Nothofagus pumilio (Poepp & Endl.) Krasser, known as “lenga” is the most important timber wood species in southernmost Patagonia (Argentina). Humicolopsis cephalosporioides Cabral & Marchand is a soil fungus associated with Nothofagus pumilio forests, which has outstanding cellulolytic activity. However, there is no information about the ability of this fungus to use organic substrates other than cellulose, and its ability to produce different enzyme systems, as well as its response to temperature.
AimsThe aim of this study was to examine the role of H. cephalosporioides in degradation processes in N. pumilio forests in detail by evaluating the in vitro ability of four isolates of this fungus to grow and produce different lytic enzyme systems, and their response to incubation temperature.
MethodsThe ability of the fungi to grow and produce enzyme systems was estimated by inoculating them on agar media with specific substrates, and the cultures were incubated at three temperatures.
ResultsA differential behavior of each strain in levels of growth and enzyme activity was found according to the medium type and/or incubation temperature.
ConclusionsA intra-specific variability was found in H. cephalosporioides. Likewise a possible link between the saprotrophic role of this fungus in N. pumilio forests and the degradation of organic matter under stress conditions, such as those from frosty environments, was also discussed.
Nothofagus pumilio (Poepp & Endl) Krasser (N. pumilio), conocido como «lenga», es la especie maderable más importante en el extremo sur de Patagonia (Argentina). Humicolopsis cephalosporioides Cabral & Marchand es un hongo del suelo asociado a bosques de N. pumilio, que tiene una actividad celulolítica excepcional. Sin embargo, no hay información acerca de la capacidad de este hongo para utilizar otros sustratos orgánicos distintos de la celulosa, o para producir diferentes sistemas enzimáticos, así como su respuesta a la temperatura.
ObjetivosEl objetivo de este estudio fue profundizar en el rol que Humicolopsis cephalosporioides tiene en los procesos de degradación en los bosques de N. pumilio a través de la evaluación de la capacidad in vitro de 4 aislamientos de este hongo para crecer y producir diferentes sistemas enzimáticos líticos y su respuesta a la temperatura de incubación.
MétodosLa capacidad de los hongos para crecer y producir sistemas enzimáticos se estimó a través de su inoculación sobre medios de agar con sustratos específicos, siendo incubados a 3 temperaturas.
ResultadosSe observó un comportamiento diferencial de cada cepa en el crecimiento y la actividad enzimática de acuerdo con el tipo de medio o la temperatura de incubación.
ConclusionesSe observó variabilidad intraespecífica en Humicolopsis cephalosporioides. Asimismo, se discutió la posible relación entre el rol saprotrófico de este hongo en los bosques de N. pumilio y la degradación de la materia orgánica en condiciones estresantes, como las existentes en ambientes fríos.
Soil is a fundamental resource of the forests. The productivity of forest soil is mainly related to its capacity to support plant growth, being most often measured in volume of trees produced.25 Management practices can affect the soil productivity and the level of timber harvesting that the forest can sustain, as well as its habitat and biodiversity associated.20 Although the forest soil microbiota as a whole is involved in mineralization of organic matter, it has been reported that fungi play a greater role than bacteria in the decaying processes that occur on the forest floor.30 Furthermore, forest soil-fungi also perform many other complex tasks relating to soil formation, nutrient availability and recycling, as well as tree metabolism and growth.24
Nothofagus pumilio (Poepp & Endl.) Krasser (Nothofagaceae), known as “lenga”, is the most important timber wood species in the southernmost Patagonia. It lives on volcanic soils present on the mountain slopes and on shallow soils in South of Tierra del Fuego (Argentina), growing until 56° S. Therefore, this tree tolerates several abiotic stresses such as low temperatures (−20°C), frost and fire and also biotic stresses as those caused by defoliating insects and pathogenic agents. All these factors alter the forest and each of its components, which also modify productivity of soil and its resources, including the diversity and activity of associated microorganisms.5,19,20,33
Humicolopsis cephalosporioides Cabral & Marchand (anamorph Ascomycota, Insertae Sedis) was identified as one of the soil fungi with high cellulolytic activity in Tierra del Fuego (Argentina).10,17,18 However there is no information about the ability of H. cephalosporioides to use other organic substrates, its enzyme systems and its role in nutrient cycling. Recently, we isolated four strains of H. cephalosporioides from soils of N. pumilio harvested by shelter wood-cut management. Therefore, the aim of this study was to get a better insight into the role of H. cephalosporioides in degradation processes in forests from Tierra del Fuego by evaluating its ability to grow and produce different lytic enzyme systems as well as its response at incubation temperature.
