Candida parapsilosis is recognized as a species complex: Candida parapsilosis sensu stricto, Candida orthopsilosis and Candida metapsilosis are three distinct but closely related species.
AimsTo determine the species and antifungal susceptibility of members of the C. parapsilosis complex, isolated from clinical samples.
MethodsIsolates identified as C. parapsilosis complex by VITEK® 2 system were included. Antifungal susceptibility test was done using the VITEK® 2 semi-automated system. The distribution of the species in the complex was determined by multiplex PCR.
ResultsAmong the seventy-seven C. parapsilosis complex isolates, C. parapsilosis sensu stricto (57.1%) was the commonest species, followed by C. orthopsilosis (40.2%) and C. metapsilosis (2.5%). All three species were susceptible to amphotericin B, caspofungin and micafungin. Among C. parapsilosis sensu stricto isolates, 16% were resistant to fluconazole while 2.2% showed dose dependent susceptibility. Also, 18.2% of C. parapsilosis sensu stricto isolates showed dose dependent susceptibility to voriconazole.
ConclusionsC. parapsilosis sensu stricto was the most commonly isolated member of the C. parapsilosis complex and it showed high resistance to fluconazole. A high prevalence of C. orthopsilosis (40.2%) was also noted.
Candida parapsilosis se reconoce como un complejo de especies compuesto por Candida parapsilosis sensu stricto, Candida orthopsilosis y Candida metapsilosis, tres especies distintas pero estrechamente relacionadas.
ObjetivosEstablecer las especies y la sensibilidad antifúngica de aislamientos del complejo C. parapsilosis procedentes de muestras clínicas.
MétodosSe incluyeron los aislamientos identificados como complejo C. parapsilosis por el sistema VITEK® 2. El estudio de la sensibilidad antimicótica se realizó mediante el sistema semiautomático VITEK® 2. La distribución de las especies del complejo se estableció mediante PCR múltiple.
ResultadosEntre los 77 aislamientos del complejo C. parapsilosis, C. parapsilosis sensu stricto (57,1%) fue la especie más común, seguida por C. orthopsilosis (40,2%) y C. metapsilosis (2,5%). Las tres especies fueron sensibles a anfotericina B, caspofungina y micafungina. Entre los aislamientos de C. parapsilosis sensu stricto, el 16% fue resistente al fluconazol, mientras que el 2,2% mostró sensibilidad dependiente de la dosis. Además, el 18,2% de los aislamientos de C. parapsilosis sensu stricto mostró una sensibilidad al voriconazol dependiente de la dosis.
ConclusionesC. parapsilosis sensu stricto fue la especie más aislada del complejo C. parapsilosis y mostró una elevada resistencia al fluconazol. C. orthopsilosis fue también aislada con una alta prevalencia (40,2%).
Candida parapsilosis has emerged as the third most commonly isolated species from blood cultures of critically ill patients admitted to Indian intensive care units.4 It is known to persist in hospital environments, is spread by contaminated hands, and prior colonization is not a prerequisite for causing invasive infection.2 In 2005 Tavanti et al. recognized C. parapsilosis as a complex of species comprising C. parapsilosis sensu stricto, Candida orthopsilosis and Candida metapsilosis.11 Since the three species are phylogenetically and morphologically indistinguishable, only molecular techniques like multiplex PCR, randomly amplified polymorphic DNA analysis, and analysis of internal transcribed spacer sequences of DNA encoding ribosomes can reliably differentiate them.1 Another closely related species, Lodderomyces elongisporus, is also often misidentified as C. parapsilosis by phenotypic methods.1 Previous studies have shown C. parapsilosis sensu stricto to be the most commonly isolated species from clinical specimens among the members of the C. parapsilosis complex.1 The members of the complex are also known to differ in their antifungal susceptibilities and virulence.12 The aim of the study was to determine the prevalence of the three closely related species (C. parapsilosis sensu stricto, C. orthopsilosis, C. metapsilosis) comprising C. parapsilosis complex, as well as the phenotypically closely related species L. elongisporus using a single multiplex PCR (mPCR) assay.
