To investigate the relationship between the Sperm Motility Index and the presence of reactive oxygen species in semen.
MethodsWe retrospectively analyzed Sperm Motility Index measured by the Sperm Quality Analyzer and reactive oxygen species levels in 92 semen samples of infertile male patients who visited the Ebina Ladies’ Clinic between September 2011 and June 2012. Using the same semen samples, we also analyzed 8 sperm parameters measured by computer-assisted semen analysis and validated the relationship with the Sperm Motility Index.
ResultsThe presence of reactive oxygen species in semen was positive in 19 samples and negative in 73 samples. In the reactive oxygen species-positive group, there was a significant negative correlation between the logarithm of reactive oxygen species level and Sperm Motility Index (p = 0.039).
ConclusionsThis is the first study to find a significant negative correlation between Sperm Motility Index and reactive oxygen species level. This result indicates that the presence of reactive oxygen species in semen may inhibit the fertilization ability of spermatazoa as measured by the Sperm Motility Index.
Investigar la relación entre el índice de movilidad espermática y la presencia de especies reactivas del oxígeno en el semen.
MétodosAnalizamos retrospectivamente el índice de movilidad espermática medido por el analizador de calidad del esperma y los niveles de especies reactivas del oxígeno en 92 muestras de semen de varones estériles que visitaron Ebina Ladies’ Clinic entre septiembre de 2011 y junio de 2012. Con las mismas muestras de semen también analizamos 8 parámetros del esperma medidos mediante un análisis de semen asistido por ordenador y validamos la relación con el índice de movilidad espermática.
ResultadosLa presencia de especies reactivas del oxígeno en el semen dio positivo en 19 muestras y negativo en 73. En el grupo positivo para especies reactivas del oxígeno hubo una correlación negativa significativa entre el logaritmo de nivel de especies reactivas del oxígeno y el índice de movilidad espermática (p = 0,039).
ConclusionesEste es el primer estudio que encuentra una correlación negativa significativa entre el índice de movilidad espermática y el nivel de especies reactivas del oxígeno. Este resultado indica que la presencia de especies reactivas del oxígeno en el semen podría inhibir la capacidad de fecundación de los espermatozoides según la medición del índice de movilidad espermática.
Recently, the Sperm Quality Analyzer® (SQA), a useful instrument to assess sperm quality, has been widely used in the field of male infertility. The Sperm Motility Index (SMI) is a single value obtained via the SQA that reflects the number of sperm, intensity of movement, viability, and concentration of motile sperm in a semen sample. The SMI is a measurement of optical density fluctuations caused by motile cells that has been reported to correlate statistically with motile sperm parameters.1–3 One study reported that the SMI could be used to rule out oligozoospermia and asthenozoospermia by setting a threshold value.4 Based on previous studies, the SMI is a simple and useful quantifiable measure of sperm quality and activity.
Reactive oxygen species (ROS) in semen were first reported by Aiken in 1987.5 According to the study, immature sperm and leukocytes in semen are considered the origin of ROS. The presence of ROS affects sperm function and fertilizing ability through several mechanisms, such as deterioration of sperm motility, DNA fragmentation, and lipid peroxidation of sperm plasma membranes.6–8 Iwasaki et al. reported that ROS was detected in the semen of 40% of male infertile patients.9 Another group suggested that the combination of ROS level and total antioxidant capacity could predict idiopathic male infertility.10,11
Although both SMI and ROS are useful to assess sperm quality and activity, no previous study has examined the relationship between these two parameters. The aim of this study was to examine the relationship between SMI and ROS in infertile males to determine the utility of measuring ROS levels in semen.
Materials and methodsWe examined the nonprocessed semen samples of 92 male patients who visited the Ebina Ladies’ Clinic between September 2011 and October 2012. Written informed consent was obtained from all patients for their data to be used for research purposes. At the patients’ first visits, the SMI of the semen samples was measured using SQA-V® (Medical Electronic Systems, Israel). Using the same semen samples, a computer-assisted semen analyzer (CASA) was also used to determine 8 semen parameters: concentration, motility, straight-line velocity (VSL), curvilinear velocity (VCL), linearity, mean amplitude of lateral head displacement (mALH), beat-cross frequency (BCF), and progressive motility.
