The introduction of alternative systems in vivo is very important for cancer patients who are treated with gonadotoxic treatment. In this study, we examine the progression of the spermatogenesis process after human spermatogonial stem cell (SSCs) transplantation in vivo and in tissue culture conditions.
Materials and methodsHuman SSCs were obtained from a Testicular Sperm Extractions (TESE) sample, and characterization of these cells was confirmed by detecting the promyelocytic leukemia zinc finger (PLZF) protein. These cells, after being labeled with Di-alkyl Indocarbocyanine (DiI), were transplanted to adult azoospermia mouse testes treated with Busulfan 40mg/kg. The host testicular tissue culture was then considered a test group and in vivo transplant a control group. After 8 weeks, immunohistochemical, morphometric and molecular studies were performed.
ResultsThe results of morphometric studies indicated that the mean number of spermatogonia, spermatocytes, and spermatids in the test groups was significantly lower than in the control group (P<0.05) and most of the cells responded positively to DiI tracing. Immunohistochemical study in both groups revealed expression of PLZF, Synaptonemal complex protein 3 (SCP3) and Acrosin Binding Protein (ACRBP) proteins in spermatogonial cells, spermatocyte and spermatozoa, respectively. Also, PLZF, Transition Protein 1 (TP1) and Tektin-1 (Tekt1) human-specific genes had a significant difference in the between test groups and control groups (P<0.05) in molecular studies.
ConclusionThese results suggest that the conditions of testicular tissue culture after transplantation of SSCs can support spermatogenesis resumption, as well as in an in vivo condition.
La introducción de sistemas alternativos in vivo es muy importante para los pacientes con cáncer que son tratados con tratamiento gonadotóxico. En este estudio, examinamos la progresión del proceso de espermatogénesis después del trasplante de células madre espermatogoniales en condiciones de cultivo de tejidos in vivo.
Materiales y métodosSe obtuvieron células madre espermatogoniales humanas a partir de una muestra de extracciones de esperma testicular y se confirmó la caracterización de estas células mediante la detección de la proteína de dedo de cinc con leucemia promielocítica. Estas células, después de marcarse con di-alquil indocarbocianina (DiI), se trasplantaron a testículos de ratón con azoospermia adulta, tratados con busulfán 40mg/kg. A continuación se consideró el cultivo del tejido testicular del huésped como un grupo de prueba, y el trasplante in vivo como un grupo de control. Transcurridas 8 semanas, se realizaron estudios inmunohistoquímicos, morfométricos y moleculares.
ResultadosLos resultados de los estudios morfométricos indicaron que el número medio de espermatogonias, espermatocitos y espermátidas en los grupos de prueba fue significativamente menor que el grupo de control (P<0/05) y la mayoría de las células respondieron positivamente al rastreo de DiI. La inmunohistoquímica en ambos grupos reveló la expresión de las proteínas de dedo de cinc con leucemia promielocítica, proteína 3 del complejo sinaptonemal (SCP3) y proteína de unión a la acrosina (ACRBP) en células espermatogoniales, espermatocitos y espermatozoides, respectivamente. Además, los genes humanos específicos de proteína de dedo de cinc con leucemia promielocítica, transition protein 1 (TP1) y Tektin-1 (Tekt1) reflejaron una diferencia significativa entre los grupos de prueba y los grupos de control (P<0/05) en los estudios moleculares.
ConclusiónEstos resultados sugieren que las condiciones del cultivo de tejido testicular tras el trasplante de células madre espermatogoniales pueden apoyar la reanudación de la espermatogénesis, así como la condición in vivo.
Human germ cells experimental manipulation is ethically restricted and the spermatogonial stem cell (SSCs) transplantation, the biological assay system necessary to evaluate stem cell activity, has not been confirmed in humans. Xenogeneic transplantation of human SSCs to testes of laboratory animals decrease ethical concerns related to human subjects and also, is an appropriated alternative option for human SSCs evaluation. Since the transplantation is best developed in the mice and is readily available, the mouse is technically the most promising recipient species for human SSCs transplantation. This point is very important in cancer patients who are exposed to gonadotoxic treatments since the risk of cells returning to cancer patients prior to treatment. In vitro culture systems that reproduce the male germ cells are various.1 This seems to be for shortening a complex process to smaller parts for experiments, manipulations, and understanding of it at the cellular and molecular level.2 In vitro cultures have a good opportunity to manipulate the paracrine environment and also to examine the effect of the role of each growth factor individually on the spermatogenesis.3 The three dimensional (3-D) testicular tissue culture systems will be appropriate for spermatogenesis progress due to the maintenance of the 3-D structure of the seminiferous tubules and interstitial tissue.4 It seems that this system can be used to induce and resume the spermatogenesis by SSCs transplantation, in order to produce mature sperm and an application that is a high-level reproductive medicine target.5,6 Reports on the potential of testicular tissue culture systems have recently begun to be published. Unfortunately, a few studies have reported tissue culture optimization. Sato et al. used immature mouse testicular tissue fragments to reach fully functional spermatozoa.7 They isolated mice germ cells, labeled with Green Fluorescent Protein (GFP) and transplanted to immature azoospermia testicular tissues by in vitro transplantation (IVT) under tissue culture conditions. After 6 weeks, they reported functional sperm extraction that could be used for assisted reproductive technology (ART). In 2013, Yokonishi et al. introduced the system of testicular tissue culture on the agarose gel with a report of sperm obtaining.8 They split the immature mouse testicular tissue into small pieces and place them on the agarose gel in culture conditions. In 2010, Gohbara et al. also reported the release of round haploid spermatids by culturing of the testicular tissue on the agarose gel.9 In another report, Sato et al. put the immature mouse testis tissue that contains only gonocytes and spermatogonial cells (SCs) precursor under tissue culture system on an agarose gel and, that leads to the full progression of spermatogenesis. They obtained yielded adult mature sperm able to fertilized by microinjection. They also placed immature mouse testicular tissue after freezing and thawing in a tissue culture system and reported complete progress in spermatogenesis.10 In the present study, human SSCs were transplanted in IVT and in vivo to mature mouse azoospermia testis and placed on an agarose gel in a 3-D tissue culture system. In the previous reports, there isn’t an achievement to the differentiation of human SSCs in xenotransplantation for different reasons. It may be related to the difference between the niche of germ cells in human and non-human primates. However, in this study, we evaluated the progress of spermatogenesis induction by human SSCs in mouse testis as a host.
Materials and methodsSample preparation and freezing-thawing protocolAccording to timeline chart of protocol (Fig. 1), Samples were obtained from the rest of the TESE of 4 azoospermia patients after the completion of the treatment. All stages of this research were based on the approval of the research ethics committee of Tarbiat Modares University with the registration ID IR.TMU.REC.1394.68. For the freezing and thawing of testicular tissues, the protocol of the Honaramooz et al.11 was used. In this way, the small parts of the testicular tissue were placed in a special freezing medium and then placed in a programmable freezer (Planner Cryo 360. 1/7-UK). The machine automatically processes all the freezing steps slowly and stepwise, and finally, the frozen tissue is ready to leave the machine and put in liquid nitrogen.
