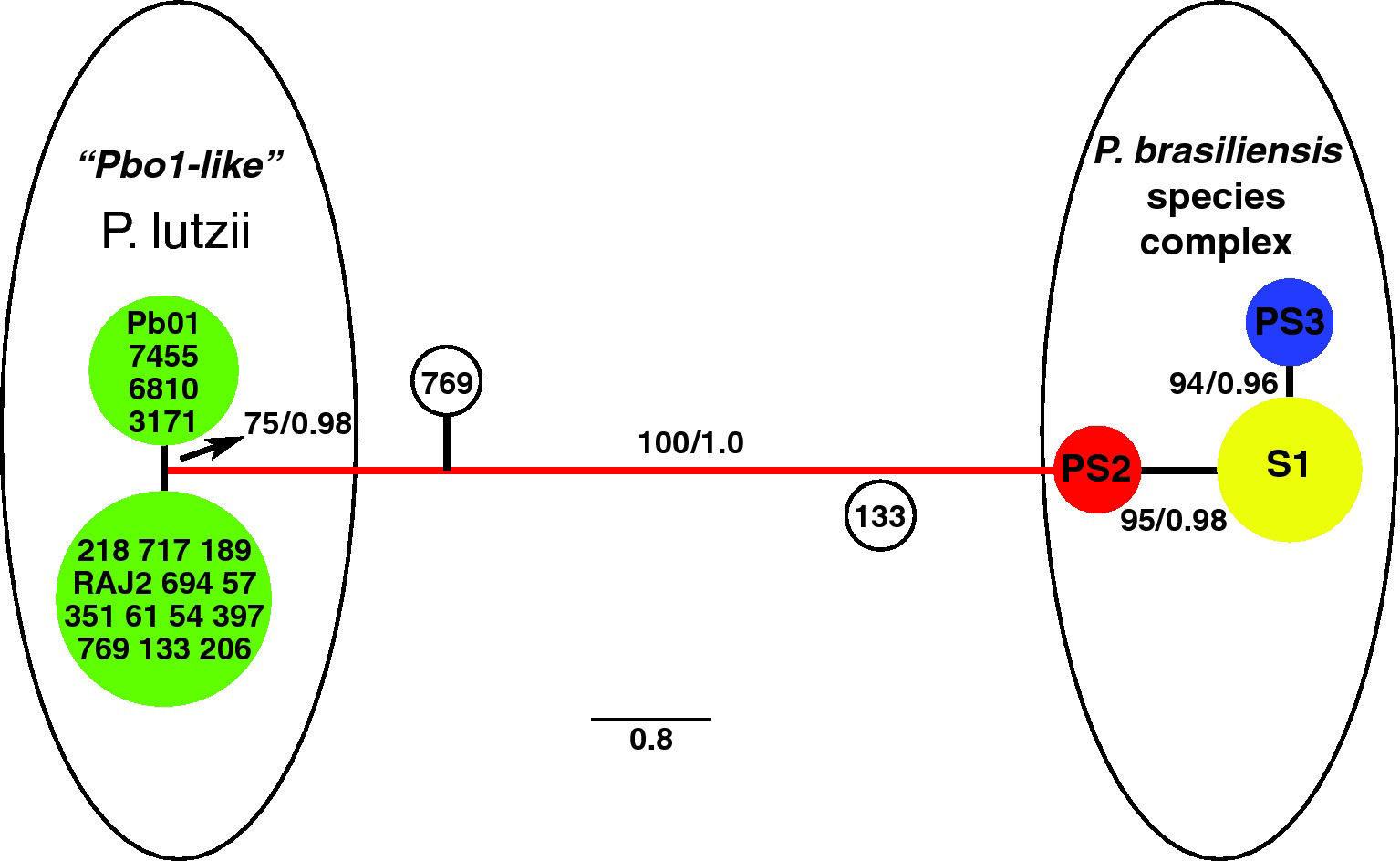
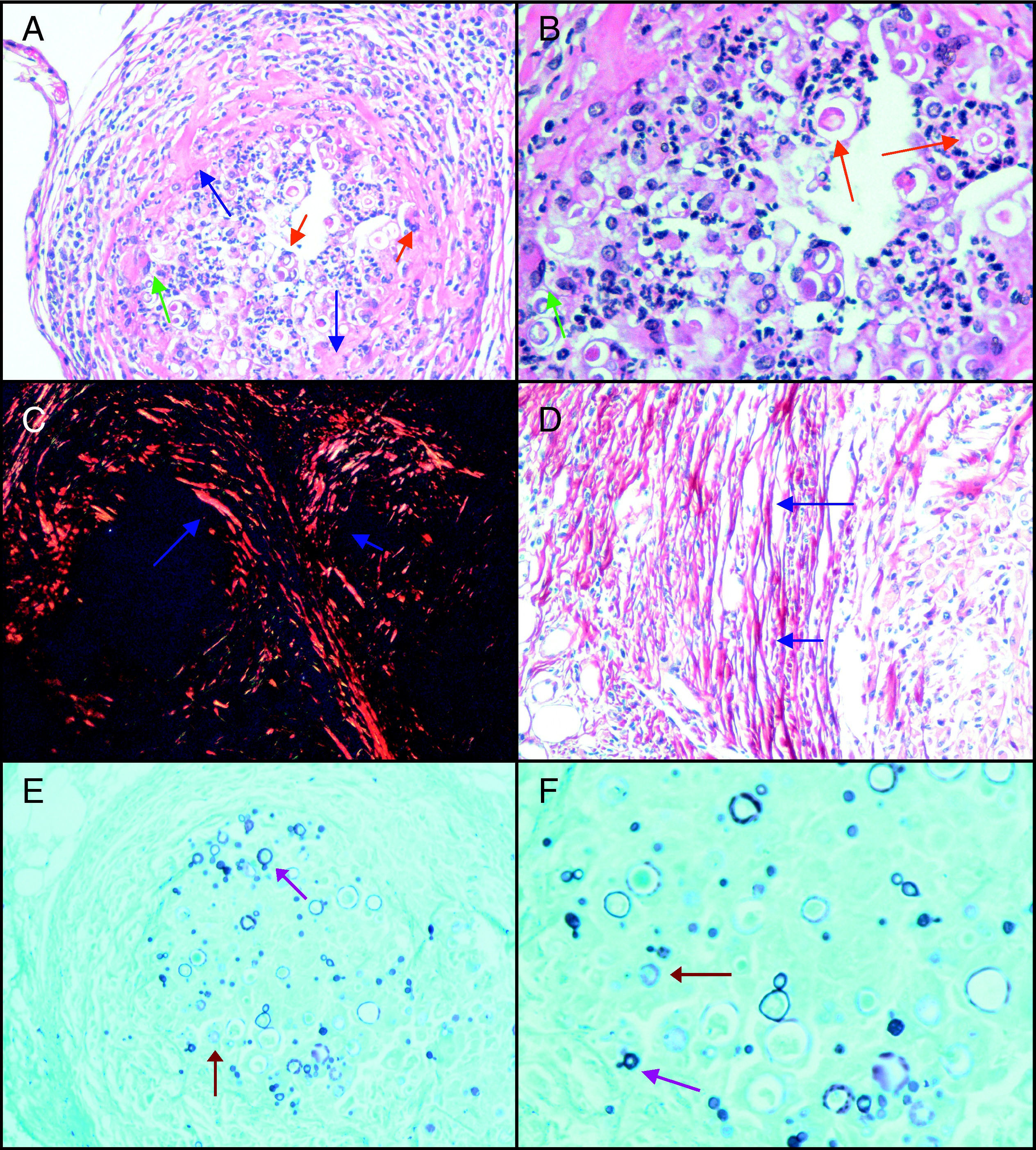
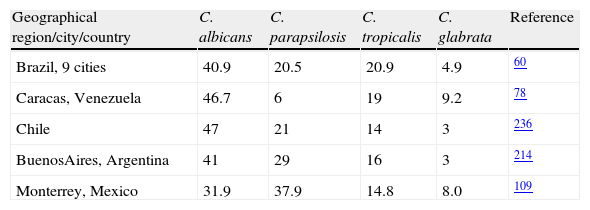
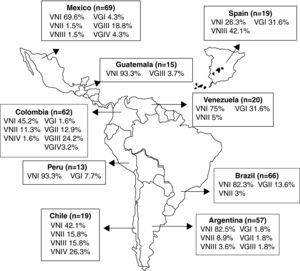
![Bayesian unrooted phylogram showing the relationship between the isolates from the three phylogenetic species S1, PS2, PS3 and the isolates from the “Pb01-like” cluster. Eight concatenated loci, comprising 3,565 nucleotides, from dataset 1 (fks-exon2, fks-exon3, chs2-exon1, chs2-exon2-4, gp43-promoter-exon1, gp43-exon2, arf and α-tubulin). This is a consensus unrooted tree highlighting the distance that separate the three phylogenetic species of P. brasiliensis from the “Pb01-like” (Paracoccidioides lutzii) group. The scale means the numbers of substitutions per site analysed [modified from 255]. Reproduced by permission. Bayesian unrooted phylogram showing the relationship between the isolates from the three phylogenetic species S1, PS2, PS3 and the isolates from the “Pb01-like” cluster. Eight concatenated loci, comprising 3,565 nucleotides, from dataset 1 (fks-exon2, fks-exon3, chs2-exon1, chs2-exon2-4, gp43-promoter-exon1, gp43-exon2, arf and α-tubulin). This is a consensus unrooted tree highlighting the distance that separate the three phylogenetic species of P. brasiliensis from the “Pb01-like” (Paracoccidioides lutzii) group. The scale means the numbers of substitutions per site analysed [modified from 255]. Reproduced by permission.](https://static.elsevier.es/multimedia/11301406/0000002800000001/v1_201305061352/S1130140610001117/v1_201305061352/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)