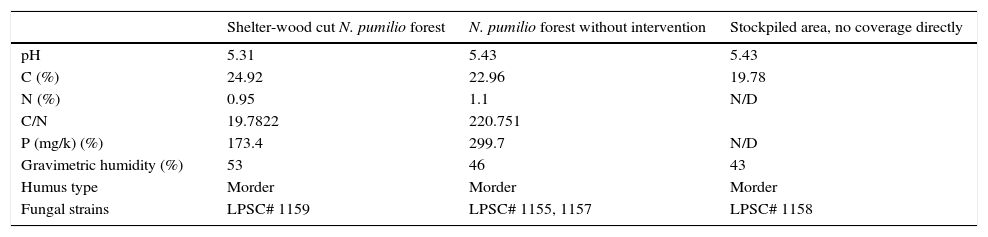
Materials and methodsSampling sites, soils and isolation of the fungiSoil composite samples (two replicates with five sampling points), representative from the first 5cm depth, were collected in stand of N. pumilio forest that was harvested by shelter-wood cut practice 50 years ago1,20 in Tierra del Fuego, Argentina (54°49′48″ S–68°21′35″ W). The soil type sampled has been classified as Litosols or litic Criortents.7,34 Sites and their soil properties are indicated in Table 1. For fungal isolation, the soil samples were processed according to Elíades and colleagues and soil particles were placed in Petri dishes on corn meal-agar medium incubated at 25°C until the development of fungal colonies.6 The strains were identified as H. cephalosporioides according to Marchand and colleagues (Table 1), and deposited in the culture collection of Spegazzini Institute (LPSC).17 Stock cultures were kept at 4°C on 2% (wv−1) agar-malt extract slants.
Characteristics of the sampling soils. Data are mean values (n=3).
| Shelter-wood cut N. pumilio forest | N. pumilio forest without intervention | Stockpiled area, no coverage directly | |
|---|---|---|---|
| pH | 5.31 | 5.43 | 5.43 |
| C (%) | 24.92 | 22.96 | 19.78 |
| N (%) | 0.95 | 1.1 | N/D |
| C/N | 19.7822 | 220.751 | |
| P (mg/k) (%) | 173.4 | 299.7 | N/D |
| Gravimetric humidity (%) | 53 | 46 | 43 |
| Humus type | Morder | Morder | Morder |
| Fungal strains | LPSC# 1159 | LPSC# 1155, 1157 | LPSC# 1158 |
N/D: not determined.
Inoculation was carried out by using 6-mm diameter agar plugs of each isolate, which were cut from cultures grown on malt agar extract medium, on Petri dishes with agar media supplemented with different specific substrates: carboxymethylcellulose (CMC) 0.5%,14 apple pectin 0.5%,14 Xylan 1%,3 Casein 0.5%,13 Tween® 20 (polyoxyethylene sorbitan monolaurate Merck®) 1%,11 Starch 20%,3 Guaiacol 0.18%29 and Chitin-Azure® (Sigma®, C 3020) 0.08%.12 Three replicates of each isolate on each culture medium was incubated in the dark for 10 days at three temperatures: 5±1°C, 15±1°C and 25±1°C.32 Fungal growth was estimated considering development (cm) under each treatment. The hydrolytic enzyme activity was estimated qualitatively according to the presence of a halo of clearance through hydrolase action and was expressed semiquantitatively as the ratio between the hydrolytic-halo and colony diameters.3,30
Statistical analysisThe experimental design was completely at random, considering a fixed effects model. All results are shown as mean±standard deviation (SD). The differences in fungal growth between treatments were analyzed by a one-way analysis of variance (ANOVA) and means were contrasted by the Fisher's least-significant-difference multiple-range-test (at P≤0.05), using the InfoStat software. In order to establish differences in enzyme activity between the two main factors (isolate type and incubation temperature) as well as their interactions, ANOVA was also performed as described.