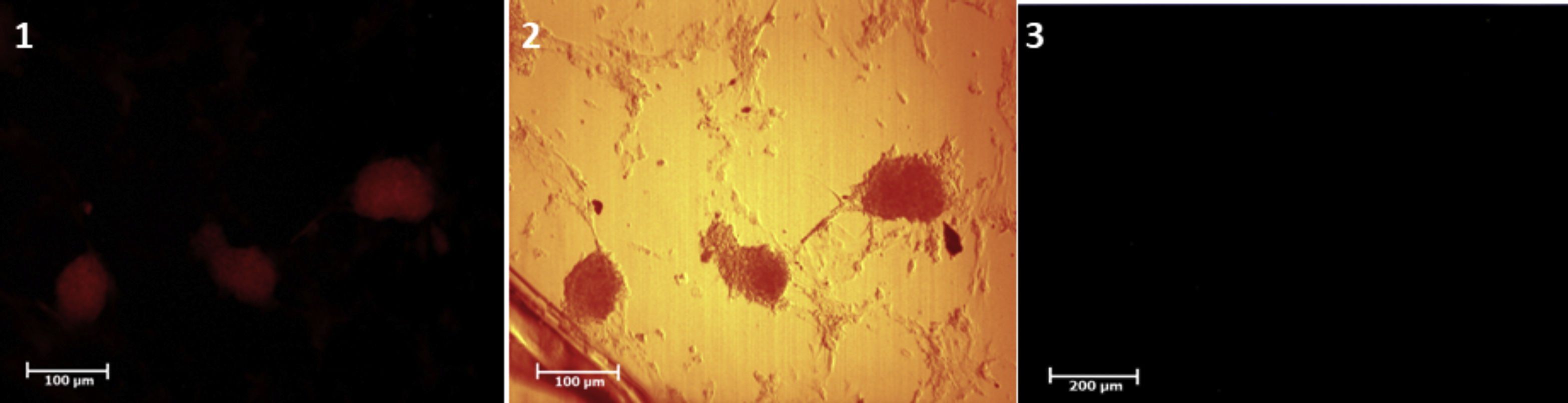
Isolation, culture and confirmation of identification of SSCsSSCs were isolated by Mirzapour et al. protocol under two steps of enzymatic digestion with trypsin (0.5mg/ml, Sigma, USA), collagenase (0.5mg/ml, Sigma, USA) and DNase (0.05mg/ml, Sigma, USA) enzymes.12 Because of the small number of SSCs present in TESE biopsy, after digestion, in order to enrich and enhance the SSCs, these cells were cultured two weeks. The identification of isolated and purified SSCs was investigated by tracing the PLZF protein as a stem cell marker in colonies derived from the cell suspension.
Preparation of agarose support layer for tissue cultureTo provide an agarose support layer we were using the Yokonishi et al. method.8 The agarose solution 1.5% (Invitrogen, USA) was prepared and then sterilized. At the time of using the 1×1×0.5mm agarose components in culture containing about 4/5 of the Alpha Minimum Essential Medium (α-MEM) (Bio-Ideal, I.R.I) containing 10% Knockout Serum Replacement (KSR) (Gibco, UK), progesterone (Invitrogen, UK) at a maximum concentration of 60ng/ml, beta-estradiol (Pepro Tech, USA) maximum concentration 30ng/ml and Epithelial Growth Factor (EGF) (Pepro Tech, USA) at a maximum concentration of 20ng/ml, Fibroblast Growth Factor (FGF) (Pepro Tech, USA) maximum concentration of 10ng/ml, Human glial cell line-derived neurotrophic factor (GDNF) (Pepro Tech, USA) maximum concentration of 10ng/ml and Leukemia Inhibitory Factor (LIF) (Royan, I.R.I) maximum concentration of 10ng/ml. The tissue parts were gently placed in the middle of the agarose layer so that they did not float. The culture medium was changed twice a week.
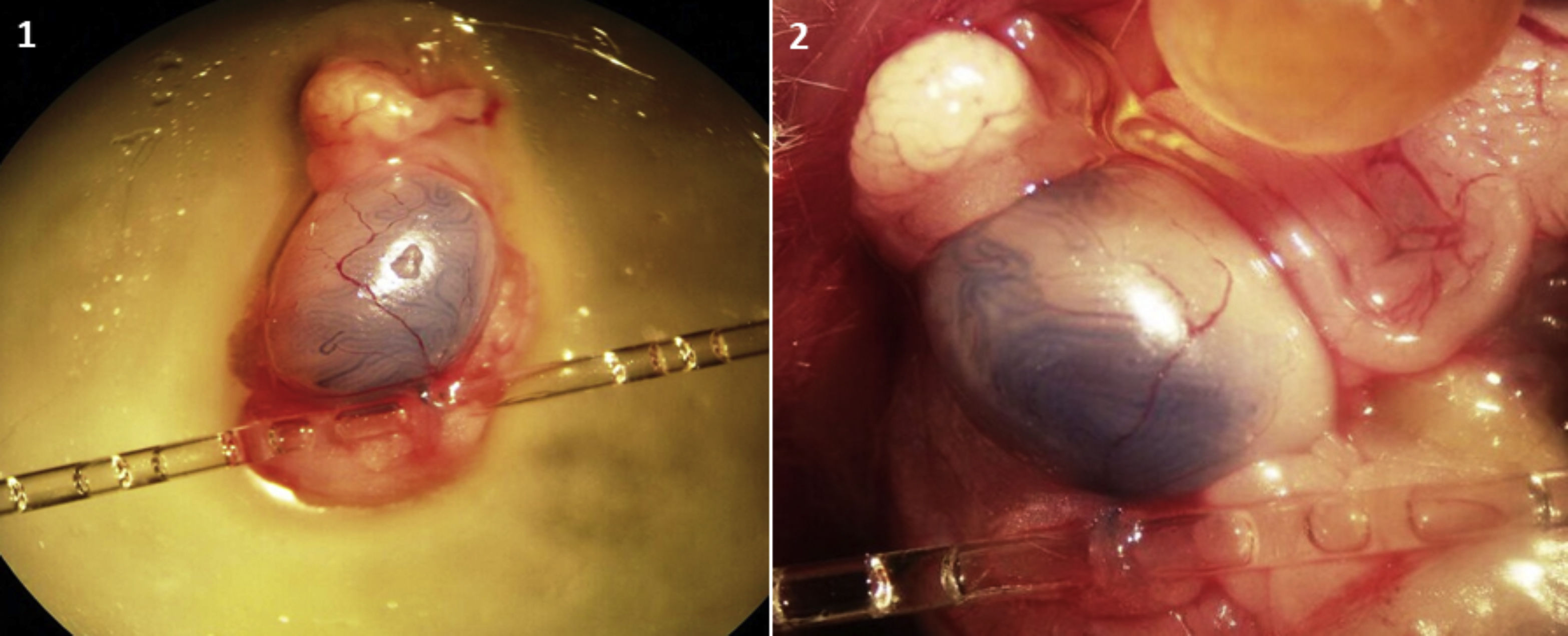
In vitro transplantation of SSCs to the testesTo detect the transplanted cells and distinct them from endogenous cells, the cells cultured with a density of about 80% before transplantation for 5min exposed to DiI color (Eugene.OR, USA) a 2μg/1ml phosphate-buffered saline (PBS) was placed at room temperature and then placed at 4°C in a dark place for 20min after 3 times washing in the medium, they were transplanted into the host testis. To prepare the mouse azoospermia model, 40mg/kg busulfan was used. This caused after 4 weeks, adult mouse testes to be virtually empty of spermatogenesis (Fig. 2). For transplantation procedure, using a glassy needle, entered to efferent ductuli and injected cells to the end of the efferent ductuli and the early ret of the testis (Fig. 3). A 10μl cell suspension containing 105 cells was spread into seminiferous tubules and filled about 40–80% of host testis. In the IVT group, according to Sato et al. protocol,7 SSCs were transplanted to exited host testes below the stereo microscope then after a few times (10min), host testes were cute to small pieces and placed in 3-D tissue culture system on the agarose support layer. In the in vivo group, SSCs were transplanted to host testes in the body of the host mouse then preserve host testes in the body by the suture surgery area.
