Spermatogonial stem cells (SSCs) are able to form embryonic stem-like cells (ES-like cells) and embryonic bodies (EBs). Low-intensity ultrasound stimulation (LIUS) has positive effects on the growth and differentiation of the different cells. In this study, we tried to investigate the effects of LIUS on SSC differentiation to ES-like cells.
Materials and methodsSSCs were isolated from neonatal mice and their identification was confirmed by tracking of PLZF, Oct-4, and C-Kit proteins. The SSCs and Sertoli cells were co-cultured in DMEM/F12 supplemented with 15% FBS and LIF. SSCs stimulated by LIUS with 200mW/CM2 intensity. Characterization of obtained ES-like cells was confirmed with Sox2, Oct-4, and SSEA-1 immunofluorescence staining. Also, real-time PCR was performed to analyse the expression of c-Myc and Nanog genes in ES-Like Cells and Stra8, Piwil2 and Plzf genes in SSCs after 21 days of the in vitro culture.
ResultsOur results showed c-Kit, PLZF and Oct-4 proteins were expressed positively in SSCs and Sox2, Oct-4, SSEA-1 in the ES-like cells by immunocytochemistry. The results of flow cytometry showed a significant increase in expression of c-Myc and Nanog in ES-like cells compared to SSCs (p<.05), whereas the Stra8, Piwil2, and Plzf became down-regulated during 21 days of culture. ES-like markers cell SSEA-1, Sox2 and Oct-4 were increased in the LIUS group compared to the control group (p<.05).
ConclusionThe results indicated that ES-like cells with pluripotency characteristics were derived from SSCs.
Las células madre espermatogoniales (SSC) pueden formar células embrionarias de tipo madre (células similares a ES) y estructura de cuerpos embrionarios (EB). La estimulación por ultrasonido de baja intensidad (LIUS) tiene efectos positivos sobre el crecimiento y la diferenciación de las diversas células. En este estudio, tratamos de investigar los efectos de LIUS en la colonización y la viabilidad de células similares a ES.
Materiales y métodosLas SSC se aislaron de ratones neonatales y su identificación se confirmó mediante el seguimiento de las proteínas PLZF, Oct-4 y c-kit. Las SSC y las células de Sertoli se cultivaron conjuntamente en DMEM/F12 suplementado con 15% de FBS y LIF. Las SSC estimuladas por LIUS continúan con una intensidad de 200 mW/cm2. La caracterización de las células similares a ES obtenidas, se confirmó con tinción con inmunofluorescencia Sox2, Oct-4 y SSEA-1. Además, se realizó una PCR en tiempo real para analizar la expresión de los genes c-Myc y Nanog en células similares a ES y genes Stra8, Piwil2 y Plzf en SSC, después de 21 días de cultivo in vitro.
ResultadosNuestros resultados mostraron que las proteínas c-kit, PLZF y Oct-4 se expresaron positivamente en SSC y Sox2, Oct-4, SSEA-1, en las células similares a ES por inmunocitoquímica. Los resultados de la citometría de flujo mostraron un aumento significativo en la expresión de c-Myc y Nanog en células similares a ES, en comparación con las SSC (p < 0,05), mientras que Stra8, Piwil2 y Plzf disminuyeron en 21 días. Las células marcadoras similares a ES, SSEA-1, Sox2 y Oct-4 se incrementaron en el grupo LIUS, en comparación con el grupo control (p < 0,05).
ConclusiónLos resultados indicaron que las células similares a ES, con características de pluripotencia, se derivaron de las SSC.
SSCs (spermatogonial stem cells) are the base of sperm production and male fertility. Similar to other stem cells, SSCs are rare in testis tissue, representing only 0.03% of all germ cells in rodent testis.1 Neonatal and adult mice SSCs are able to develop into multipotent cells when cultured under specific conditions in vitro.2–5 In addition, the efficient and long-term culture of SSCs is now possible allowing sufficient cells for regenerative therapy to be generated.6 Recently, some groups of researchers have independently found successfully formation of ES-like cells (embryonic stem-like cells) by exposing human testicular cells to specific ES conditions in vitro.7–9 Another study shows that ES-like cells derived from testis are capable to differentiate into neuroepithelial like cells that may provide a cellular reservoir usable for neurodegenerative disorders.10 Pluripotency potential of these ES-like cells has been demonstrated by their capacity to contribute to germ-line chimeras and differentiation into tissues that belong to all three embryonic germ layers. ES-like cells express a blend of germ line and embryonic stem cell markers.2,3,11,12 Recent studies show that new methods such as mechanical waves, especially LIUS (low-intensity ultrasound stimulation) have an influence on the proliferation and colony formation of cells. Now, the positive effect of these waves enhances growth, proliferation, differentiation and etc. These waves increase the proliferation of human umbilical cord-derived mesenchyme stem cells,13 hematopoietic stem cells14 and Adipose-derived stem cells.15 Also, these waves increase the proliferation of chondrocytes16,17 and fibroblast.18 Another study shows that The LIUS of mouse SSCs increased of Integrin-α6 and Integrin-β1gene expressions after Continues and pulsed LIUS.19 In relation to previous results showing the pluripotency of ES like cells derived from testis, the aim of this study was to evaluate the differentiation capacity and alteration in gene expression patterns during the in vitro differentiation of mouse SSCs into ES like-cells following exposure to LIUS.
