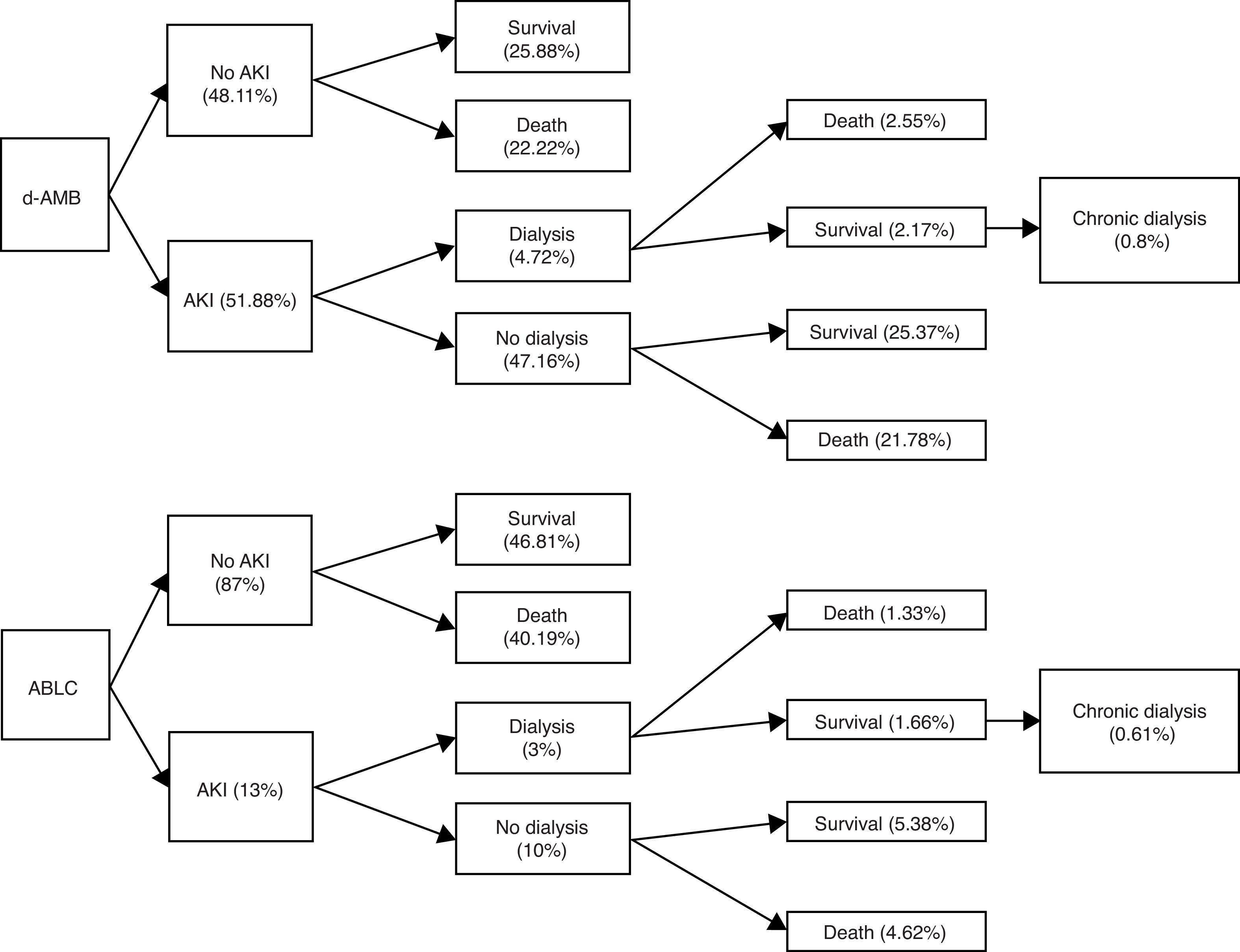
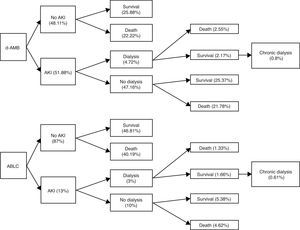
Clinical use of lipid formulations of amphotericin B is markedly limited by acquisition costs in developing countries.6,7 The aim of this study is to assemble a model of cost-effectiveness of amphotericin B lipid complex (ABLC) in patients with invasive mycoses in a public Brazilian hospital. This is a pharmacoeconomic analysis from the payers’ perspective based on a retrospective observational study published previously about the incidence of acute kidney injury (AKI) in patients using deoxycholate amphotericin B (d-AMB).8 One hundred and six adult patients were included. The incidence of AKI with ABLC was estimated through another cohort,1 and in-hospital mortality rate was used as primary endpoint. In the model, the outcome of patients who received ABLC was considered similar to that of patients who received d-AMB, according to a previous publication5 (Fig. 1). The probability of evolving to chronic hemodialysis (HD) after developing AKI that required acute HD was estimated according to a previous study of Duran et al.2 Finally, in order to predict the 10 years outcome of every patient under chronic HD after discharge we used reported data from the publication of Gomez et al.3 In Brazil, patients under chronic HD are retired, and the retirement pension fee is the 80% of the mean salary of the last five years (US$ 302.34; minimum salary of the Parana State in Brazil).
Sensitivity analysis was performed considering ±25% of used costs.4 Only the direct cost of amphotericin B (updated to December 2016) was included, (ABLC=US$ 360.53/50mg d-AMB=US$ 36.61/50mg). Costs of chronic HD values were considered those paid by Brazilian public health system to public hemodialysis clinics per HD session (US$ 74.76).