An optical microscope equipped with an ophthalmologic eye lens was used to measure the various structural parameters in the sections prepared from the host testes.2 A total of 5 sections, spaced equally apart, were selected from successive sections of each testis. After Hematoxylin and Eosin (H&E) staining, 10 seminiferous tubules with rounded or close-circle sections randomly selected were used to evaluate the testicular parameters. Therefore, 50 seminiferous tubules were selected from each group. The number of SCs, spermatocyte, and spermatid per unit volume was measured in each testis. Two histological sections were prepared from each recipient testis with an interval of 12μm to obtain the percent of tubules with SSCs subsiding on the seminiferous tubules.
Immunohistochemical studiesThe immunohistochemistry method was used to prove the nature of the cells claimed to be germ cell types. For this purpose, the testicular tissue fragments, in addition to the tracing of DiI, were subjected to immunohistochemistry after tissue processing. The PLZF,13 Synaptonemal complex protein 3 (SCP3),14 and Acrosin Binding Protein (ACRBP)15 antibodies were used to confirm the nature of the SSCs, spermatocyte, and spermatozoa, respectively. The procedure of immunocytochemistry was performed according to the previous study.8 Briefly, the cells were grown on the glass slides and fixed for 20min in 4% paraformaldehyde at room temperature, before rinsing with PBS. After permeabilization by 0.2% Triton X-100 (MP Biomedicals, USA) for 1h to facilitate antibody penetration, the slides were washed with PBS supplemented with 0.2% bovine serum albumin. Nonspecific antigens were blocked with 10% normal goat serum (Vector Laboratories, USA). The slides were then incubated overnight at 37°C with a human monoclonal antibody. The slides were washed with PBS and then the second antibody was applied for 2h at room temperature in the dark.
Molecular studies using Real Time PCRIn order to prove the presence of different types of germ cells and to prove that these cells aren’t due to endogenous spermatogenesis, testicular fragments of the experimental groups were studied by Oct-4, Tekt1 and Tnp1 genes to SSCs, spermatocyte, and spermatozoa, respectively. The protocols of Real-Time PCR (polymerase chain reaction) were done based on the previous study.16 The humanity of primers designed to district endogenous cells from human germ cells.
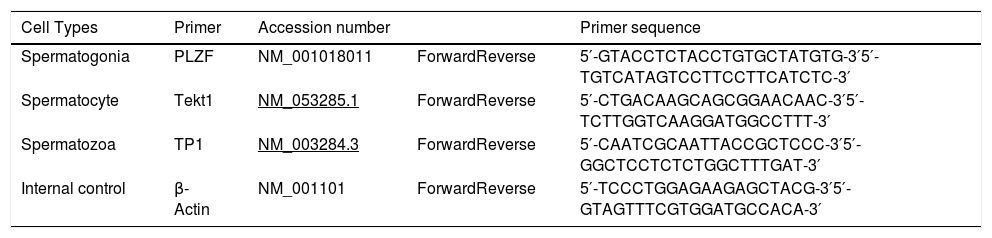
Design, order and prepare primersIn order to design the primers used in Real-Time PCR, the gene sequences from Oct-4, Tekt1 and Tnp1 were obtained from The National Center for Biotechnology Information (NCBI) database and the sequence of their exons and introns was determined. Primer design was done using Primer3 Online Software. Real-time PCR primers were designed to have at least one of the Forward or Reverse primers in the interconnection region between the two exons to prevent possible contamination of the genomic DNA in Real-Time PCR. Designed primers are blasted to confirm their accuracy and reproduce only the genes’ mRNA sequences. The sequences of the Real-Time PCR primers of Oct-4, Tekt1, and Tnp1 genes are shown in Table 1.
The list of used primers in Real Time PCR studies.
| Cell Types | Primer | Accession number | Primer sequence | |
|---|---|---|---|---|
| Spermatogonia | PLZF | NM_001018011 | ForwardReverse | 5′-GTACCTCTACCTGTGCTATGTG-3′5′-TGTCATAGTCCTTCCTTCATCTC-3′ |
| Spermatocyte | Tekt1 | NM_053285.1 | ForwardReverse | 5′-CTGACAAGCAGCGGAACAAC-3′5′-TCTTGGTCAAGGATGGCCTTT-3′ |
| Spermatozoa | TP1 | NM_003284.3 | ForwardReverse | 5′-CAATCGCAATTACCGCTCCC-3′5′-GGCTCCTCTCTGGCTTTGAT-3′ |
| Internal control | β-Actin | NM_001101 | ForwardReverse | 5′-TCCCTGGAGAAGAGCTACG-3′5′-GTAGTTTCGTGGATGCCACA-3′ |
All quantitative data in this study were presented as mean±standard deviation. One-way ANOVA, T-test, and Tukey tests were used for statistical analysis. The significance level was considered to be P<0.05.