The study was conducted from December 2015 to February 2017 in a tertiary care 1300-bed hospital in South India. Seventy-seven non-repetitive clinical isolates identified as C. parapsilosis complex by VITEK® 2 system (bioMérieux, Marcy I’ Etoile, France) were collected from different clinical specimens like, blood, urine, sputum, pus, skin, wound tissue and nail. Antifungal susceptibility for fluconazole, amphotericin B, caspofungin, micafungin, flucytosine, and voriconazole was done using the VITEK® 2 semi-automated system (AST-YSO1 cards, bioMérieux). Susceptibility of C. parapsilosis complex was interpreted using CLSI M27-S4 guidelines.7 The minimum inhibitory concentrations (MIC) of all fluconazole-resistant isolates were confirmed using Etest.
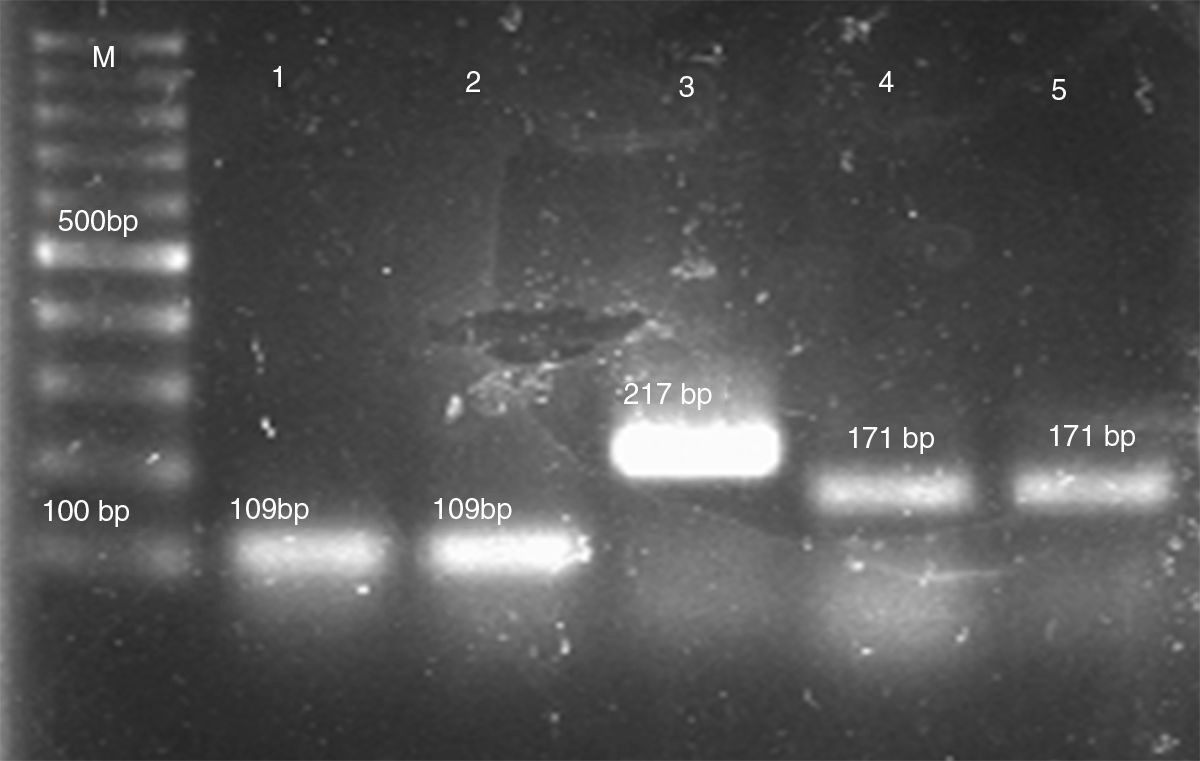
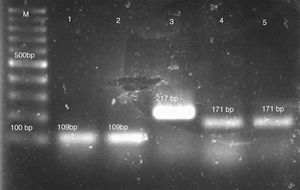
Genomic DNA was extracted using an established CTAB method. Briefly, three to five isolated colonies were placed in 1.5-ml microcentrifuge tubes containing 700μl of 2×CTAB buffer (100mM Tris–HCl [pH 8], 1.4M NaCl, 25mM EDTA, 2% CTAB). After vortexing the cell mixture, 1 volume of phenol–chloroform was added. The mixture was vortexed again and centrifuged at 12,000×g for 5min. DNA was precipitated from the upper aqueous phase by adding an equal volume of isopropanol and centrifuging at 12,000×g for 10min. After washing the DNA pellet once in 70% ethanol, it was resuspended in 100μl of TE buffer (10mM Tris–HCl [pH 8.0], 1mM EDTA).13 Species characterization of C. parapsilosis complex