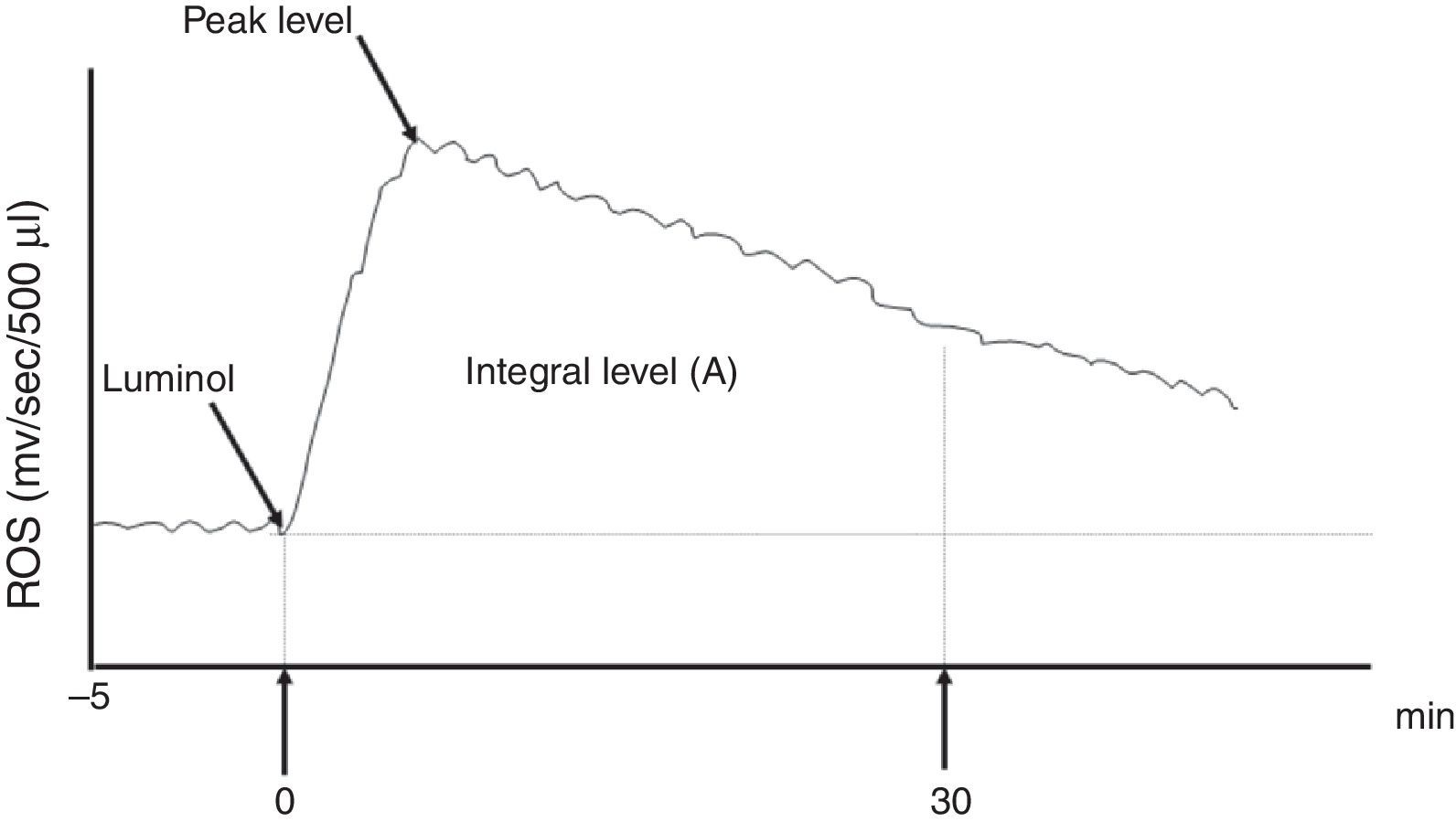
The ROS production levels in whole semen were measured using a computer-driven LKB Wallac 1251 Luminometer™. Chemiluminescence was recorded after the addition of 40μL of 4mM luminol (5-amino-2,3-dihydro 1,4-phtalazine-dione) to 500μL of whole semen. When the luminescence was equal to or greater than 0.1mV/s at peak value, ROS production in the sample was considered positive.9 The integrated values of ROS were also used to clarify the differences between ROS positive and negative cases. The definite integral level of ROS between 0 and 30min after the addition of luminol was expressed by the unit mV/30min/106 spermatozoa and considered the ROS level of the sample (Fig. 1).