ResultsFour strains, which were identified as H. cephalosporioides according to Marchand and colleagues, were isolated from soil particles from sites with different forest management practices (Table 1).17
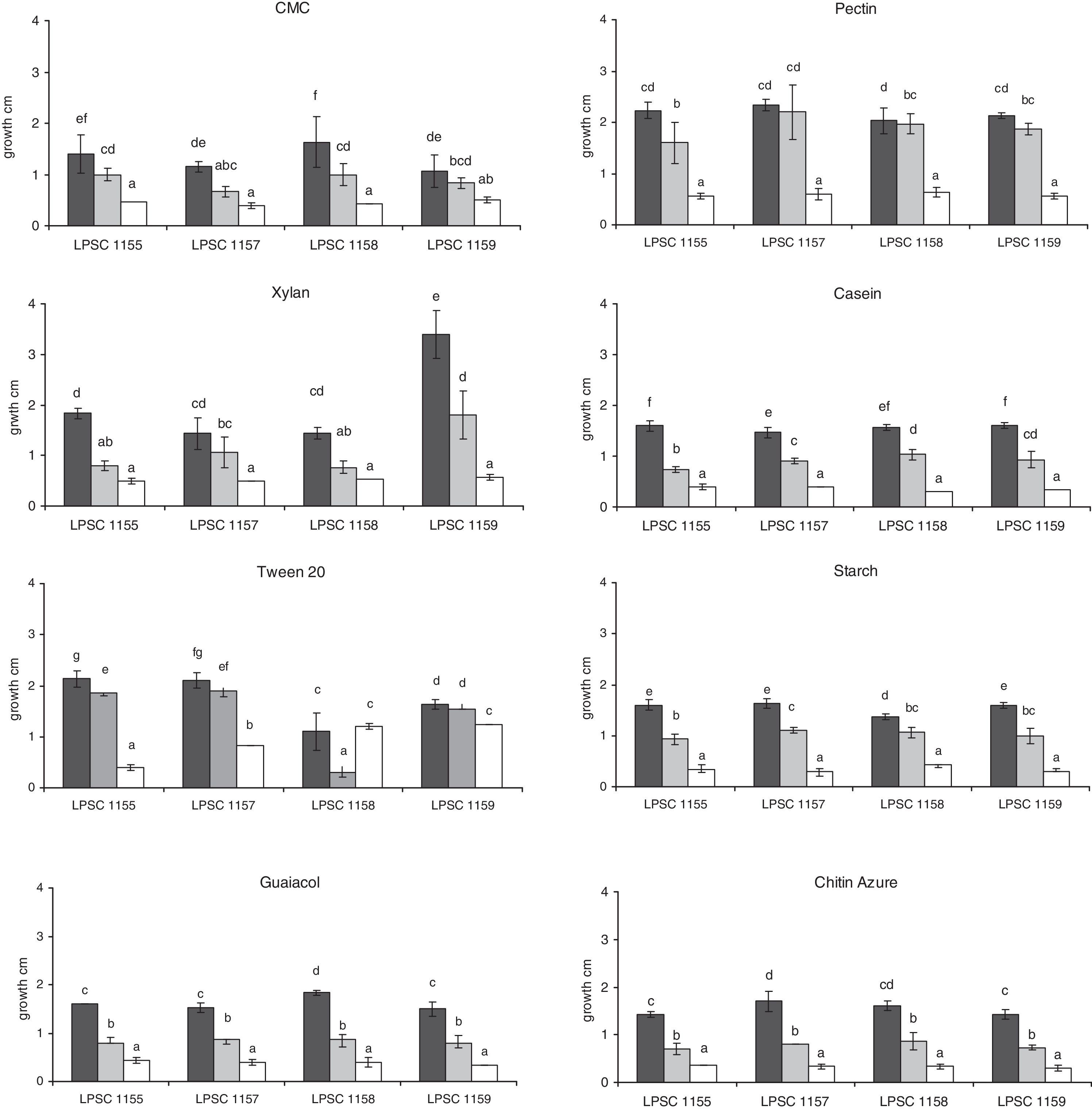
We analyzed the growth and the activity of different enzyme complexes of four isolates of H. cephalosporioides grown on different solid media at three incubation temperatures. While all isolates grew on all culture media at the three incubation temperatures (Fig. 1), a differential behavior of each strain was found according to the medium type and/or incubation temperature. Isolate LPSC 1159 showed the highest growth when it was cultured on the medium with xylan at 25°C, which was also significantly different compared to that from other isolates grown at the same temperature. In most of the isolates, a higher diameter was also observed at 25°C when compared with that at 5 and 15°C on tested media. However, a similar growth at 15 and 25°C was found on media with CMC, pectin and Tween 20 as well on one with pectin, Tween 20 and xylan for isolate 1159 and 1157, respectively. Furthermore, both isolates also did not show differences in growth on CMC at 5 and 15°C according to their putative role in litter-cellulose degradation. A similar behavior in growth was also found for the isolate 1155 and 1158 on xylan at 5 and 15°C. Surprisingly, a similar growth was found on the isolate 1158 when cultured on Tween 20 at 5 and 25°C, though it was different compared to that at 15°C. However, equivalent diameters on the same medium at 5°C were also found for the isolate 1159.
