Colletotrichum truncatum is the most common pathogenic fungus associated with soybean anthracnose, a prevalent disease in Argentina. Pectinolytic enzymes are involved in the pathogenicity of a wide range of plant pathogenic fungi.
ObjectivesTo explore pectinolytic enzyme production in Argentinian Colletotrichum strains isolated from diseased soybean plants from different geographic locations, as a preliminary step to establish the biological role of the pectinolytic enzymes in the Colletotrichum spp.–soybean system, yet unknown.
MethodsTen strains were screened for in vitro pectinolytic enzyme production on a defined medium based on pectin as carbon source.
ResultsAll isolates were able to grow in this medium and polymethylgalacturonase (PMG), polygalacturonase (PG) and pectin lyase (PL) activities were detected. On the whole, the peak of polygalacturonases activities preceded the day of maximum growth, while PL activity reached its highest level afterwards. Strain BAFC 3097 (from Santa Fe province) yielded high titles of the three enzymes (1.08U/ml PG, 1.05U/ml PMG, 156U/ml PL), after a short incubation period (7–10 days). Low synthesis of polygalacturonases in cultures containing glucose as unique carbon source suggests that these enzymes are constitutive in contrast with PL, which was not detected.
ConclusionsThe disparity observed in enzyme production among strains cannot be related to fungal growth, since no major differences in mycelial yield were found; it was not connected with their geographic origin, but might be associated with differences in virulence among strains not yet evaluated.
Colletotrichum truncatum es el hongo patógeno más comúnmente asociado con la antracnosis de la soja, enfermedad de alta prevalencia en Argentina. Las enzimas pectinolíticas se relacionan con la patogenicidad de un amplio rango de hongos fitopatógenos.
ObjetivosInvestigar la producción de enzimas pectinolíticas por cepas aisladas de plantas de soja enfermas de diferentes regiones de nuestro país, y con ello contribuir a la caracterización fisiológica de dichos aislamientos como paso preliminar para esclarecer el aún desconocido rol biológico de las enzimas pectinolíticas en la interacción Colletotrichum spp-soja.
MétodosSe investigó la producción in vitro de enzimas pectinolíticas, en un medio sintético con pectina como fuente de carbono, de diez aislamientos de C. truncatum.
ResultadosTodas las cepas crecieron en dicho medio, detectándose actividades polimetilgalacturonasa (PMG), poligalacturonasa (PG) y pectin liasa (PL). En general, el pico de galacturonasas precedió al día de máximo crecimiento, en cambio el de PL se registró posteriormente. La cepa BAFC 3097 (originaria de la Provincia de Santa Fe) produjo altos títulos de las tres enzimas tras 7–10 días: 1,08U/ml PG, 1,05U/ml PMG, 156U/ml PL. C. truncatum, cultivado en un medio con glucosa como fuente de carbono, produjo PG y PMG (pero no PL), aunque su síntesis disminuyó marcadamente sugiriendo que estas enzimas son constitutivas.
ConclusiónLa disparidad registrada en la producción enzimática entre cepas no puede atribuirse al crecimiento fúngico; tampoco se corresponde con su distribución geográfica; pero podría relacionarse con diferencias en su virulencia, que aún no se han investigado.
Soybean (Glycine max) production in Argentina has increased over the last two decades. More than 90% of the cropped area is located in the northern Pampeana region. This area includes southwest of Cordoba, centre and south of Santa Fe and north of Buenos Aires provinces. Losses have increased due to diseases associated with monocropping, no-till systems and genetic uniformity of cultivars.46
Several species of Colletotrichum cause plant diseases so-called anthracnoses throughout the world, causing economically significant diseases of cereals, grain legumes, vegetables, forage legumes, fruit crops and perennial crops. Their ability to cause latent or quiescent infections places them amongst the most important post-harvest pathogens.2 These imperfect fungi belong to the subdivision Deuteromycotina (form-class Deuteromycetes, form-subclass Coelomycetidae, form-order Melanconiales, form-family Melanconiaceae) with 39 “accepted” species,40 which continue to be revised and clarified by molecular taxonomic techniques.