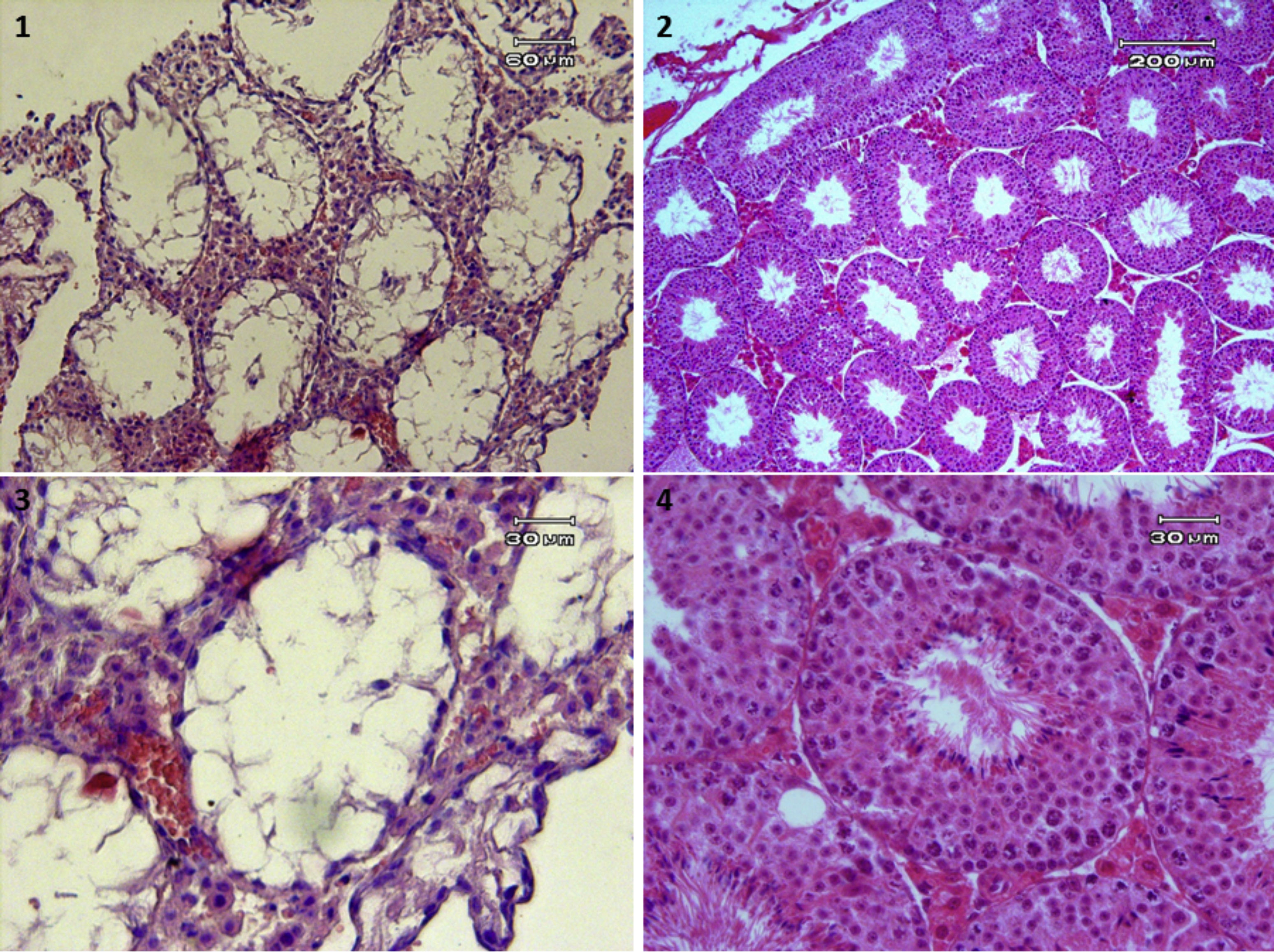
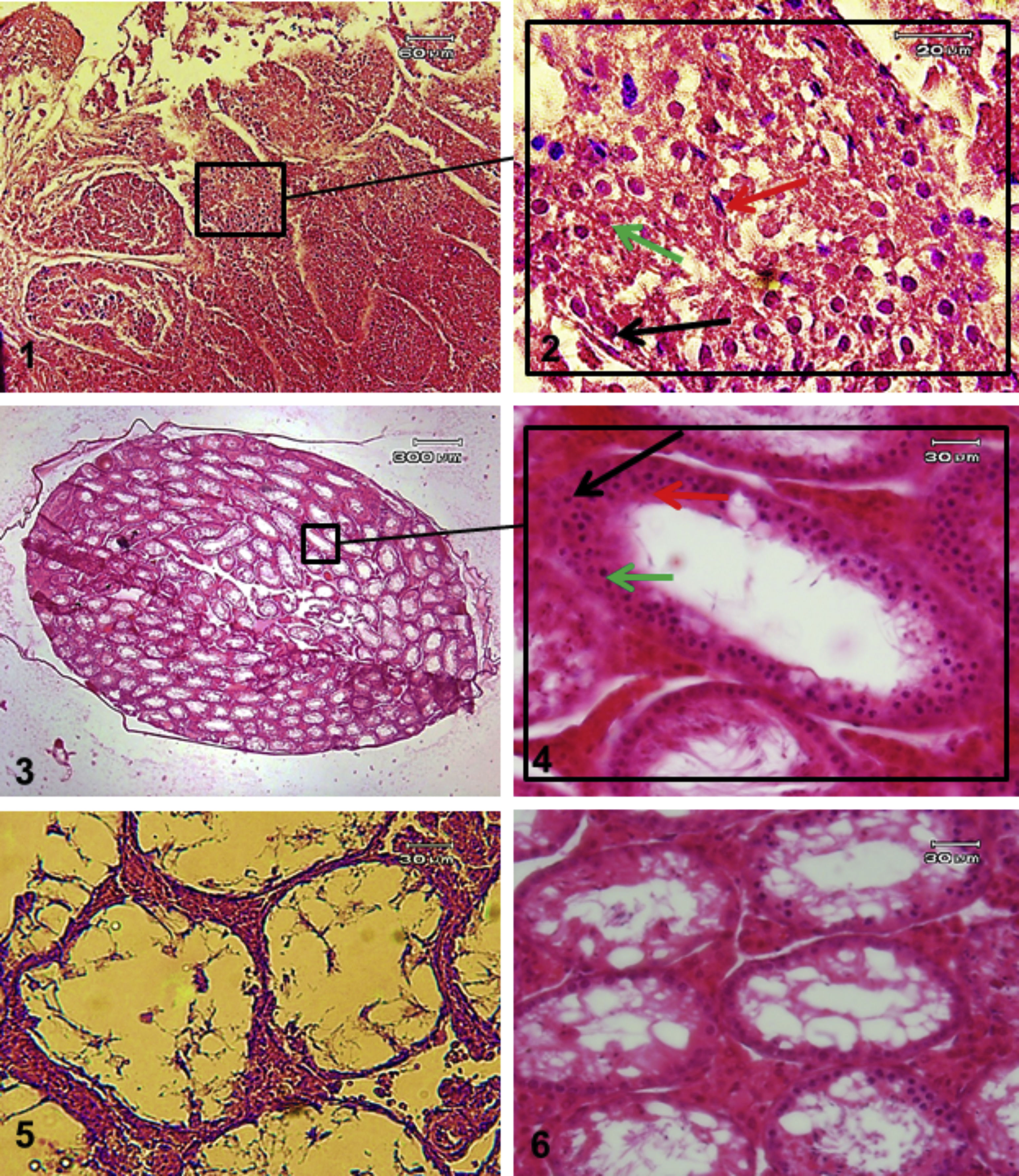
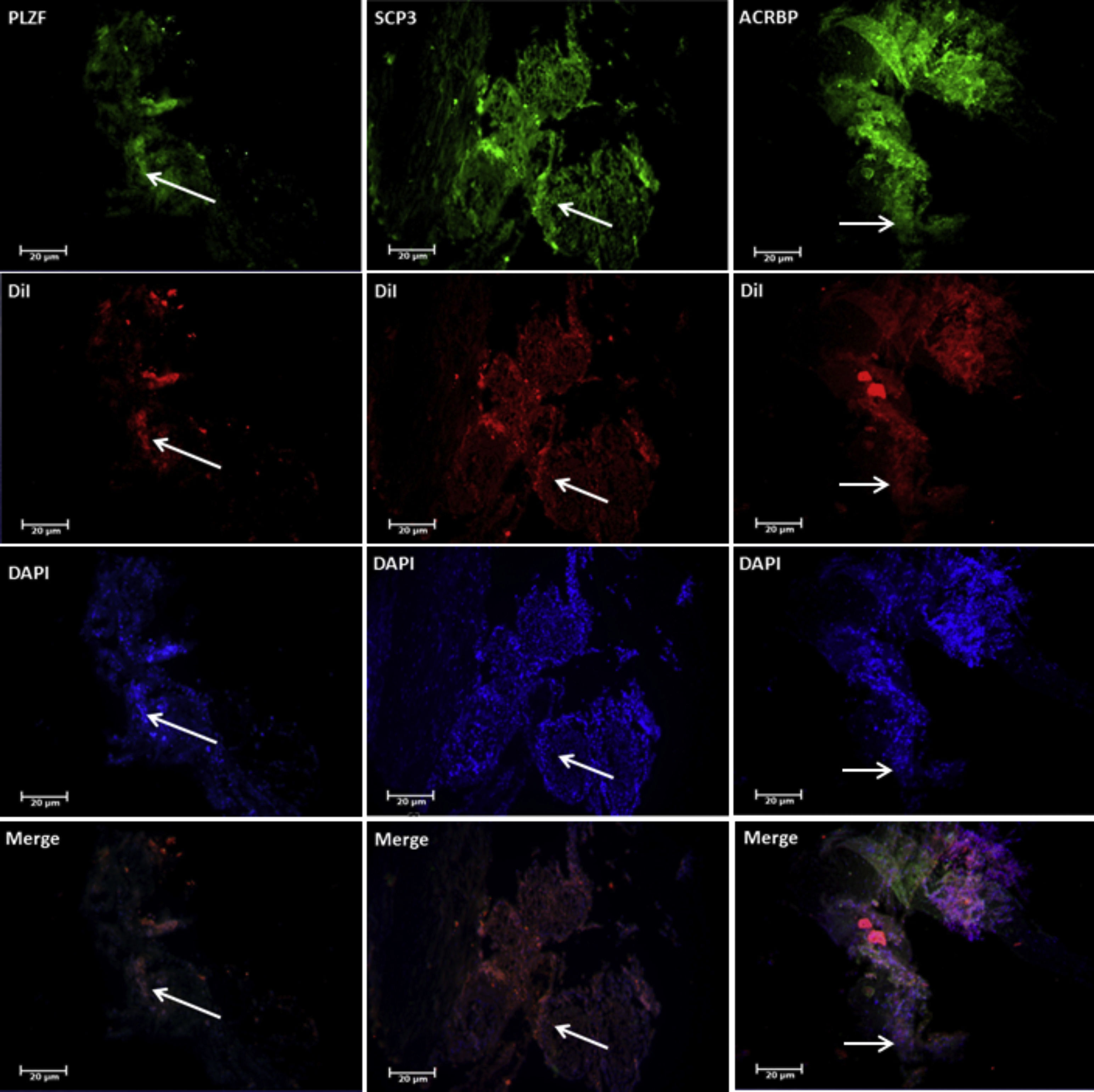
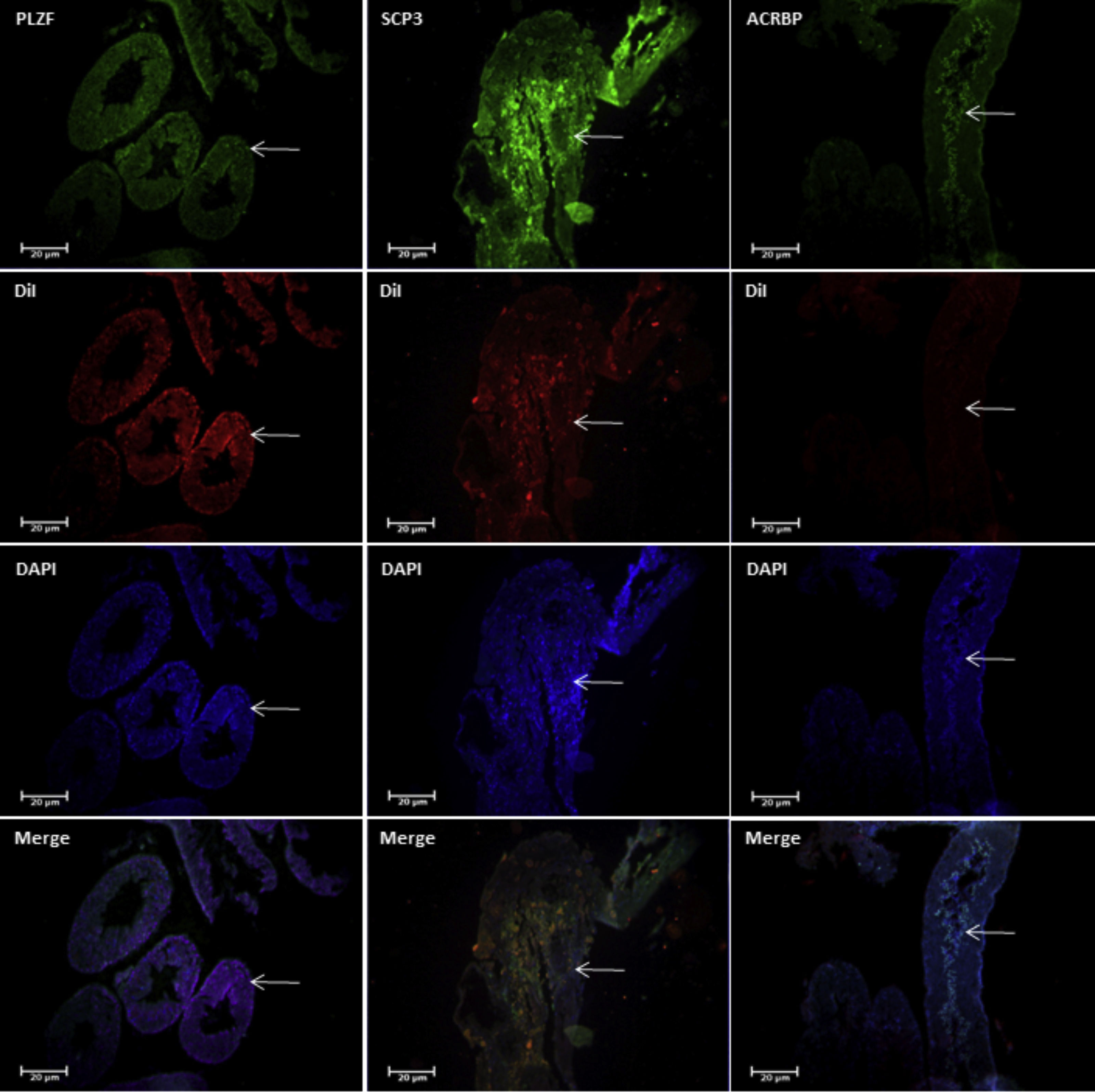
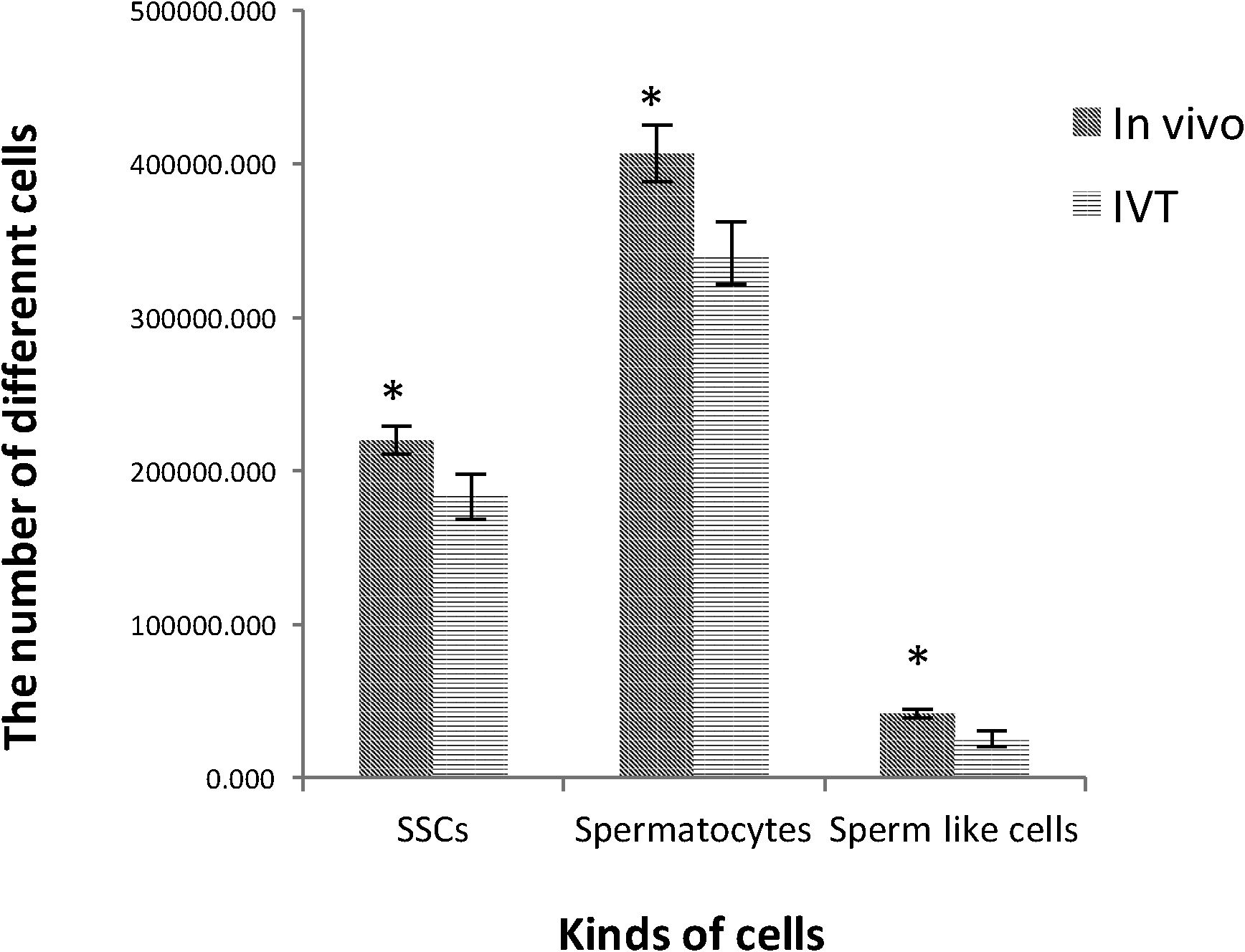
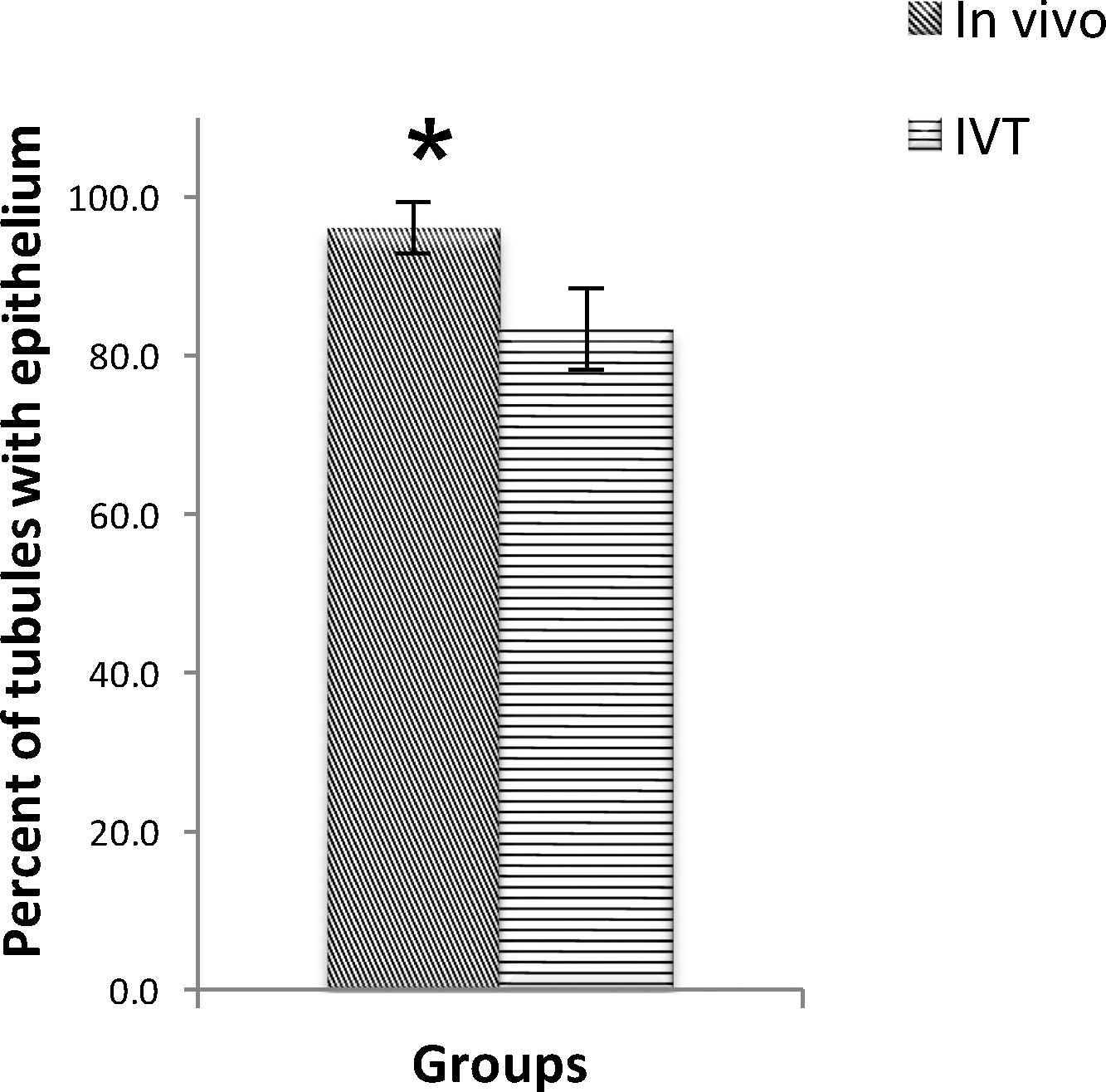
ResultsThe TESE biopsies were subjected to two stages of enzymatic digestion after the preparation steps, and finally, a cell suspension containing SSCs was put under culture conditions. The identification of the SSCs was confirmed by tracing the PLZF protein in the colonies derived from the cultured cell suspensions (Fig. 4). Eight weeks after transplantation, the groups were evaluated in different studies. The results of histological evaluations showed a progression of spermatogenesis and relative repair of tubules epithelium in both groups