Materials and methodsGeneration and culture of ES-like cellsIn this experimental study, ES-like cells were generated from 3 to 5 days neonatal mouse testis.20 All of procedures were according to animal ethical clearance of Tarbiat Modares University. Both testes of neonatal mouse were removed and were decapsulated. After mechanical dissection, testes were done under two-step enzymatic digestion with DMEM/F12 (Pan-Biotech, UK) which contained; 0.5mg/ml Collagenase-Dispase (Sigma, USA), 0.5mg/ml Trypsin (Sigma, USA), and 0.05mg/ml DNase (Sigma, USA) at 37°C for 30–45min. The obtained dissociated cells that included SSCs and Sertoli cells were incubated in differential plate method for SCs (Spermatogonial Cells) purification. After SCs purification, these SCs were co-cultured with sertoli cells at 34°C and 5% CO2, and in a humidified atmosphere, in DMEM/F12 (Pan-Biotech, UK) supplemented with 15% FBS (Gibco, UK), 1mM l-glutamine (Gibco, UK), 0.1mM Nonessential Amino Acids (Gibco, UK), 0.1mM β mercaptoethanol (Sigma–Aldrich), and 1000units/ml LIF (Sigma–Aldrich).10,20,21
Ultrasound stimulation designOur ultrasound device (PHYSIOMED, Germany) was designed with the following parameters: frequency: 1MHz, intensity: 200mW/cm2, Time: 200s, duration: 5 days. These design parameters were chosen based on the previous biological and clinical studies.14,19 LIUS applied by a transducer to SSCs cultured in an enclosed sterile conventional 3.5cm tissue culture plate in an incubator with 32°C temperature and 5% CO2. It was transmitted through the bottom of the well via coupling gel between the transducer and the Plate.19 After LIUS stimulation, the cells cultured for 21 days. To investigate the proliferation rate, the mean number of whole cells per volume on day 7th was considered. The colonization of ES-like cells was assessed on 7th day with number and diameter of colonies by Invert-phase microscope (Zeiss, Germany) equipped with ocular grid.
Quantitative real-time polymerase chain reaction (RT-qPCR)To confirm generation of ES-like cells, Real-Time PCR was performed to analyze the expression of a subset of pluripotency markers as well as germ cell-specific genes. Nanog and C-Myc were assessed as pluripotency genes in ES-like cells and Stra8, PLZF, and Piwill2 were assessed in SSCs as stem cell markers on day 21. Total RNA was extracted from the cells using RNX PlusTM (Cinnagen, Iran) according to the manufacturer's recommendations. In order to remove genomic contamination, RNA was treated with DNase I using a kit from Fermentas (Fermentas, Lithuania). Concentrations of RNA were determined using UV spectrophotometer (DPI-1, Qiagen). The cDNAs were synthesized from 500ng DNase treated RNA samples with a Revert Aid TM first-strand cDNA synthesis kit (Fermentas, Lithuania) using oligo dT primers. For PCRs, primers were adapted from others21–25 and synthesized by Cinnagen Company (Iran). PCRs were carried out using Master Mix (Cinnagen, Iran) and SYBR Green I (Fluka, Switzerland) in a Rotor-Gene 3000 thermo cycler (Corbett, Australia). The PCR program started with an initial melting cycle, 4min at 94°C, to activate the polymerase and followed by 40 cycles as follows: a melting step (20s at 94°C), an annealing step (30s at 57°C), and an extension step (30s at 72°C). After completing the PCR run, the quality of the reactions was confirmed by melting curve analyses. Efficiency was determined for each gene using a standard curve (the logarithmic dilution series of testis cDNA). For each sample, the reference gene (b2M) and the target gene were amplified in the same run. Ratio of gene expression was determined using the Comparative CT (cycle threshold) method.