The graph of ROS level measurement. Chemiluminescence was recorded after the addition of 40μL of 4mM luminol (5-amino-2,3-dihydro 1,4-phtalazine-dione) to 500μL of whole semen. The definite integral of the ROS levels between 0 and 30min (A) after the addition of luminol were expressed by the values mV/30min/106 spermatozoa and considered the ROS level of the sample.
The samples were divided into an ROS-positive group and an ROS-negative group. The SMI, ROS level, and 8 semen parameters were compared between the two groups using univariate analysis. The correlation between SMI and each semen parameter was calculated using the Spearman rank correlation coefficient. In the ROS-positive group, the correlation between log rank ROS level and SMI was also examined. For all of the statistical tests, p≤0.05 was considered significant.
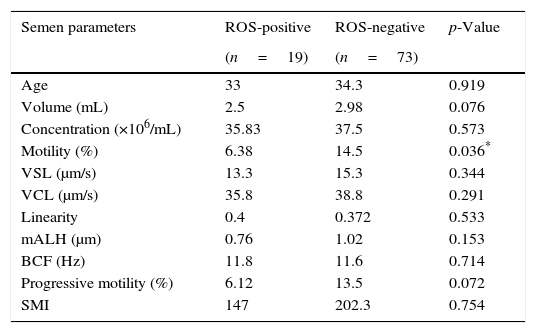
ResultsOut of 92 semen samples, 19 samples were positive for ROS (20.7%), and 73 samples were negative for ROS (79.3%). The mean age of patients in each group was 33±7.47 (range 26–51) and 34.3±4.4 (range 25–45) years, respectively (p=0.919). A comparison of each semen parameter between the ROS-positive group and the ROS-negative group is shown in Table 1. Motility was significantly higher in the ROS-negative group than in the ROS-positive group (13.5% vs. 6.12%, p=0.036). There were no significant differences between the two groups for the remaining 7 sperm parameters.
The comparison of each semen parameter between reactive oxygen species (ROS)-positive and ROS-negative groups.
| Semen parameters | ROS-positive | ROS-negative | p-Value |
|---|---|---|---|
| (n=19) | (n=73) | ||
| Age | 33 | 34.3 | 0.919 |
| Volume (mL) | 2.5 | 2.98 | 0.076 |
| Concentration (×106/mL) | 35.83 | 37.5 | 0.573 |
| Motility (%) | 6.38 | 14.5 | 0.036* |
| VSL (μm/s) | 13.3 | 15.3 | 0.344 |
| VCL (μm/s) | 35.8 | 38.8 | 0.291 |
| Linearity | 0.4 | 0.372 | 0.533 |
| mALH (μm) | 0.76 | 1.02 | 0.153 |
| BCF (Hz) | 11.8 | 11.6 | 0.714 |
| Progressive motility (%) | 6.12 | 13.5 | 0.072 |
| SMI | 147 | 202.3 | 0.754 |
VSL, straight-line velocity; VCL, curvilinear velocity; mALH, mean amplitude of lateral head displacement; BCF, beat-cross frequency.