but it was shown that in the in vivo group the amendment of epithelium and form of seminiferous tubules was better than IVT group (Fig. 5). The results of host testes immunohistochemistry in both groups shown that the repaired epithelium cells express positively PLZF, Synaptonemal complex protein 3 (SCP3) and Acrosin Binding Protein (ACRBP) proteins, which are the human-specific proteins of SSCs, spermatocytes, and spermatozoa, respectively (Figs. 6 and 7). The results of the morphometric studies were shown the number of spermatogonial cells (SCs), spermatocytes and spermatids, or sperm-like cells per each testis, in the in vivo group were higher than the in vitro group significantly, as same as the percentage of seminiferous tubules with epithelium (P<0.05) (Fig. 8). The number of SCs in the in vivo and IVT groups were (220×103±8.4×103) and (183×103±15×103) respectively that shown the significant high number of SCs in the in vivo group (P<0.05). In the next, the number of spermatocytes in the in vivo and IVT groups were (407×103±18×103) and (341×103±20×103) respectively that shown the significant high number of spermatocytes in the in vivo group (P<0.05). Finally, the number of spermatids in the in vivo and IVT groups were (416×103±2×103) and (256×103±5×103) respectively that shown the significant high number of spermatids in the in vivo group (P<0.05). Also, the percent of repaired seminiferous tubules were shown a significant difference between groups (Fig. 9). The percent of tubules with epithelium in the in vivo and IVT groups were (96±3.2%) and (83.3±5.2%) respectively, that show the significant difference between groups (P<0.05).