Immunocytochemistry analysisFor immunocytochemistry, cells in each group were washed with phosphate-buffered saline (PBS) at PH 7.4 and fixed in 4% par formaldehyde (PFA) for 30min at room temperature (RT). Fixed cells were permeabilized with 0.2% Triton X-100 (MP Biomedicals, USA) for 10min at RT followed by 3 times washing with PBS. To block unspecific binding of the antibody, cells were incubated with 10% goat serum (Vector Laboratories, USA) for 30min at RT. Then, cells were incubated with primary antibodies overnight at 4°C. Primary antibodies including: Rabbit Polyclonal antibody Oct-4, SSEA-1, Sox2; the following day, cells were washed twice with PBS and incubated with the appropriate secondary antibody. Antigens were visualized using appropriate fluorochrome-conjugated secondary antibodies including: PE: Donkey to Rabbit IgG (200:1; Abcam) and FITC: Goat anti Rabbit IgG (200:1; Abcam). After two times washing with PBS for 5min, cells were mounted with 4, 6-diamidino 2-phenylindole (DAPI)/PBS and Images were captured with an Olympus phase contrast microscope (BX51, Olympus, Tokyo, Japan).
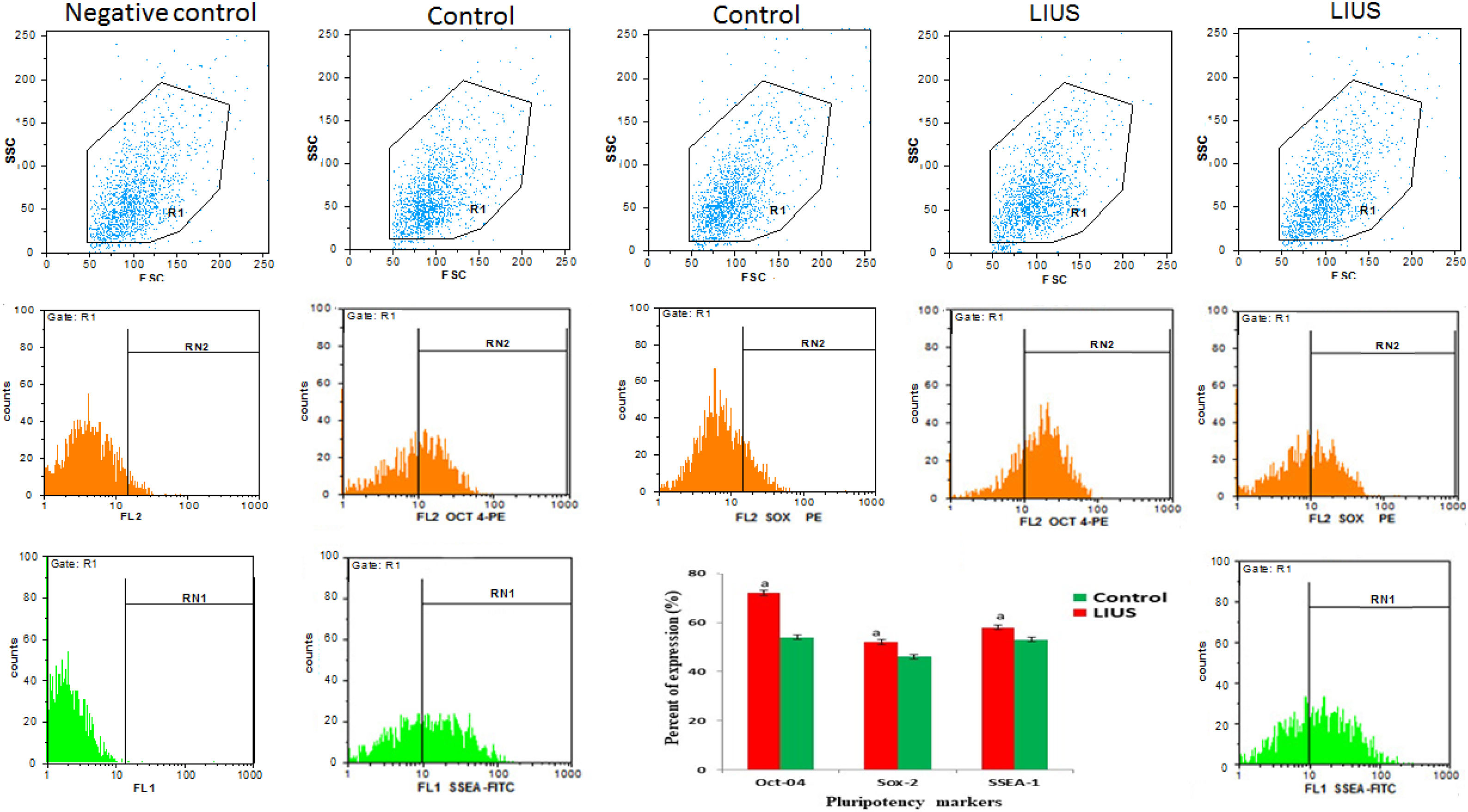
Flow cytometryCells were washed with PBS and treated with trypsin/EDTA (Sigma, USA) for 5min to form single cells. The suspension collected by centrifugation at 2000 RPM for 5min, then were fixed in 4% PFA for 10–15min at 4°C for stabilizing proteins, followed by permeablizing of cells in detergent (0.2% Triton X-100). Fixation/permeabilization procedures had to be on ice. Then, it was washed by adding 2ml of PBS, and centrifuged at 2000 RPM for 5min. The supernatant was discarded and the cells were suspended in goat serum for 45min to block nonspecific antibody binding. Cells were labeled with primary antibodies overnight at 4°C in dark room. Antibodies including: Rabbit Poly Colonal Antibody Oct-4, SSEA-1, And Sox-2: (1:50; Abcam) and Rabbit Poly Colonal Antibody Oct-4, PLZF, C-Kit: (1:50; Abcam). Then they were washed three times by PBS. The following day, cells were incubated with the appropriate secondary antibody and antigens were visualized using appropriate fluorochrome-conjugated secondary antibodies included: PE, Donkey to Rabbit IgG (200:1; Abcam) and FITC, Goat anti Rabbit IgG (200:1; Abcam). This incubation had to be done in dark, followed by three times washing by PBS. Analysis was performed as soon as possible using PARTEC CyFlow space flowcytometer (GERMANY) and FlowJo software (WinMDI 2.9, J. Trotter).