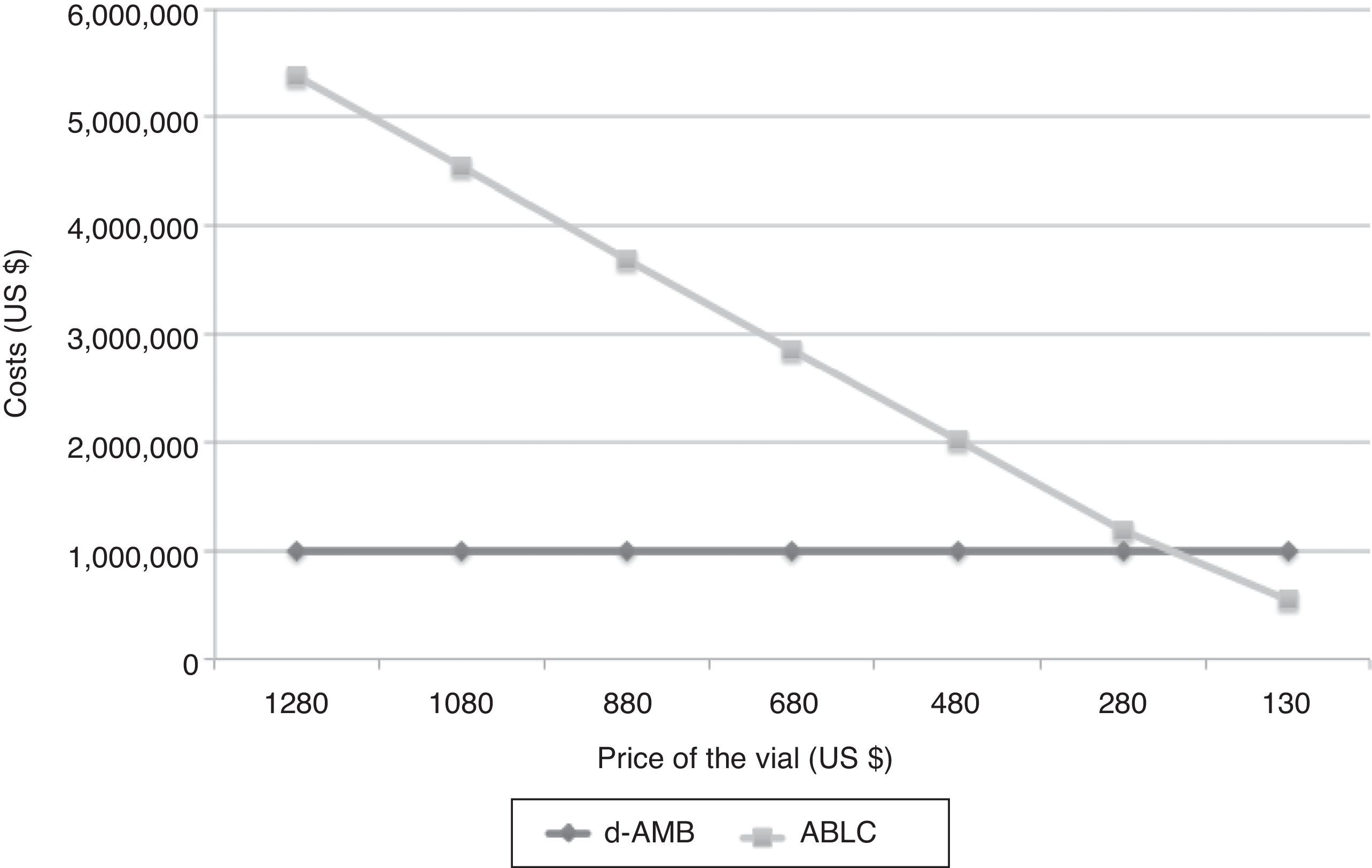
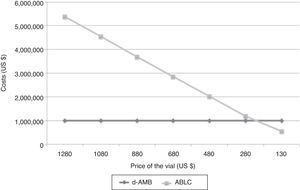
From 106 patients, five were submitted to acute HD due to acute renal failure attributed to treatment with d-AMB (4.72%). Global in-hospital mortality rate was 46%. It may be inferred that 2.17% of the patients were discharged from hospital after acute HD. Considering the previous study of Duran et al.,2 we estimated that 0.8% of these patients treated with d-AMB would be under chronic HD. Considering 106 patients, the total cost of d-AMB, chronic HD and retirement would be US$ 54,343.66, US$ 27,000.35, and US$ 98,331.33, respectively. On the other hand, if the same group would have received ABLC, total cost of ABLC, chronic HD and retirement would be US$ 1,605,228.17, US$ 10,125.13, and US$ 36,874.25, respectively. The break-even value of ABLC to be cost-effective in comparison with d-AMB is US$ 67.61, a value very different from the current value of US$ 360.56 per vial (Fig. 2).
Despite the high cost of chronic HD and retirement, direct cost of lipid formulations in Brazil is too high to be considered cost-effective. However, a subset of patients with early renal dysfunction should be re-analyzed in the future because ABLC induces less renal injury and consequently fewer patients would be on chronic HD. This aspect is important for a future re-evaluation of the cost of these patients not to be included in the model as well as costs beyond 10 years of life.
Conflicts of interestThis study was supported by TEVA, that had no influence at all on the content of the paper. Felipe Tuon receives grants from TEVA and is a CNPQ researcher.
We thank Clea Ribeiro and Group of the Service of Epidemiology of the Hospital de Clínicas da UFPR.