was performed by multiplex PCR using a previously published protocol.1 The PCR consisted of species-specific forward primers for C. parapsilosis sensu stricto, C. orthopsilosis, C. metapsilosis and L. elongisporus and a common reverse primer resulting in products of 171bp, 109bp, 217bp and 258bp respectively (Fig. 1).
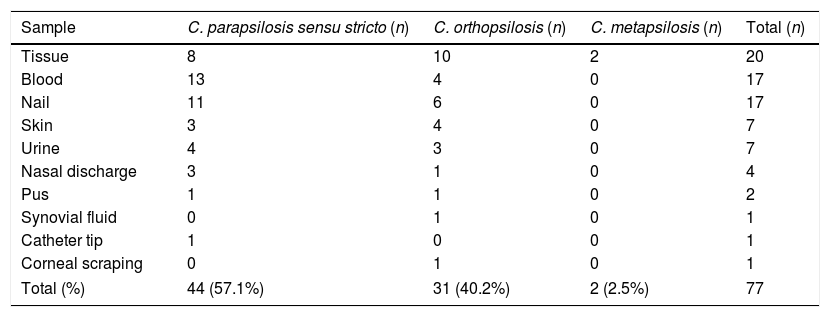
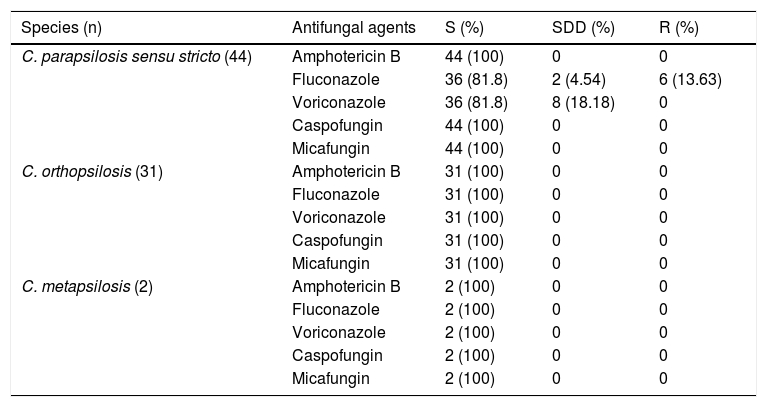
Seventy-seven C. parapsilosis complex isolates were included in the study. Out of these, 26% (20/77) were recovered from wound tissue samples, 22% (17/77) each from blood and nail samples, and 9% (7/77) each from skin and urine samples. Species distribution in various samples is represented in Table 1. Male patients accounted for 54.5% of the cases. There was a solitary case of corneal ulcer caused by C. orthopsilosis. The most common species isolated was C. parapsilosis sensu stricto (57.1%) followed by C. orthopsilosis (40.2%) and C. metapsilosis (2.5%). None of the isolates were identified as L. elongisporus. All three species of C. parapsilosis complex were susceptible to amphotericin B, caspofungin and micafungin (Table 2). Among C. parapsilosis sensu stricto isolates 16% were resistant to fluconazole while 2.2% showed dose dependent susceptibility (SDD). Notably, all isolates (18.2%) that were either resistant or SDD to fluconazole were also SDD to voriconazole. Though all isolates were susceptible to caspofungin the MICs of 68% of the C. parapsilosis sensu stricto isolates were on the higher side (1μg/ml).