Data analysisEach data point represents the average of three separate experiments with three repeats in each experiment. The one-way ANOVA and Tukey post hoc tests used to determine the statistical significance of observed differences in the mean values among our groups using the SPSS statistical software (SPSS 16.0 Production Mode Facility). P value less than 0.05 was considered significance.
Ethical considerationCurrent study was conducted under the protocol approved by the animal experimentation committee of Medical Sciences Faculty in Tarbiat Modares University.
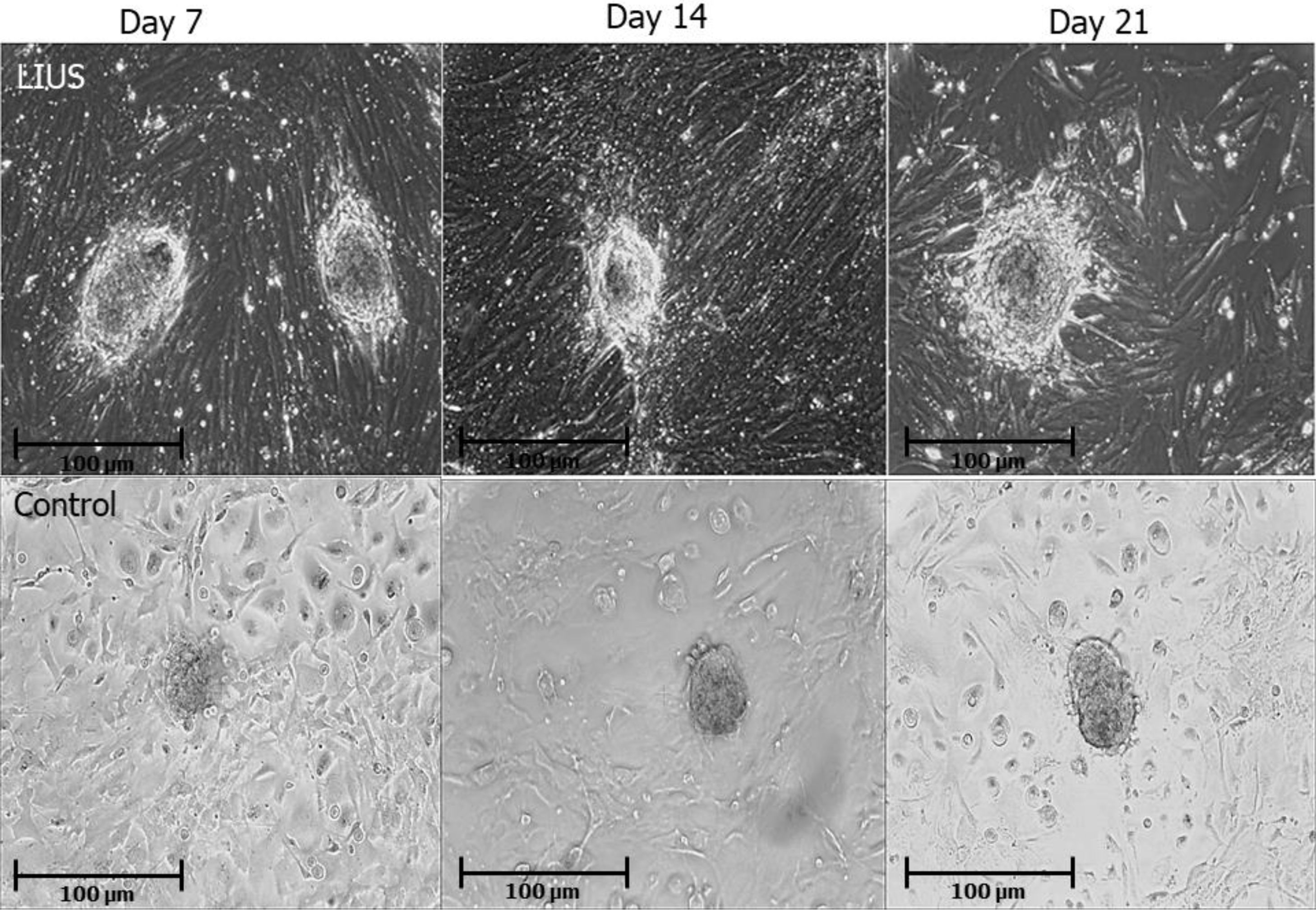
ResultsAfter purification of SSCs and sertoli cells, we culture these cells as co-culture. Most of sertoli cells were attached to the growing surface and SSCs were attached n the sertoli cells 48 hr of post plating. These cells proliferated and created a monolayer of cells. Our result showed after several days, SSCs formed colonies and these colonies were passaged every 7 days (Fig. 1). After ultrasound stimulation, ES-like colonies with sharp edge that resembled ES-cell appeared within 3 weeks (at passages 3rd). ES-like cells were confirmed by immunoflorescent & Immunocytochemistry staining and quantitative real-time q-PCR analysis.