Soybean anthracnose is an economically important disease widely distributed in every soybean area production.37 Even though soybean is susceptible to the pathogen throughout all stages of development, symptoms become more noticeable in crop maturation, and anthracnose is included in the complex commonly known as late season diseases (LSDs). LSDs are presently the most damaging soybean diseases in Argentina, as they affect the plants during the yield generation period.
Although taxonomy and nomenclature in the group is confusing,18 the species most frequently associated with soybean anthracnose is Colletotrichum truncatum24,12 (teleomorph Glomerella truncata).1 Other species of Colletotrichum involved are C. coccodes, C. destructivum (teleomorph Glomerella glycines), C. gloesporioides (teleomorph Glomerella cingulata) and C. graminicola (teleomorph Glomerella graminicola).2
Cellulose, hemicellulose, lignin and pectin are the main components of plant cell wall. Pectin, a heteropolysaccharide defined as galactosyluronic acid-rich polymers, is composed of α-1-4 linked galacturonate chains with high percentage of methyl esterification. Pectinolytic enzymes, capable of degrading pectin and leading to maceration of plant tissues, are the first enzymes secreted by most fungal pathogens when attacking plant cell walls.7,19 Pectin degradation can be attained by the combined action of several enzymes such as pectin methylesterases and pectin depolymerases, including hydrolases and lyases, such as polymethylgalacturonase and pectin lyase. Degradation involves the breakdown of polygalacturonic acid through two enzimatic processes: lyases split the α-1-4 glycosidic bond between galacturonic acid residues by trans-limitation, while polygalacturonases catalyze a hydrolytic cleavage.36
The role of pectin degrading enzymes in causing cell-wall degradation is so important that it determines the virulence of many pathogens.8,7,35 In a number of systems, correlations have been established between the presence of pectinolytic enzymes, disease symptoms and virulence.10
Several Colletotrichum species, including C. truncatum29; C. lindemuthianum27 and C. destructivum,22 are intracellular hemibiotrophic pathogens and have a restricted host range, suggesting that host specifity in Colletotrichum might be associated with intimate contact between infective hyphae and living host cells.2C. truncatum establishes an initial biotrophic interaction that lasts approximately 24h followed by a secondary necrotrophic development.28,31 Two endopolygalacturonases were characterized in C. lindemuthianum: endopolygalacturonase 1 (endoPG1), released during saprophytic growth on pectin medium and induced during necrotrophic colonization, and endopolygalacturonase 2 (endoPG2), not detected in culture fluids, and associated with fungal cell wall components5 and wall degradation at the site of penetration. Limited cell wall degradation might be necessary for the beginning and preservation of the biotrophic growth by reducing cellular damage. During the necrotrophic phase, the production of endoPG1 is accompanied by other pectinases such as pectin lyases45 and probably pectin methyl esterases, which produce synergist and extensive wall dissolution. The pectinolytic enzymes of Colletotrichum species have been investigated in several crops, including avocado (C. gloeosporioides),43 bean (C. lindemuthianum),44,45,16 rubber (C. acutatum)11 and pea (C. truncatum).29 As far as we know, there is only one previous report on pectinolytic enzyme production by Colletotrichum spp. isolated from soybean plants.6 Although pectinolytic enzymes are involved in the pathogenicity of a wide range of plant pathogenic fungi, their biological function in the Colletotrichum spp.–soybean system is unknown. In the present work, by exploring in vitro pectinolytic enzyme production, we wish to contribute to the physiological characterization of Argentinean C. truncatum. strains isolated from diseased soybean plants from different geographic locations, as a preliminary step to establish the role of the pectinolytic enzymes in the Colletotrichum spp.–soybean interaction.
