The knowledge of the diversity of yeasts that make up the skin microbiota of human beings is essential for the efficient monitoring of infections to which a person may be predisposed.
AimsThis study identified yeasts comprising the genital skin microbiota of patients attending the Dermatology Service at the Hospital São Paulo-UNIFESP, Brazil.
MethodsSamples were collected from the genital region of each patient and cultured on Sabouraud dextrose agar. Individual colonies were carefully transferred to tubes daily. Yeasts were identified based on classical methodologies and confirmed using a commercial kit.
ResultsEighty-three patients were included in the study. Approximately 80% were women and 20% were men. The average age was 55 years. Hypertension, diabetes, kidney transplant and AIDS were the main underlying diseases reported by the patients. The most prevalent yeasts were Candida parapsilosis (36.1%), Rhodotorula mucilaginosa (9.2%), Rhodotorula glutinis (8.3%), Candida tropicalis (5.5%) and Trichosporon inkin (1.8%). Approximately 78% of the isolates were obtained in pure cultures. Trichosporon inkin was isolated only from women, in contrast to literature describing a high prevalence in males.
ConclusionsOur results suggest that Candida albicans is not the main yeast found on genital skin as previously thought, and opportunistic pathogens such as C. parapsilosis, C. tropicalis, Rhodotorula spp. and T. inkin make up the genital skin microbiota, representing a risk for infection in immunocompromised subjects. These results also indicate that women are carriers of T. inkin, the etiological agent of white piedra and trichosporonosis.
El conocimiento de la diversidad de las levaduras que conforman la microbiota de la piel de los seres humanos es fundamental para un eficaz seguimiento de las infecciones.
ObjetivosEn este estudio se identificaron las levaduras que componían la microbiota de la piel de los genitales de pacientes que acudieron al Servicio de Dermatología del Hospital São Paulo - Unifesp, Brasil.
MétodosSe recogieron muestras de la región genital de cada paciente y se cultivaron en agar dextrosa Sabouraud. Las colonias individuales se transfirieron cuidadosamente a tubos. La identificación de las levaduras se basó en metodologías clásicas y se confirmó utilizando un kit comercial.
ResultadosOchenta y tres pacientes fueron incluidos en el estudio. Aproximadamente el 80% eran mujeres y el 20% eran hombres. La edad promedio fue de 55años. La hipertensión, la diabetes, el trasplante renal y el sida fueron las principales enfermedades subyacentes de los pacientes. Las levaduras más frecuentes fueron Candida parapsilosis (36,1%), Rhodotorula mucilaginosa (9,2%), Rhodotorula glutinis (8,3%), Candida tropicalis (5,5%) y Trichosporon inkin (1,8%). Aproximadamente el 78% de los aislamientos crecieron en cultivo puro. Trichosporon inkin fue aislado únicamente en mujeres, en contra de la literatura que describe una alta prevalencia en los hombres.
ConclusionesNuestros resultados sugieren que Candida albicans no es la principal levadura que se encuentra en la piel de los genitales, como se pensaba, y los agentes patógenos oportunistas C. parapsilosis, C. tropicalis, Rhodotorula spp. y T. inkin conforman la microflora de la piel genital, lo que representa un riesgo inminente de infección para los pacientes inmunocomprometidos. Estos resultados también indican que las mujeres son portadoras del agente etiológico de la piedra blanca y de la tricosporonosis, T. inkin.