SSCs were culture on the sertoli cells feeder layer in two conditions: LIUS treatment (upper row) and control (without LIUS treatment) (lower row). The morphology of ES-Like cells colonies derived from SSCs On in LIUS group were more shaped and expanded with sharp edge, which is not similar to control group.
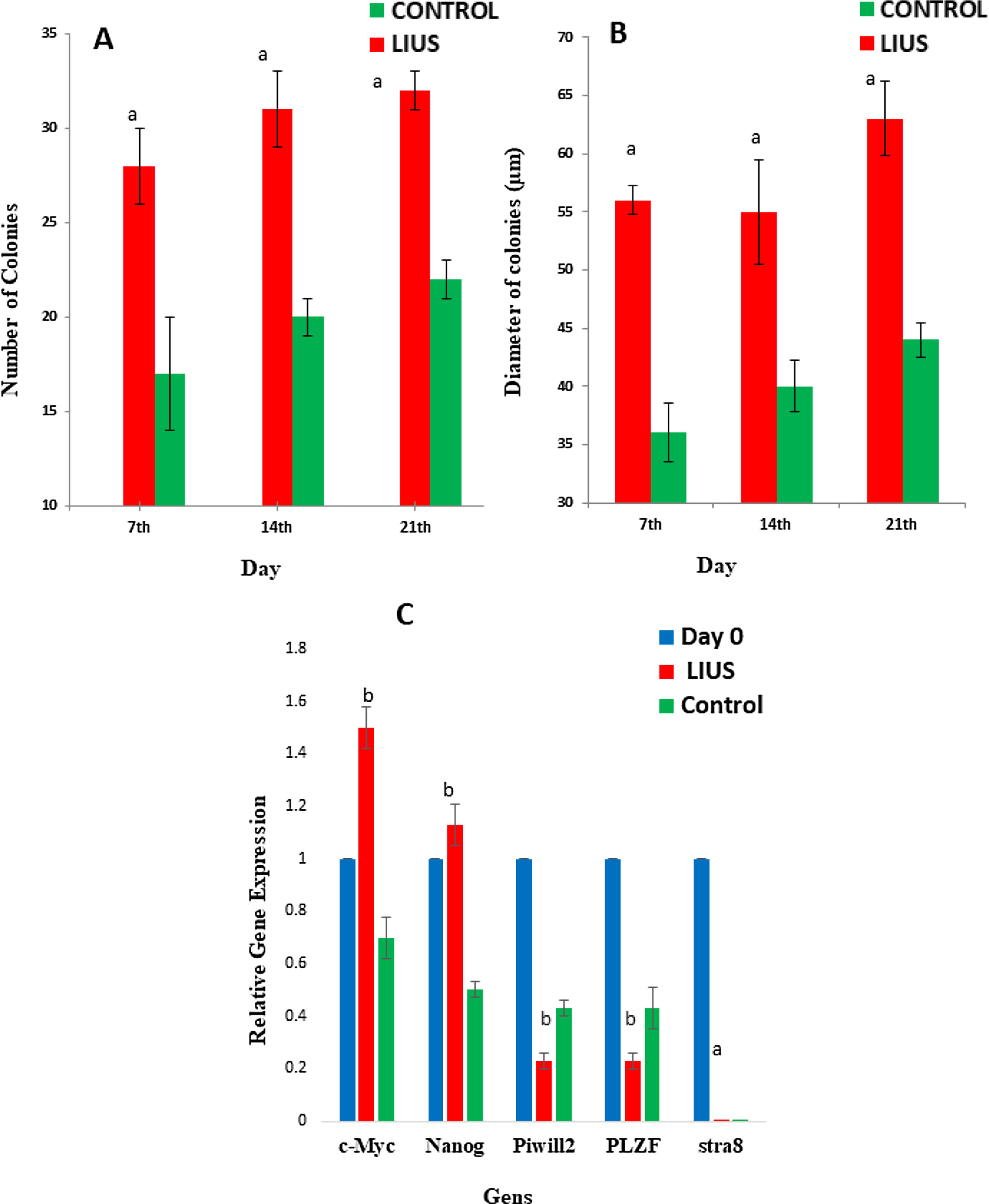
On days 7th, 14th, 21st the findings showed that the colonization increased in LIUS groups compared to control (without LIUS treatment) groups (P<0.05). The average number of colonies in 7th, 14th, 21st days in LIUS group was 28±3, 3±1, 32±1 and in control group were 17±2, 202, 221, respectively. Regarding the diameter of colonies, there were significant differences between groups (P<0.05) on days 7th, 14th, 21st (Fig. 2 A). Also, the average diameters of colonies in LIUS groups were 56±1/2, 55±4/5, 63±3/2 and in control group were 36±2/5, 40±2/2, 44±1/5 (Fig. 2 B).