Material and methodsMicroorganismsStrains BAFC 3093–3102 (BAFC: Mycological Culture Collection of the Department of Biological Sciences, Faculty of Exact and Natural Sciences, University of Buenos Aires) of the anamorphic species C. truncatum were used in these experiments. The isolates were obtained from lesions of stems and pods of symptomatic soybean plants from seven localities in the provinces of Buenos Aires (3099, 3100, 3101), Santa Fe (3093, 3096, 3097, 3098), Chaco (3102), La Rioja (3095) and Misiones (3094). Pieces of symptomatic tissue bearing immature acervuli were surface sterilized in 1.5% sodium hypochlorite for 2min, rinsed twice in sterile water and incubated in humid chambers at 25–27°C under 12h near ultraviolet light (nuv) and 12h of darkness, until liberation of masses of conidia, which were cultured in potato–dextrose agar (PDA) plates at 25–27°C in darkness. Hyphal tips were aseptically transferred to PDA and incubated at 25–27°C under nuv light/darkness (12/12h) to obtain pure cultures. Stock cultures were maintained on potato–dextrose agar slants at 4°C.
Basal culture mediumPectin from apple, 10g; asparagine monohydrate, 4g; MgSO4·7H2O, 0.5g; H2KPO4, 0.5g; HK2PO4, 0.6g; CuSO4·5H2O, 0.4mg; MnCl2·4H2O, 0.09mg; H3BO3, 0.07mg; Na2MoO4·2H2O, 0.02mg; FeCl3, 1mg; ZnCl2, 3.5mg; thiamine hydrochloride, 0.1mg; distilled water up to 1l. Final pH: 3.5. In one assay pectin was replaced by glucose, the initial pH of this medium being 6.2.
Culture conditions100ml Erlenmeyer flasks with 20ml of medium were inoculated with one agar plug (0.25cm2) and cut out from a colony grown on Bacto-agar 2%. Incubation was carried out at 23±1°C under stationary conditions. Cultures were harvested at different incubation periods, filtered through a filter paper using a Büchner funnel and dried overnight at 70°C. Dry weight of mycelia was determined. The culture supernatants were used as enzyme sources.
Enzyme assaysPolygalacturonase activity (endo plus exo activity) was assayed by following the release of reducing groups from 0.1% apple pectin (polymethylgalacturonase—PMG) or polygalacturonic acid (polygalacturonase—PG) in 50mM sodium acetate buffer of pH 4.8 according to the Somogyi–Nelson method.39 One unit of enzymatic activity was defined as the amount of enzyme releasing 1μmol of galacturonic acid per min at 37°C. Enzyme activity was expressed as EU/ml of culture filtrate. Pectin lyase (PL) activity was assayed by the thiobarbituric acid method.30 The reaction mixture contained 3ml of pectin (1.2% in 0.05M Tris HCl buffer, pH 8.0) and 2ml of culture filtrate. The mixture was incubated for 1h at 30°C. After incubation, 1.5ml of 1N HCl and 3ml of 0.04M thiobarbituric acid were added and kept at 100°C for 20min. Afterwards, absorbance was measured at 550nm. In all assays, boiled enzyme and substrate mixtures were used as controls. One unit of lyase activity was defined as the amount of enzyme causing an increase of 0.01 in absorbance during 30min. Results are the average of three triplicate experiments with a standard error lower than 5%.