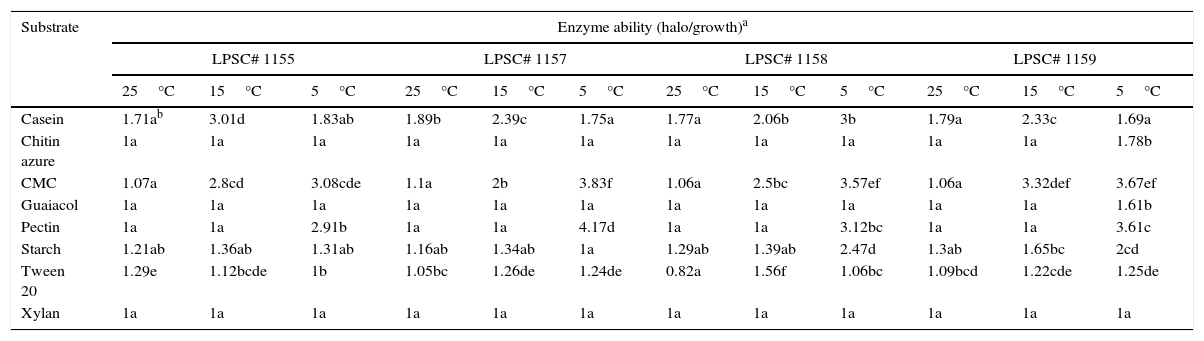
At the three tested temperatures, all the isolates showed a broad spectrum of enzyme ability when they were inoculated on several media (Table 2). However, significant differences in some enzyme activities were found between the different isolates and incubation temperatures. The proteolytic, oxidative and chitinolytic activities were different among the isolates tested (Table 3). In terms of incubation temperature variation, the cellulolytic, proteolytic, lipolytic, oxidative and chitinolytic activities showed significant differences in their levels. A different response in level of enzyme activity to temperature was found compared to that for growth. An intense activity was observed on media with casein, CMC and pectin for all isolates tested. This high level of activity was only found at temperatures below 25°C, the pectinase activity being higher at 5°C compared to that at 15°C. Isolates 1157 and 1158 also showed higher cellulolytic activity at 5°C than at 15°C. However, the proteolytic activity was higher at 15°C, although in the isolate 1158 it was not significantly different compared to that at 5°C. An interaction between the isolate type and incubation temperature also occurred for this latter activity as well as for lipolytic, oxidative and chitinolytic activities, which indicates that the enzyme level also depended on the specific combination of both of these variables. Furthermore, amylolytic activity was higher at 5°C for the isolates LPSC 1158 and 1159.