Evaluation of colonization in LIUS and control groups in different days includes the number of colonies (A) and the diameter of colonies (B) was showed. And (C) is the ratio of pluripotency genes expression: Nanog, C-Myc, and spermatogonial genes: Stra-8, Plzf, and Piwill2 in ES-Like cells in LIUS and control groups on day 21st. Relative expression was shown as the ratio of target gene to 0th day. (a) Significant differences compared to control group (P<0.05). (b) Significant differences compared to control group and day 0 (P<0.05).
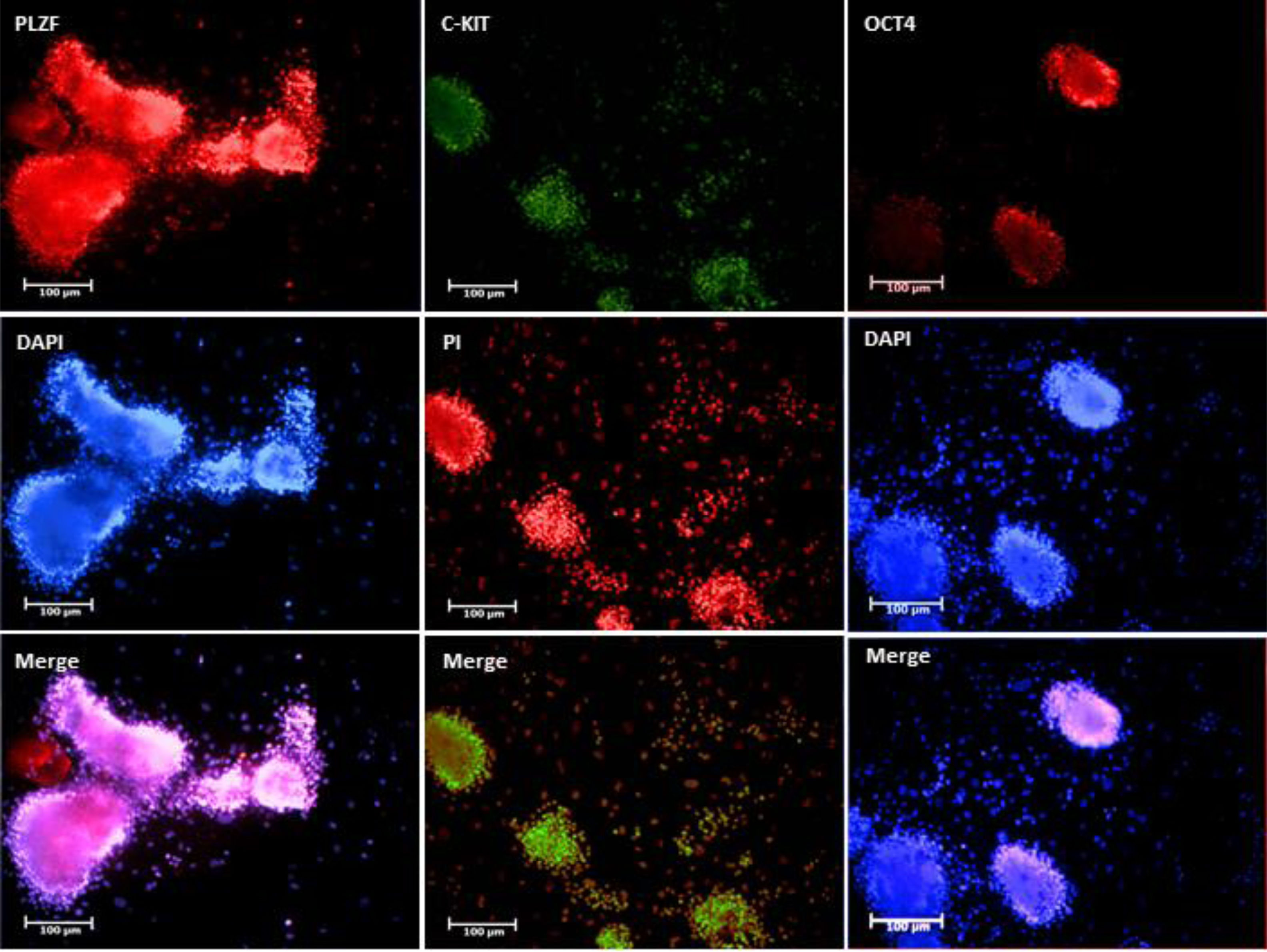
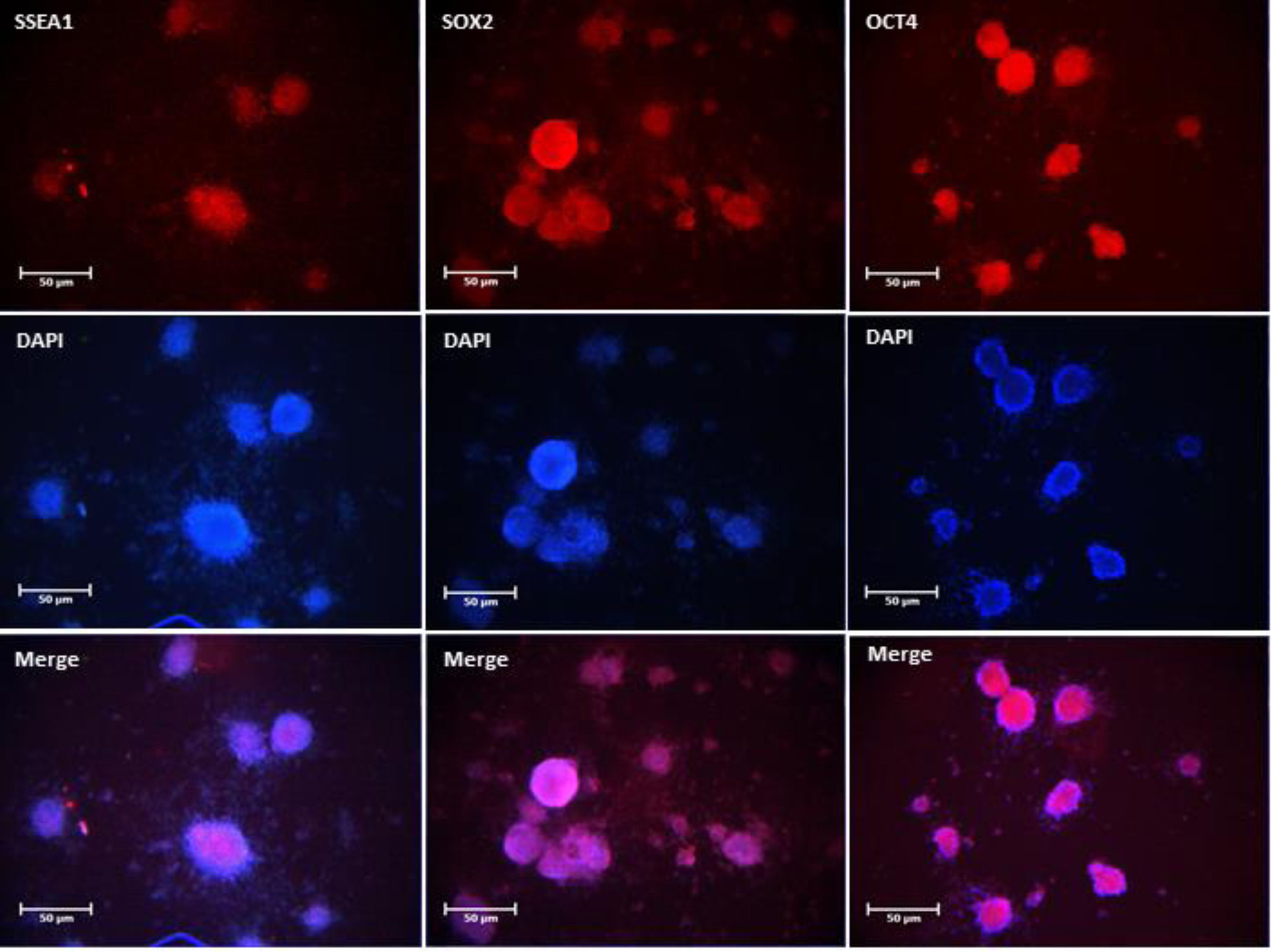
Isolated SSCs were positive to PLZF, Oct-4and c-Kit protein detecting respectively (Fig. 3). The obtained ES-like cell colony expressed the SSEA-1, Sox2 and Oct 4 marker (Fig. 4). On day 21st the findings showed that the expression of a subset of pluripotency markers increased in LIUS group compared to control groups (P<0.05) (Fig. 5). Result showed that in control group (54/66±4/4) % of ES-like cells were positive to Oct4 and (72/92±5/4)% in LIUS groups. Also, (46/60±3/9)% of ES-like cells were positive for sox2 in control group and (52/02±4/4)% were positive in LIUS group. Furthermore, (53/95±4/3)% of ES-like cells had positive reaction for SSEA-1 in control groups and (58/71±5/4) % in LIUS groups (Fig. 5). The whole results of flowcytometery were presented in Fig. 5.