H&E staining of tissue sections IVT and in vivo transplantation groups. Tissue section of IVT group (1 and 2). Tissue section of in vivo transplantation group (3 and 4). Azoospermia mouse testis in firs day (5) and after 8 weeks without transplantation in the mouse body (6) as control group to in vivo group. Black arrow suggested: SCs, green arrow suggested: spermatocyte and red arrow suggested: Long spermatid or sperm cells.
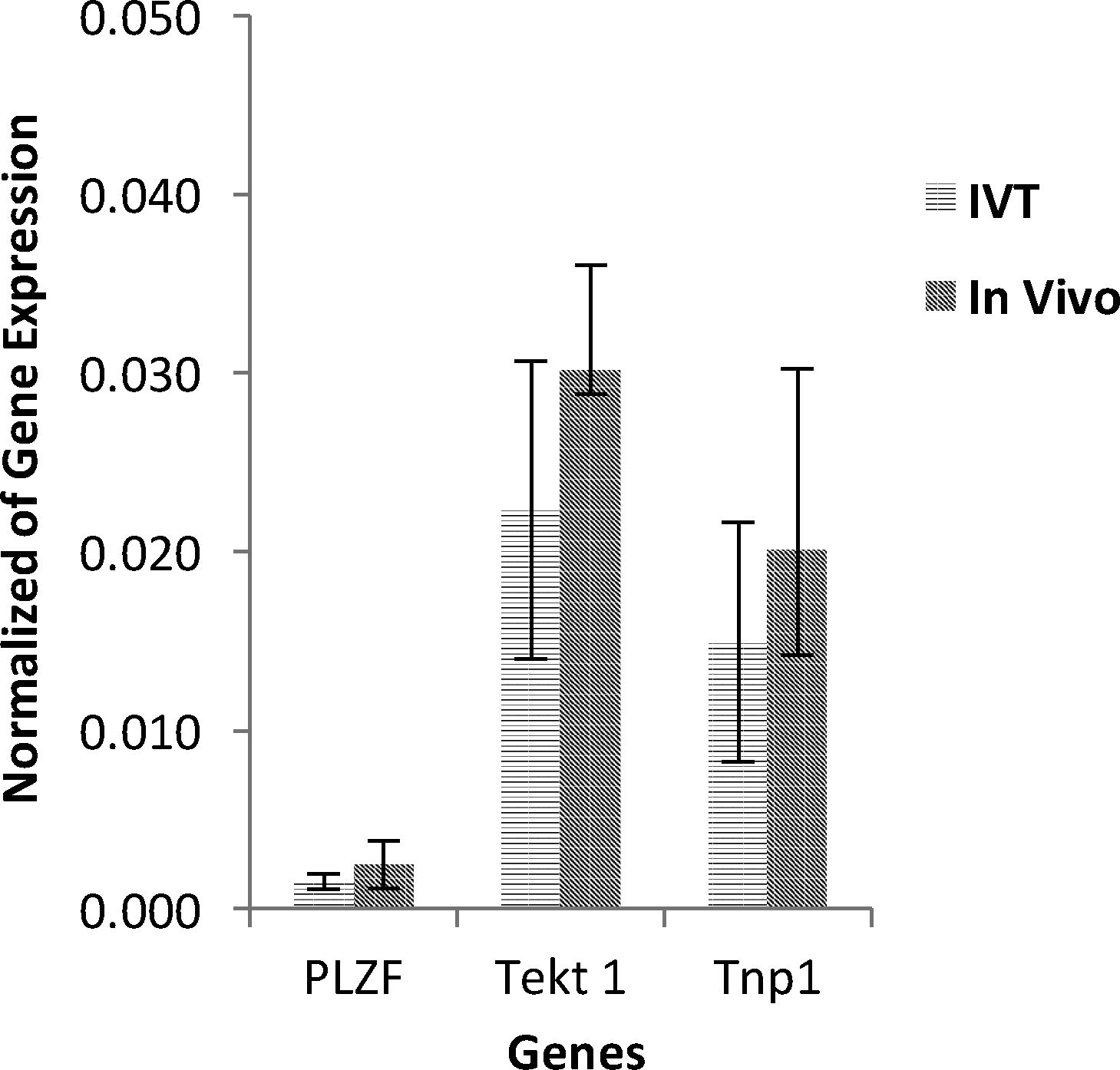
The Real-Time PCR results of host testes were shown that PLZF, Tekt1, and Tnp1genes, respectively, of SSCs, spermatocytes and spermatids or spermatozoa were expressed positively in the in both groups without significant difference (P<0.05) (Fig. 10).