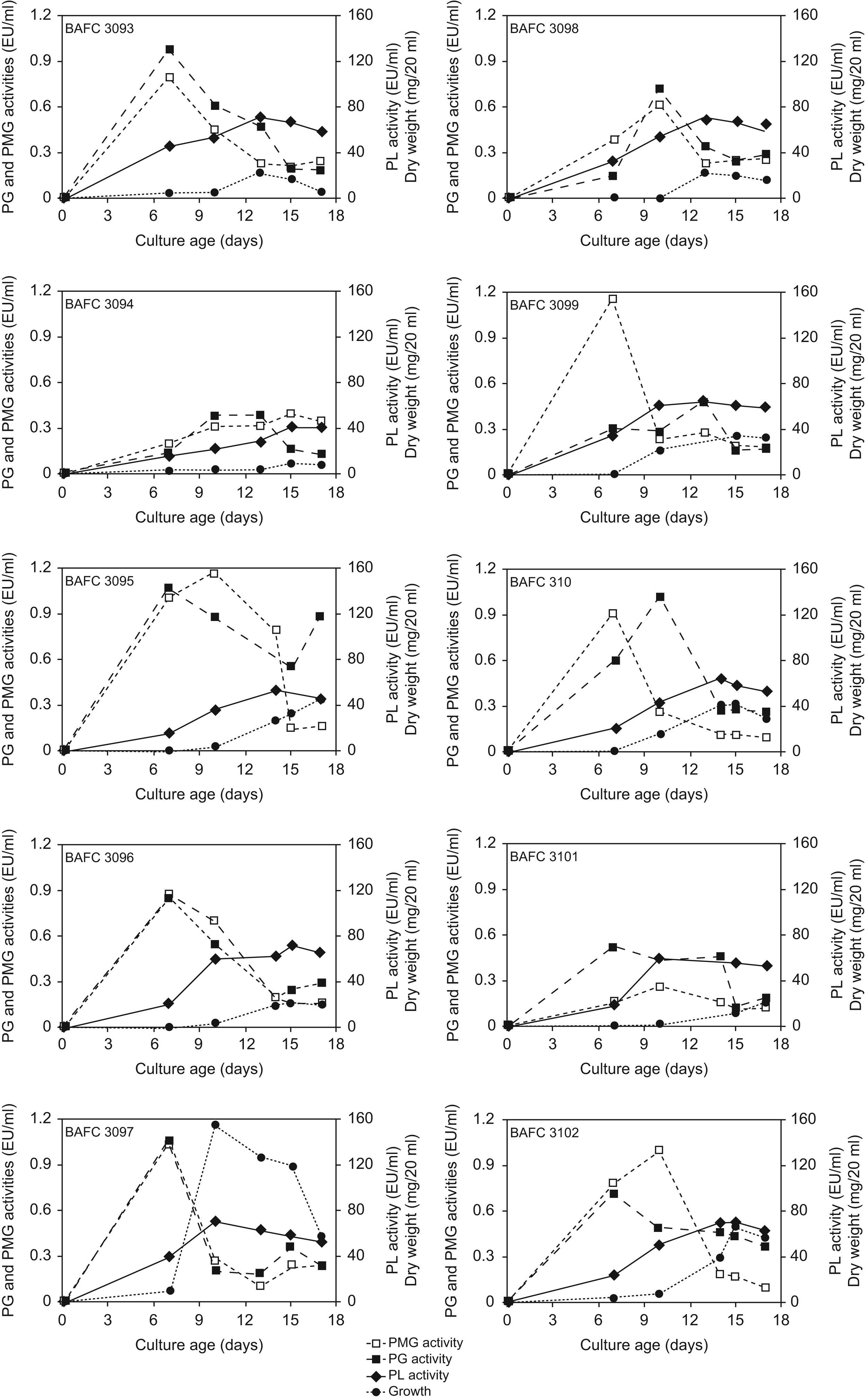
Results and discussionKinetics of in vitro production of extracellular pectinolytic enzymes by ten fungal strains from Colletotrichum isolated from soybean plants from Argentina that showed typical anthracnose symptoms was characterized in a synthetic medium based on pectin and asparagine as carbon and nitrogen sources, respectively. Fig. 1 depicts the relationship between their growth and pectinolytic enzyme production. All isolates were able to grow in the mentioned medium and produced a complex of enzymes having the potential to degrade α-1-4 bonds in pectic substances hydrolytically, as well by a trans-eliminative mechanism. The disparity observed in enzyme production among strains cannot be attributed to fungal growth, since no major differences in mycelial yield were found. Maximal growth values were around 50–70mg/20ml of medium, and were usually registered after 10–15 days of cultivation. In general, the peak of PG and PMG activities preceded the day of maximum growth. On the contrary PL activity reached its highest level simultaneously with or after the peak of growth. As detected in this work also in C. lindemuthianum44 and Rhizoctonia solanii23 the PG peak preceded the one of PL, and in Fusarium oxysporum f. sp. melonis26 and Botrytis cinerea25 PG activities peaked during growth, whereas the highest levels of PL were detected during autolysis. The earlier production of galacturonases during C. truncatum in vitro cultivation coincides with their postulated role in pathogenesis. Initially, several endoPG isoforms facilitate biotrophic development without causing extensive tissue maceration, while other isoenzymatic forms predominate in the necrotrophic stage. On the contrary PL, which appears later, is more likely to play an important role during the necrotrophic phase of tissue colonization.44,15
Among the strains assayed, C. truncatum BAFC 3097 rendered high levels of the three enzymes, after a short incubation period (7–10 days; 1.08U/ml PG, 1.05U/ml PMG, 156U/ml PL). Strains BAFC 3095 and 3100 also demonstrated high production of PG, PMG and PL (Fig. 1).