In vitro extracellular ability of H. cephalosporioides cultures grown on agar media supplemented with different specific substrates at three incubation temperatures.
| Substrate | Enzyme ability (halo/growth)a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPSC# 1155 | LPSC# 1157 | LPSC# 1158 | LPSC# 1159 | |||||||||
| 25°C | 15°C | 5°C | 25°C | 15°C | 5°C | 25°C | 15°C | 5°C | 25°C | 15°C | 5°C | |
| Casein | 1.71ab | 3.01d | 1.83ab | 1.89b | 2.39c | 1.75a | 1.77a | 2.06b | 3b | 1.79a | 2.33c | 1.69a |
| Chitin azure | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1.78b |
| CMC | 1.07a | 2.8cd | 3.08cde | 1.1a | 2b | 3.83f | 1.06a | 2.5bc | 3.57ef | 1.06a | 3.32def | 3.67ef |
| Guaiacol | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1.61b |
| Pectin | 1a | 1a | 2.91b | 1a | 1a | 4.17d | 1a | 1a | 3.12bc | 1a | 1a | 3.61c |
| Starch | 1.21ab | 1.36ab | 1.31ab | 1.16ab | 1.34ab | 1a | 1.29ab | 1.39ab | 2.47d | 1.3ab | 1.65bc | 2cd |
| Tween 20 | 1.29e | 1.12bcde | 1b | 1.05bc | 1.26de | 1.24de | 0.82a | 1.56f | 1.06bc | 1.09bcd | 1.22cde | 1.25de |
| Xylan | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a |
Analyses of variance (ANOVA) on different enzyme activities of isolates at a determined incubation temperature with the interactions between these variables.
| Casein | Chitin Azure | CMC | Guaiacol | Pectin | Starch | Tween 20 | Xylan | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F value | P | df | F value | P | df | F value | P | df | F value | P | df | F value | P | df | F value | P | df | F value | P | df | F value | P | |
| LPSC | 3 | 10.22 | <0.0002 | 3 | 49 | <0.0001 | 3 | 22 | ns | 3 | 121 | <0.0001 | 3 | 3.66 | ns | 3 | 5.06 | ns | 3 | 0.62 | ns | 3 | 0 | ns |
| T | 2 | 61.42 | <0.0001 | 2 | 49 | <0.0001 | 2 | 136.6 | <0.0001 | 2 | 121 | <0.0001 | 2 | 280.1 | ns | 2 | 4.83 | ns | 2 | 15.56 | <0.0001 | 2 | 0 | ns |
| LPSC×T | 6 | 34.26 | <0.0001 | 6 | 49 | <0.0001 | 6 | 3.61 | ns | 6 | 121 | <0.0001 | 6 | 3.66 | ns | 6 | 2.93 | ns | 6 | 12.22 | <0.0001 | 6 | 0 | ns |
ns=not significant; T=temperature.
N. pumilio forests in the province of Tierra del Fuego, Argentina, are an ecological region where stressful conditions prevail, such as a sub-Antarctic climate (with temperatures ranging between 0°C and 9°C) with frequent snow that covers the soil, frequent freeze-thaw cycles, strong winds and high solar incidence.21,26,28 Fungi growing in forests from cold climates are exposed to several stresses, as low temperatures and frost, the main determinants of fungal abundance and activity. However, several fungal species considered as psychrophilic or psychrotolerant forms can grow, reproduce and tolerate low temperatures and frost due to a high level of adaptation that allows them to withstand extreme conditions.8,27 Temperature is a key climatic parameter in the forests and their soils, being biological processes regulated by it.9 It modulates productivity of forests through the physiology of their trees as well as the microorganisms associated, since these latter are involved in the soil formation, litter transformation and organic matter mineralization.22,31 In a survey made in these forests with different management practices, survey aimed at identifying fungal assemblages associated to cold soils and studying their seasonal variation pattern, H. cephalosporioides was the dominant fungus (data not shown). It is a soil microorganism specific from Nothofagus forests that degrades organic matter with an outstanding cellulolytic ability.10,18 However, there is no information about the ability of this fungus to use other organic substrates, its enzyme systems and its role in nutrient cycling at several incubation temperatures, which may contribute to an understanding of how this fungus thrives at similar conditions from where it grows and degrades in nature. Since Pietikainen23 suggested that fungi were more adapted to low-temperature conditions than bacteria, H. cephalosporioides might have a key role in the organic matter degradation in the soil of N. pumilio in Tierra del Fuego (Argentina). All isolates tested showed ability to grow in vitro on other organic substrates in addition to a cellulose-like compound at 5, 15 and 25°C. Although there are no data about growing optimum temperature for this species, these results suggest than H. cephalosporioides isolates are psychrotolerant forms of mycobiota in soils of N. pumilio forests as reported by Ruisi27 for mycobiota of Antarctica, including ones from Western Antarctic Peninsula.15,16 These localities share similar biogeographic and climatic features