The whole results of Flow Cytometry of ES-Like cells on 21st day for Oct4, Sox2, SSEA-1 markers as pluripotency potential of these cells. The results were shown in both LIUS and control groups. The quantitative results of flow-Cytometry were shown in chart. (a) Significant differences compared to control group (P<0.05).
These results demonstrated that the expression of pluripotency genes Nanog, C-Myc, Plzf and Piwill2 had significantly increased in LIUS group's compared to control groups on day 21st (P<0.05). The expresión of Stra8, gene had no significant differences between groups (P>0.05) (Fig. 2D).
DiscussionNew researches have shown the derivation of pluripotent stem cells from neonatal and adult mouse testis that can differentiate to all cell types.22–24 These studies proved that ES-like cells were generated without genetic manipulation but under specific culture conditions.10,20 Our research demonstrated that ES-like cells originated from SSCs, express high levels of the pluripotency genes Nanog and C-Myc. Furthermore, germ cell-specific genes such as Stra8, Mvh, and Piwill2 are downregulated which confirms pervious results.2,10,20 Our results showed that ES-like cells derived from SSCs share many molecular and cellular characteristics with embryonic stem cells; on cellular level, these cells have the same morphology with the sharp edged colonies and can form embryonic body structure after transfer to bacteriological Plate.25 In this study, we investigated the effects of LIUS on germ cell specific and pluripotency genes as same as colonization, proliferation and survival rates of cells during 21 days of culture in vitro. Our results showed that SSCs can proliferate and form colonies on the feeder layer of sertoli cells in related to previous studies.2,10,20 Consistent with present data, an earlier study demonstrated that stimulating osteoblasts and chondrocytes with LIUS transiently increased the expression of specific integrins, namely α5 and β1 integrin26 and alpha 6 and beta1 integrin.19 Our findings obtained from in vitro study strongly revealed that LIUS could improve the number of mouse ES-like cells and their colonies. While viability rates in LIUS treatment decreased in compared to control group (P<0.05). Mohaqiq et al (2018) stimulated mouse SSCs by LIUS and LIUPS (Low Intensity Ultrasound Pulsed Stimulation) and reported that the proliferation and colonization rates were increased during 21 days of culture in vitro. Integrin family proteins provide a link between extra cellular matrix and intracellular cytoskeletal components as well as actin filaments. Integrin proteins were thought to have function by undergoing conformational changes that activate them and reveal their ligand binding site. Integrins can bind to cytoskeletal components and other signaling molecules, while they activate several intracellular signaling pathways in response to mechanical stress such as sound waves, thus enabling the cells to react to changes in their physical environment.27 Integrin proteins family act as sensitive mechanoreceptors on the surface of cells. Ultrasound waves produce mechanical stimulation which has been transferred to adherent cells via interactions with the ECM. Increase in integrin expression was observed in the cells after LIUPS treatment. It was shown to activate a number of downstream kinases including focal adhesion kinase (FAK), phosphatidylinositol 3-kinases (PI3K) and mitogens activate protein kinase,28 indicating that LIUS with their effect on transmembrain proteins, such as integrins, might be able to stimulate more SSCs to divide and it can enter these cells to mitotic process through regulation of self-renewal or differentiation pathway. Our results of LIUS effects on ES-like cells showed that these waves had a useful short time effect on proliferation and colonization during 21 days culture.
ConclusionWe have demonstrated a novel continuous LIUS mediated effect on ES-like cells proliferation, colonization and survival rates during 21 days culture. We also concluded that LIUS unregulated ES-like cell genes and proteins expression.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis study is sponsored financially by the Tarbiat Modares University.
Conflict of interestWe wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
The present study is part of the MSc thesis of Anatomical Sciences at Tarbiat Modares University, Medical sciences faculty and sponsored by Tarbiat Modares University.