The term “normal microbiota” is given to those microorganisms that inhabit the skin and mucosal surfaces of healthy individuals.3 The microbiota is classified as resident, when the organism is found regularly in certain areas of the body, or transient, when it remains in the tissue for hours, days or weeks. The fungal microbiota that makes up the body surface of living beings is constitutively dynamic. Periodic changes may be observed. These changes are largely due to environmental factors such as geographic location, sanitation, and some climatic conditions, such as temperature and exposure to UV light.2
The fungi that are present in the human superficial microbiota may be variable. Among the yeast genera that colonize humans, we are particularly interested in Candida, Rhodotorula, Malassezia, and Trichosporon.3,8,16,24,28,32 The members of the Malassezia genus are the most studied yeasts on the skin of patients with pityriasis versicolor and in seborrheic areas of the body in some healthy individuals.9,14,32 The skin and mucosal surfaces act as barriers against infections caused by pathogenic yeasts. Fatty acids, pH, renewal of epithelial cells and normal microbiota contribute to the resistance of the host. However, under certain immunosuppressive conditions, some yeasts can overcome this barrier and play a pathogenic role.29
The emergence of less common but important fungal pathogens has contributed to the increase in the morbidity and mortality of immunocompromised patients. New advances in medicine, such as the advent of new surgical techniques, more efficient diagnostic methods, and the development of new drugs, have promoted the survival of patients with diseases regarded as fatal. Patients with AIDS, cancer, tissue transplants, and diabetes are important and growing groups of immunosuppressed patients who have a large predisposition to infection with opportunistic pathogens. Despite the normally low virulence of the fungi, acute disseminated and often fatal infectious processes are observed among these patients.33
Candida species are the fourth most common agent diagnosed in nosocomial hematologic infections.33 Although this pathogen continues to be important, other fungal agents have been found. Despite increasing reports of fatal infections by new microbial strains, the epidemiology, pathogenesis, virulence-associated factors, prevention and treatment remain poorly understood. Among these pathogens are non-Candida yeasts such as Trichosporon and Malassezia.10,22,27 The aim of this study was to isolate and identify the yeasts that make up the genital skin microbiota of patients at the dermatology outpatient clinic of the Hospital São Paulo, UNIFESP (Brazil).
Materials and methodsPatientsEighty-three patients of both genders volunteered to participate in this study. Each subject received information about the study and its goals and signed a consent form. Next, each patient completed and signed a questionnaire that included questions about gender, ethnicity, sexual orientation, occupation, symptoms and underlying diseases. The interviewed patients were classified into three age groups: young adults (age 15–39 years), adults (age 40–63 years) and elderly (age greater than 64 years).
SamplesSamples were obtained using the carpet technique.18 A square carpet (4cm×4cm) that was previously sterilized was rubbed on the genital region of the examined patients. These carpets were pressed onto Sabouraud dextrose agar (SDA) medium containing 0.5% chloramphenicol in Petri dishes and incubated at 32°C. Readings were made every 24h for 15 days. Each yeast colony was isolated into a tube containing SDA medium and identified using classical methods.15,16 The protocols employed in this research followed the ethical standards of the Committee for Research Involving Humans of the Universidade Federal de São Paulo and were in accordance with the Helsinki Declaration of the World Medical Association.
Isolate identificationFor the identification of Candida albicans, Candida tropicalis, and Candida krusei, samples were plated on CHROMagar Candida™ medium (Difco™) according to the manufacturer's instructions. To confirm the identification of C. albicans isolates, they were subjected to germ tube and chlamydospore formation tests. Differentiation to Candida dubliniensis was based on the ability to grow at 42°C. Macroscopic characteristics of the colonies were recorded according to each gender. The isolates were tested for the production of urease4 and stained with methylene blue and Indian ink for direct examination. Clinical strains of C. albicans and Cryptococcus neoformans were used as positive and negative controls, respectively. Unidentified isolates underwent an auxanogram and a zymogram. The following carbon sources were used: dextrose, maltose, saccharose, galactose, trehalose, cellobiose, xylose, raffinose, melibiose, l-arabinose, sorbitol, myo-inositol, and potassium nitrate (KNO3). The suspension turbidity was adjusted to 5 on the McFarland scale. The plates were incubated at room temperature for 24–48h. The same sources were used for the fermentation tests. The tubes were incubated at 37°C until the 14th day of incubation, and readings were obtained every day. Definitive identification of the yeasts was confirmed using the commercial kit ID 32C™ (bioMérieux™, São Paulo, SP, Brazil).
Statistical analysisThe variables were described based on their absolute and relative values. The significance of the association between the variables present in the sample was evaluated using Fisher's exact test, and α≤0.05 was considered acceptable.