DiscussionThe transplantation of SSCs to azoospermia testis model has been carried out by various researchers on various animal models. In the present study, we would like to compare the progress of spermatogenesis after human SSCs in vivo transplantation and IVT plus tissue culture of host testes. The results of histological studies and the DiI tracing in the host testis 8 weeks after transplantation indicated successful homing of transplanted SSCs on the basic membrane of the seminiferous tubules as same as the results of Mohaqiq et al. (2019)2 and repairing epithelium to the cells in the pulled in lumen centers in both in vitro culture and in vivo condition. The probability that these cells are located in the center of the seminiferous tubules with the nature of haploid spermatid or sperm-like cells was confirmed by the results of Immunohistochemical and molecular studies in both groups. This result indicates that human stem cells are capable of proliferation and differentiation during the post-transplantation period in the mouse host testes. In contrast to our results, previous researchers in xenotransplantation of human SSCs have different results in various levels of surviving cells or differentiation after transplantation. Some researchers were shown that they had allowed for identifying human SSCs that can migrate to the base of host mouse seminiferous epithelium, where daughter cells proliferate and survive for a long time.17–19 These results indicate the key characteristics of SSCs and are important and essential when human SSCs is to be used for male fertility restoration by transplantation. So, xenotransplantation can be a logical functional detection method suit for human SSCs.18,20 In 2012 Zohni et al. shown they have found that human germ cells possess the ability to migrate through and colonize the host seminiferous epithelium for at least 4 months of transplantation.21 These observations suggested that donor human germ cells did not merely survive after transplantation, but some of them were replenished and/or proliferated, suggesting the presence of long-term self-renewing cells that sustain colonies of human germ cells, although they couldn’t rule out the possibility that these colonies enlarged simply because committed cells in selected colonies proliferated. In 2011, Izadyar et al. shown that human spermatogonia expressing SSEA-4, which also express ITGA6, have a higher repopulation potential after xenotransplantation than nonexpressing cells, indicating that SSEA-4 is a putative human SSC marker.22 In 2002 Nagano et al. shown that mouse testes were colonized by human testis cells.19 They found human spermatogonia in 16 of 22 (73%) host testes. Human SSCs survived in mouse testes for at least 6 months and proliferated during the first month after transplantation. They notified that no human differentiating spermatogonia were identified, and meiotic differentiation did not occur in mouse testes. In 2000, an attempt to transplant human germ cells into testes of mice has been reported.23 The results indicated that no human germ cells survived in mouse testes, suggesting that human male germ cells possibly died in the mouse testis environment due to incompatibility between human germ cells and mouse tubular somatic cells, or immunological rejection. In contrast, previous studies of Nagano et al. demonstrated that nonhuman primate (baboon) SSCs can colonize testes of immunodeficient mice for at least 6 months.24
In continue, previous xenotransplantation evaluations using several experimental animals as donors and mice as hosts have indicated that the SSCs of many animal species can colonize mouse testes.25–28 On the basis of the results of non-human xenogeneic transplantation, may hypothesize that the biological properties of SSCs have been well conserved among species and that stem cells of a variety of species, including human, can be maintained in mouse testes. Nagano et al. reported, On the basis of their observations, human spermatogonial stem cells are thought to have proceeded through the following stages after transplantation into mouse testes19: A: was recognized by mouse Sertoli cells. B: migrated to the stem cell niche on the seminiferous tubule basement membrane. C: proliferated for a short time, and D: only a small fraction of undifferentiated spermatogonia or stem cells survived for 6 months.
The findings of the IVT group are consistent with the results reported by Sato et al. in 2013. Sato and colleagues were done IVT of mouse SSCs into immature azoospermic mice testis that these transplanted cells sat down on a membrane of seminiferous tubules after 7–14 days. Their marking was to track Acrosin GFP in transplanted cells. They reported that after 40–50 days spermatids or sperm cells appeared.7 However, the exact mechanism of the alignment of transplanted cells on the membrane has not yet been completely transparent.29 In 2008 Shinohara et al. suggested strongly that B1-intergin is involved in the first several weeks of SSCs homing and colonization.30 In 2019 Mohaqiq et al. was shown that transplanted human SSCs to mouse host testes by IVT could conclude to homing of these cells after 2 weeks in tissue culture of host testes, as same as in vivo transplantation.2 In 2011, Sato et al. conducted another study in which germ cell cells were transplanted into seminiferous tubules in the in vitro condition.29 After placement on the membrane of the seminiferous tubules, these cells began to mitotic and then differentiated into higher-grade germ cells and eventually reached spermatid and haploid sperm cells, which were fertile, and by microinjection, fertile ability the ovum. In the in vivo condition because of the presence of all characteristics of spermatogenesis both physically and nutritionally, it seems to take easy for spermatogenesis induction by SSCs transplantation.31,32 However, the presence of interstitial tissue concludes Myoid cells, Laydig cells and etc. are so necessary to support different mechanisms of spermatogenesis.33.34 SSCs fates are regulated by a complex interaction between growth factors secreted by the interstitial cells together with sertoli cell and SSCs.35 However, our limited immunohistochemical and molecular characteristics demonstrated in this study may suggests post miotic differentiation cells from human SSCs.
ConclusionThe results of this study indicate that the IVT system and testicular tissue culture have the ability to support the progression of spermatogenesis to obtaining haploid cells, as well as in vivo condition.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
FundingThis study is sponsored financially by the Tarbiat Modares University.
Conflict of interestWe wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
The present study is part of the Ph.D. thesis of Anatomical Sciences at Tarbiat Modares University, Medical sciences faculty and sponsored by Tarbiat Modares University.