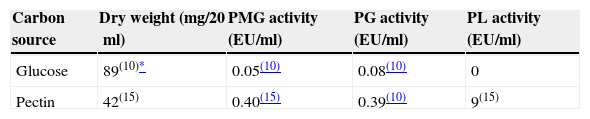
As shown in Table 1, pectinolytic enzyme production proved to be dependent on medium composition. In a medium where pectin was replaced by glucose C. truncatum BAFC 3094 attained greater growth but no PL activity could be measured, and PG and PMG activities noticeably decreased. The low synthesis of galacturonases in cultures containing glucose suggests that these enzymes are constitutive in contrast with lyase, which was not detected. Nevertheless, differences between in vivo and in vitro production, due to plant–pathogen interactions, cannot be ruled out. In a previous study, B. cinerea demonstrated constitutive production not only of endo- and exoPG but also of PL, and their patterns of in vitro production were correlated with in vivo production during the infection of bean leaves.21 Also Sclerotinia sclerotiorum produced PG and PMG constitutively in a medium with glucose as carbon source.34 Conversely trace amounts of PL activity were detected when C. lindemuthianum was cultivated in a medium with glucose, most probably corresponding to a constitutive enzyme required to start depolymerization of pectin when the organism is confronted with this substrate.16 Two regulation mechanisms are thought to occur during pectinolytic enzyme secretion by pathogenic fungi: (i) the enzyme is specifically induced by the substrate (i.e. pectin) or (ii) the enzyme is constitutive, but its expression is restricted by the presence of simple sugars (catabolite repression).3,13,14,17,38 Nevertheless, PL production may also be affected by the initial pH of the medium. The initial pH of the medium and its development during the incubation period influenced pectin and pectate lyase production in previous studies.9,41 In some anthracnoses, the launching factor to disease development is a PL produced by the fungus. High pH levels at the infection area, promoted by ammonia-releasing compounds secreted by the fungus, enhance not only the production and activity of this enzyme, but also the extent of disease.32 In this study an increase of pH of both media during culture time was detected, reaching final pHs of about 8.
PG and PL activities obtained from C. truncatum strains fall within the range of other phytopathogenic fungi grown in a medium with pectin. Thanatephorus cucumeris produced up to 25U/ml of PL20 and F. oxysporum around 180U/ml of PL4; PG-production by F. oxysporum f. sp. niveum reached a maximum of 0.4U/ml42 while C. lindemuthianum produced 0.24U/ml.17
In a previous study a comparison between pathogenic and non-pathogenic strain of C. lindemuthianum revealed significant differences in terms of PL production in liquid culture. On 92%-esterified pectin, the pathogenic strain had more PL activity and cell walls isolated from its host (Phaseolus vulgaris) induced PL only in the pathogenic strain.16 These authors hypothesize that differences in pathogenicity may in part reside both in the amount and time-course of PL production. In the present study, the disparity observed in enzyme production among strains cannot be related to fungal growth, since no major differences in mycelial yield were found, nor was it connected with their geographic origin, but might be associated with differences in virulence, not yet evaluated. Recently, when measuring genetic variability within isolates of C. truncatum from Argentina in terms of percentage of polymorphic loci we also found a high degree of polymorphism (up to 90%). With the primer used in the study we did not find any relation between the grouping of isolates and their geographic origin.33 The aim of prospect studies will be to test the hypothesis that variations in virulence among strains of C. truncatum causing anthracnose in soybean may be related to differences in pectinolytic activity.
The authors are grateful to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) and University of Buenos Aires for financial support.