ResultsSixty seven patients (80.7%) out of the 83 included in the study were female, and 16 (19.3%) were male. The average age was 53.7 years, ranging from 15 to 87 years. Several ethnic groups were represented, including 58 Caucasian (69.9%), 18 (27.7%) African, six (7.2%) mixed race and one (1.2%) Asian patient. Domestic activity was the predominant profession reported (72.3%), followed by students (6.5%), administrators and “staff” doctors (5.5%). The remaining 10.2% reported other occupations. Only 23 (27.7%) patients reported abnormal genital itching, while the remainder (72.3%) did not have any symptom. Forty-five (54.1%) were sexually active. The average frequency of sex was once a week. Among the underlying diseases reported by the individuals surveyed, hypertension was the most common and was reported by 38 (45.8%) patients. Patients with hypertension associated with diabetes represented 13.3% (n=11) of the study population, while only one individual (1.2%) reported diabetes as a single underlying disease. Five (6%) patients had experienced some type of organ transplant, and 28 (33.7%) were healthy.
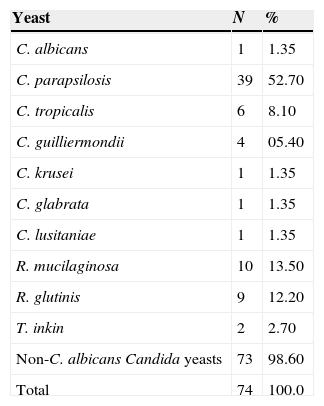
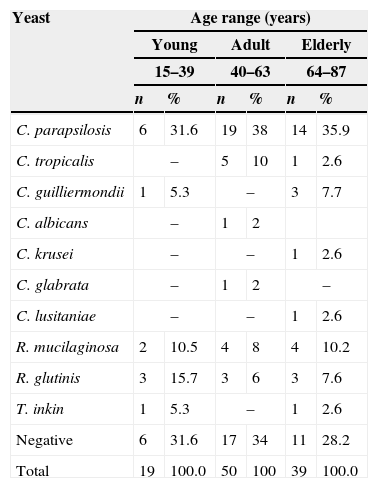
One hundred and eight isolates were obtained from rubbing carpet squares on the skin of the genital region of the 83 patients studied. More than one isolate grew on the culture plates of some patients. Seventy-four (68.5%) isolates corresponded to yeasts. The 34 (31.5%) remaining isolates were identified as bacteria or filamentous fungi that were not considered in this investigation. The genus Candida showed a remarkable prominence among the isolated yeasts. Approximately 0.9% of the isolates were C. albicans, 67.6% were non-C. albicans Candida species, and 31.5% were bacteria and filamentous fungi. The genera of the yeasts isolated in this study were Candida, Rhodotorula, and Trichosporon. Prevalent species of Candida on the genital skin of the surveyed patients were, in descending order, Candida parapsilosis, C. tropicalis, Candida guilliermondii, C. albicans, C. krusei, Candida glabrata and Candida lusitaniae. Rhodotorula mucilaginosa, Rhodotorula glutinis, and Trichosporon inkin, as well as other yeasts, were found in the present investigation. The absolute and relative values for each isolate are shown in Table 1. The greatest number of yeasts was isolated from the adult group (n=33), with slightly more yeasts than in the elderly group (n=28). The distribution of the yeast species is listed in Table 2.
Yeast species isolated from the genital region of 83 patients attending the dermatology outpatient clinic of the Hospital São Paulo, UNIFESP, Brazil (June 2006–April 2007).
| Yeast | N | % |
|---|---|---|
| C. albicans | 1 | 1.35 |
| C. parapsilosis | 39 | 52.70 |
| C. tropicalis | 6 | 8.10 |
| C. guilliermondii | 4 | 05.40 |
| C. krusei | 1 | 1.35 |
| C. glabrata | 1 | 1.35 |
| C. lusitaniae | 1 | 1.35 |
| R. mucilaginosa | 10 | 13.50 |
| R. glutinis | 9 | 12.20 |
| T. inkin | 2 | 2.70 |
| Non-C. albicans Candida yeasts | 73 | 98.60 |
| Total | 74 | 100.0 |
C.: Candida, R.: Rhodotorula, T.: Trichosporon.