Sample wise distribution of C. parapsilosis complex isolates.
| Sample | C. parapsilosis sensu stricto (n) | C. orthopsilosis (n) | C. metapsilosis (n) | Total (n) |
|---|---|---|---|---|
| Tissue | 8 | 10 | 2 | 20 |
| Blood | 13 | 4 | 0 | 17 |
| Nail | 11 | 6 | 0 | 17 |
| Skin | 3 | 4 | 0 | 7 |
| Urine | 4 | 3 | 0 | 7 |
| Nasal discharge | 3 | 1 | 0 | 4 |
| Pus | 1 | 1 | 0 | 2 |
| Synovial fluid | 0 | 1 | 0 | 1 |
| Catheter tip | 1 | 0 | 0 | 1 |
| Corneal scraping | 0 | 1 | 0 | 1 |
| Total (%) | 44 (57.1%) | 31 (40.2%) | 2 (2.5%) | 77 |
Antifungal susceptibility of C. parapsilosis sensu stricto, C. orthopsilosis and C. metapsilosis isolates.
| Species (n) | Antifungal agents | S (%) | SDD (%) | R (%) |
|---|---|---|---|---|
| C. parapsilosis sensu stricto (44) | Amphotericin B | 44 (100) | 0 | 0 |
| Fluconazole | 36 (81.8) | 2 (4.54) | 6 (13.63) | |
| Voriconazole | 36 (81.8) | 8 (18.18) | 0 | |
| Caspofungin | 44 (100) | 0 | 0 | |
| Micafungin | 44 (100) | 0 | 0 | |
| C. orthopsilosis (31) | Amphotericin B | 31 (100) | 0 | 0 |
| Fluconazole | 31 (100) | 0 | 0 | |
| Voriconazole | 31 (100) | 0 | 0 | |
| Caspofungin | 31 (100) | 0 | 0 | |
| Micafungin | 31 (100) | 0 | 0 | |
| C. metapsilosis (2) | Amphotericin B | 2 (100) | 0 | 0 |
| Fluconazole | 2 (100) | 0 | 0 | |
| Voriconazole | 2 (100) | 0 | 0 | |
| Caspofungin | 2 (100) | 0 | 0 | |
| Micafungin | 2 (100) | 0 | 0 |
S: susceptible; R: resistant; SDD: susceptible-dose dependent; breakpoints: voriconazole S/SDD/R, ≤0.12μg/ml/0.25–0.5μg/ml/≥1μg/ml; fluconazole S/SDD/R, ≤2μg/ml/4μg/ml/≥8μg/ml; caspofungin, micafungin S/I/R, ≤2μg/ml/4μg/ml/≥8μg/ml; amphotericin B S, ≤1μg/ml.
Since 2005, when C. parapsilosis species complex was divided into C. parapsilosis sensu stricto, C. orthopsilosis, and C. metapsilosis, several studies have reported their distribution, antifungal resistance and virulence.1,5,6,8,10–12 Recent studies have reported an increase in the prevalence of human infections caused by C. parapsilosis species complex. A large study among critically ill patients in Indian intensive care units showed this species complex was the second most common cause of candidemia.4 Studies have shown that there is a considerable variation in the distribution of C. metapsilosis and C. orthopsilosis at several geographic locations.1,5,6,8,10–12 A recently published study from India on eye infections due to Candida species reported C. parapsilosis species complex was the commonest cause (44%) followed by C. albicans.10 The distribution of C. metapsilosis and C. orthopsilosis in other clinical specimens from Indian hospitals is unknown due to non-availability of molecular techniques and MALDI-TOF for species differentiation.