to those from Tierra del Fuego (Argentina), being part of a same environmental unit, the subantarctic one.28 Some fungal species may show physiological variability among isolates of the same species. In our work, H. cephalosporioides showed this differential type of behavior depending on the medium used and the temperature of incubation. A higher growth was found when increasing the incubation temperature for most of the isolates, which might be related to the growth promotion by temperature through the activation of metabolic reactions. However no differences were found in isolates tested when they were cultured on cellulose or xylan at 5 and 15°C. Additionally, the isolates LPSC 1157 and 1159 did not show differences in growth when they were also cultured on pectin/Tween 20 at 15 and 25°C, though their growth responded differentially if they were grown on xylan or cellulose to those temperatures. Furthermore, a different growth pattern on Tween 20 was found for the isolate LPSC 1158 in response to temperature compared to that obtained with the other isolates tested. The isolate LPSC 1158 was obtained from a soil particle belonging to a forest area with high disturbance by wood exploitation, which possibly might be a source of physiologically different microorganisms compared to those isolated from non-disturbed natural forest areas. Similarly, Fenice et al.8 suggested that fungi’ growth and production of lytic enzymes by them is related to the chemical composition of the habitat, therefore it has adaptive and ecological significance. Colpaert et al.4 suggested that the origins of fungal isolates can affect the functional diversity or ecological plasticity of the fungi and thus may affect their range of tolerance to stressful effectors and their activity in ecological systems. Therefore our results suggest that there is intraspecific variation in growth among the isolates in H. cephalosporioides. It is consistent with the findings from Fenice et al.,8 who found intraspecific variations in Arthrobotrys ferox, Cladosporium herbarum, Geomyces pannorum, Phoma sp. and Verticillium lecanii, when they were grown at different temperatures. Because physiological traits can vary not only among species but also within species,2 it is highly probable that levels of enzyme activity and its response to temperature differs among fungal isolates, even when they are originated in a limited habitat area, as in this study. Similarly, versatile enzyme ability was found in all isolates of H. cephalosporioides at tested temperatures. Levels of several enzyme systems were different according to each isolate and temperature assayed as well as dependent upon the specific combination of both of these variables. Furthermore, a different response in level of enzyme activity to temperature was found compared to those about growth, since a high level of activity was only found at temperatures below 25°C, which might be related to the cold environments where these fungi grow in nature. These results suggest a possible link between the saprotrophic role of H. cephalosporioides in N. pumilio forest and degradation of organic matter under stressful conditions such as frost environments.
In conclusion, we show data on the ability of H. cephalosporioides to grow and produce several enzyme activities related to the decay of organic matter present in forest soil. Apart from the cellulolytic activity, this work shows a higher enzyme spectrum in this species: amylases, chitinases, proteases, pectinases and xylanases. Moreover, it has also been found that growth and enzyme activity, as well as response to temperature, varied in different isolates of this fungal species that lives specifically in Nothofagus spp. forests soil in Tierra del Fuego (Argentina). However, further work is required to get an isolate that produces high enzyme activity at 5°C, such as LPSC 1158, and that can degrade significantly the litter of N. pumilio, as well as if it depends on isolation origin.
This study was partially supported by grants from Agencia Nacional de Promoción Científica y Técnica (ANPCyT-PICTO N° 36861, PICT 2011 – N° 0501), Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CICPBA), Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET, PIP 112-200801-01422, PIP 112 201101 00391), and Universidad Nacional de La Plata (UNLP, 11/N 651), Argentina. Elíades L.A., Pancotto V., Moretto A. and Saparrat M.C.N. are members of Carrera del Investigador CONICET. Cabello M. is a researcher from CICPBA. Rago M. is an ad-honorem laboratory student from Facultad Ciencias Naturales y Museo, UNLP.