Distribution of the isolated yeasts by age group in 83 patients attending the dermatology outpatient clinic of the Hospital São Paulo, UNIFESP, Brazil (June 2006–April 2007).
| Yeast | Age range (years) | |||||
|---|---|---|---|---|---|---|
| Young | Adult | Elderly | ||||
| 15–39 | 40–63 | 64–87 | ||||
| n | % | n | % | n | % | |
| C. parapsilosis | 6 | 31.6 | 19 | 38 | 14 | 35.9 |
| C. tropicalis | – | 5 | 10 | 1 | 2.6 | |
| C. guilliermondii | 1 | 5.3 | – | 3 | 7.7 | |
| C. albicans | – | 1 | 2 | |||
| C. krusei | – | – | 1 | 2.6 | ||
| C. glabrata | – | 1 | 2 | – | ||
| C. lusitaniae | – | – | 1 | 2.6 | ||
| R. mucilaginosa | 2 | 10.5 | 4 | 8 | 4 | 10.2 |
| R. glutinis | 3 | 15.7 | 3 | 6 | 3 | 7.6 |
| T. inkin | 1 | 5.3 | – | 1 | 2.6 | |
| Negative | 6 | 31.6 | 17 | 34 | 11 | 28.2 |
| Total | 19 | 100.0 | 50 | 100 | 39 | 100.0 |
C.: Candida, R.: Rhodotorula, T.: Trichosporon.
The isolation of yeasts in men and women appeared uneven. Yeasts isolated from men included C. parapsilosis (39.2%), C. tropicalis (13%), R. mucilaginosa (8.7%), and R. glutinis (21.7%). Yeasts isolated from women consisted of C. parapsilosis (35.3%), C. guilliermondii (4.7%), C. tropicalis (3.5%), C. albicans, C. krusei, C. glabrata, C. lusitaniae (1.2% each), R. mucilaginosa (9.4%), R. glutinis (4.7%), and T. inkin (2.3%). A wider variety of yeast isolates was observed among the female population of white ethnicity. Domestic activity was prevalent among the reported occupations.
The patients were analyzed with respect to behavior and sexual behavior. The number and species of the yeasts isolated were higher in sexually active individuals than in inactive individuals. A greater quantity and variety of species of yeasts were observed among those patients with hypertension and hypertension associated with diabetes. As a result of our intensive study, we found no statistically significant differences between the variables studied and the yeasts isolated in this investigation.
DiscussionInvasive fungal infections have been recognized as a significant cause of morbidity and mortality in neutropenic patients. Recent advances in medicine have resulted in increased survival of patients with debilitating diseases such as AIDS, cancer, organ transplantation and diabetes, marking the appearance of a new patient population that, due to immunosuppression and longevity, is more susceptible to infections caused by emerging opportunistic pathogens.23,33
The human skin microbiota consists of a wide variety of fungal agents that may become pathogenic to their hosts. Among the risk factors for infection, colonization is the most important because it places the susceptible patient in direct contact with the infectious agent.26 National and international literature on superficial fungal microbiota are quite scarce. The relationship between infection and simple colonization is sometimes underestimated by studies reporting cases of opportunistic infections. The aim of this study was to isolate and identify yeasts composing the mycobiota of the genital skin region of 83 patients attending the dermatology service at the Hospital São Paulo, Universidade Federal de São Paulo, SP, Brazil.
In this study, we isolated C. albicans (n=1), C. tropicalis (n=6) and C. krusei (n=1) using the chromogenic medium CRHOMagar™ Candida™. The color expressed by these isolates was consistent with those described by the manufacturer, and their identity was confirmed using additional tests. Other isolated yeasts displayed a lavender color that is typical of other Candida species plated on chromogenic medium, as suggested by some authors.13 The isolates were identified using standard protocols, and their identification was confirmed using a semi-automated method. C. parapsilosis, C. guilliermondii, C. glabrata, and C. lusitaniae were the other Candida species identified. In addition, R. mucilaginosa, R. glutinis and T. inkin were identified using the commercial test ID 32 C. The isolates obtained in this study correspond to those found by other authors investigating cases of superficial infections.1,5,7 Although 23 (27.7%) patients had reported abnormal genital itching, no vaginal or vulvar signs of infection were detected when they were submitted to gynecological examination.