Though C. parapsilosis sensu stricto was found to be the most common species in our study, the prevalence of 57% is the lowest recorded when compared to previous published studies (60–95%).1–3,5,6,8,10–12 The prevalence of C. orthopsilosis (40.2%) in our study is the highest reported in literature with all previous studies having reported figures of 2–35%.1–3,5,6,8,10–12 Our findings are also not in agreement with a recently published study from India which reported a prevalence of 95.5% and 4.5% for C. parapsilosis sensu stricto and C. orthopsilosis, respectively.10 Though more than half (58%) of our C. orthopsilosis isolates were from older patients (>50 years), statistical analysis using chi-square test showed that the association was not significant (p=0.051). Two previous studies have reported C. orthopsilosis infections exclusively in older patients.3,12C. orthopsilosis is known to be more heterogenous genotypically than C. parapsilosis sensu stricto which is predominantly clonal.11 The prevalence of C. metapsilosis in our study (2.5%) is similar to what has already been reported in literature.1,5,8,12 Studies have shown that C. metapsilosis is the least virulent among the species of the C. parapsilosis complex. A recent study on expression of virulence factors found that 34.2% of C. parapsilosis sensu stricto were protease positive while none of the C. orthopsilosis and C. metapsilosis expressed protease activity.12
The members of the C. parapsilosis complex exhibit different MICs to the antifungal drugs, therefore warranting species-specific identification.1,5,8,12 None of the C. metapsilosis and C. orthopsilosis isolates were resistant to any of the antifungal agents tested, which is in contrast with previous reports.1,5,8,12 Fluconazole resistance in C. metapsilosis has been reported in many previous studies.12,14 A recent study by Chen et al. reported higher MICs to voriconazole in C. metapsilosis.6 However, caspofungin MICs of C. parapsilosis sensu stricto and C. orthopsilosis, though susceptible, were on the higher side, which is in agreement with previous studies.12,14 Resistance to fluconazole among C. parapsilosis sensu stricto isolates was 16%, which is contrary to the low levels of resistance reported in previous studies.1,5,8 All fluconazole-resistant or SDD isolates (18%) showed SDD to voriconazole also. The high azole resistance may be due to the widespread use of fluconazole in our hospital. The major limitation of our study was that broth microdilution was not used to determine the MIC values. However, previous studies have shown excellent quantitative and qualitative agreement (within 2 dilutions) for fluconazole and voriconazole between the reference CLSI or EUCAST broth microdilution and VITEK® 2 MICs.9
A recent report has described the emergence of fluconazole-resistant C. parapsilosis sensu stricto during prolonged antifungal treatment due to increase in the expression of MRR1 transcription factor resulting in overexpression of MDR1.14 Fluconazole resistance is known to emerge in settings of complicated infections, suboptimal dosing and extensive use of antifungal agents. Since C. parapsilosis complex is known to show high MIC to echinocandins, the emergence of azole resistance has significant clinical implications.
In conclusion, ours is the second study to report the species distribution of C. parapsilosis species complex from India. Though C. parapsilosis sensu stricto was the most common species isolated, the prevalence of C. orthopsilosis at 40.2% is the highest reported in literature. Resistance to fluconazole and voriconazole in C. parapsilosis sensu stricto is also high. Our data have shown that species distribution and fluconazole resistance among members of C. parapsilosis complex in India is different from those of other countries. This knowledge could be of clinical relevance in guiding therapeutic decisions.
FundingNone declared.
Conflict of interestWe declare that there is no entity (among us) with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.