Candida species are colonizing agents that may produce superficial and systemic fungal infections in immunocompetent and immunocompromised patients, respectively. C. albicans is thought to be the most frequently isolated yeast from human skin and mucous membranes; however, only one isolate of C. albicans was obtained from the genital skin region of the 83 patients studied. Abia-Bassey and Utsalo1 analyzed the same site in 1921 patients in Nigeria and collected 103 samples of C. albicans. In that study, the species was considered prevalent in the evaluated population.1,5,30 Although the literature still considers C. albicans to be the most common pathogen in cutaneous candidiasis, non-C. albicans Candida species have emerged with significant relevance in cases of invasive infections and vulvovaginitis. In our study, those species accounted for 70.2% of the Candida isolates obtained. The distribution was as follows: C. parapsilosis (52.7%), C. tropicalis (8.1%), C. guilliermondii (5.4%), and C. krusei, C. glabrata and C. lusitaniae (1.35% each).
C. parapsilosis was the most frequent Candida isolate obtained from the patients studied. According to Moreira,21 colonization by this species occurs during the postnatal period, which is a later stage than when the individual is colonized by C. albicans. Thus, horizontal transmission (person–person) is the major route of colonization by C. parapsilosis, while vertical transmission (mother–child) is more common with C. albicans, which is found earlier in the microbiota of the newborn. During youth and throughout life, the host is susceptible to changes in the prevalence of microorganisms that comprise its normal microbiota. In Brazil, a growing number of infections caused by non-C. albicans Candida yeasts have been associated with C. parapsilosis.25
Current studies show that C. tropicalis, C. guilliermondii, C. krusei, C. glabrata, and C. lusitaniae, which were also isolated in this study, have been diagnosed in opportunistic infections. This phenomenon has been associated, in part, with a reduced susceptibility of the species to antifungal drugs.5 Several studies have reported the resistance of C. krusei and C. glabrata to azoles. The increased frequency of non-C. albicans Candida species certainly reflects a change in the microbiological profiles of patients in the new millennium.7,12,19,20 The female population (n=67) showed a greater variability of yeast species than the male population (n=16). The genus Candida was prevalent in females, who naturally have a greater predisposition to developing infections of the skin and mucous membranes.7 Moreover, not even one Malassezia species was isolated in this study. However, future studies employing more suitable culture media for this purpose may clarify some important aspects of the epidemiology of pityriasis versicolor and malasseziosis on patients in the new era.14,32
Non-Candida yeasts accounted for 28.4% of the isolates in this study. This group includes R. mucilaginosa (13.5%), R. glutinis (12.2%) and T. inkin (2.7%), yeasts that are currently considered emergent and opportunistic fungal pathogens in immunocompromised patients.16,34Rhodotorula species are very abundant in natural springs and on wet surfaces but are also part of the normal microbiota of the skin. Ferrazza et al.7 isolated Rhodotorula species from the vaginal skin of patients with and without symptoms of vaginitis, so it may be inferred this yeast is part of the normal microbiota of the skin of the genital region in humans.7 Holanda et al.12 also isolated Rhodotorula sp. as an agent of vaginitis and claimed that after decades of dominance of C. albicans, other species such as those of Rhodotorula have been identified as emerging clinical agents.12R. glutinis, a yeast associated with invasive infections in immunocompromised patients, was isolated from male patients.28 The use of a central venous catheter for long periods and organ transplantation are the main risk factors for infection by these yeasts.28,34
The genus Trichosporon is a basidiomycetous yeast, like Rhodotorula and Cryptococcus, that has been associated with superficial and invasive infections. In one study, T. inkin was isolated from the genital skin region of two patients without clinical signs of superficial infection (white piedra). Similar results were found by other authors who also reported the isolation of Trichosporon spp. from the perigenital skin of healthy individuals.1,6,11 The two isolated specimens of T. inkin were obtained from two female patients, which contradicts the traditional view that this genus is restricted to the male perigenital microbiota.6,31 Two other isolates of T. inkin were also obtained from the perigenital region of two females surveyed in Nigeria.1 The prevalence remains low for both sexes when the frequency is compared to other yeasts such as Candida spp.7
The isolates obtained in this study and the characteristics presented by the studied population were statistically analyzed to determine their significance and establish relationships among them. This was not possible because of the limited sampling. Thus, the data were only compared, and no epidemiological significance was performed because the assessment would require a larger sample size.
When the yeasts were compared between age groups, a larger number of isolates were observed in the age group comprising those aged 40–63 years. In contrast, a greater variety of isolates was observed in older subjects (those within the age group from 64 to 87 years). One possible explanation for the observation that older individuals had a greater variety of yeasts is that these patients had relatively weak immune systems, predisposing them to colonization and pathogenicity of infectious agents. However, it is important to note that this problem may also be related to the number of individuals in each age group: 15–39 years (n=14), 40–63 (n=38) and 64–87 (n=31), as demonstrated by statistical analyses.2
A greater quantity and variety of specimens were obtained from yeast cultures collected from the Caucasian population. This variety may be attributed to females because this population had the most representative number (n=58). R. mucilaginosa was isolated only from individuals of white ethnicity. T. inkin was isolated only from women and was exclusively sampled from patients of black ethnicity, which may suggest a healthy carrier condition in these individuals, although more studies are necessary to confirm this hypothesis.1
When considering the professional activities of the individuals included in this study, there was a greater yeast count and variety of species in the population employed in domestic services, which accounted for a large part of the studied population (72.3%). The tissue maceration resulting from physical and chemical aggression is inherent to this population and may predispose the individuals to colonization and infection.5 Sexual behavior did not constitute a significant variable when considering the types of yeast isolated.
The most common underlying diseases were hypertension (45.8%), hypertension+diabetes (13.3%), organ transplantation (6%) and diabetes (1.2%). Hypertensive individuals (n=38) presented a greater quantity and variety of yeasts isolated from the genital region when compared to the other groups. No yeasts were isolated from patients with diabetes or organ transplantation as the underlying disease. Although there are no concrete references about the potential influence of hypertension on the predisposition to fungal infections, it is noteworthy that even among individuals with diabetes associated with hypertension it was possible to isolate emerging pathogenic yeasts such as R. mucilaginosa and T. inkin.17 The literature mentions a predisposition of diabetics to the development of complications from mycotic infections.1,2 However, there are no studies in the literature that correlate hypertension and colonization by pathogenic yeasts. Thus, further studies on this association should be encouraged. No statistically significant differences were observed between yeasts and gender, age, ethnicity or sexual habits. However, a larger population should be analyzed to assess possible epidemiological correlations between colonization by pathogenic yeasts and risk characteristics.
ConclusionBased on the findings of this investigation, it was concluded that the fungal microbiota of the skin of human beings comprises a wide variety of yeasts, although the factors that determine the permanence of these agents are not yet completely understood. Some yeasts found on the skin of the genital region are considered emergent and opportunistic, which results in a risk of infection to their hosts, especially immunocompromised patients. The high prevalence of non-C. albicans Candida species, such as C. parapsilosis, suggests that the microbiological profile of patients in the new century has changed, consistent with consecutive reports of infection described in the literature and strengthening the theory that the microbiota is the main source of infection in susceptible individuals.
Despite failing to establish a statistically significant relationship between the yeasts identified and the different variables considered in this study due to the limited number of patients included in the investigation, we hope that the findings of this research, although modest, may contribute significantly to the epidemiological study of both superficial and deep seated mycoses that affect healthy and immunocompromised patients. New studies should be developed to clarify the role that some characteristics, such as occupation, play in the risk of fungal infections.
Authors declarationThe authors declare that the contents of this article are original and have not been published previously or submitted to or subjected to consideration by any other publication, either in whole or in part. The authors also declare that there are no financial or other aspects that could be considered as a conflict of interest.
FAPESP (Proc. n°: 06/51677-7) provided financial support.