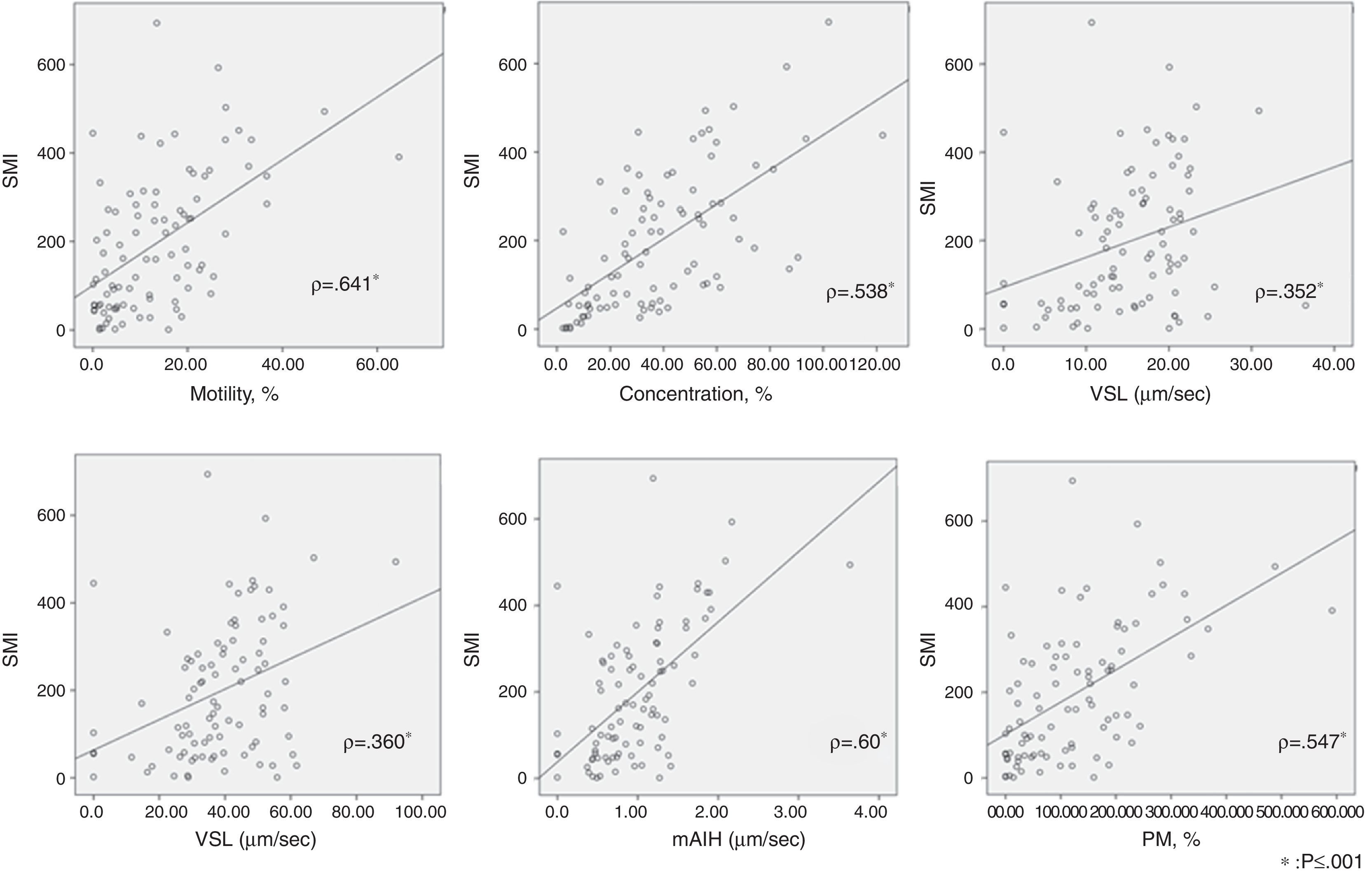
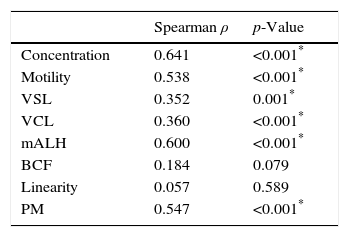
The correlation using the Spearman correlation coefficient between SMI and each semen parameter is shown in Table 2. There was a significant correlation between SMI and concentration(ρ=0.641), motility(ρ=0.538), VSL (ρ=0.352), VCL (ρ=0.360), mALH (ρ=0.60), and progressive motility(ρ=0.578), respectively (p≤0.001). Correlations between SMI and the 2 remaining parameters, BCF and linearity, were not significant. The scatterplots are shown in Fig. 2.
The correlation between SMI with each parameter measured with computer-assisted semen analysis (CASA).
| Spearman ρ | p-Value | |
|---|---|---|
| Concentration | 0.641 | <0.001* |
| Motility | 0.538 | <0.001* |
| VSL | 0.352 | 0.001* |
| VCL | 0.360 | <0.001* |
| mALH | 0.600 | <0.001* |
| BCF | 0.184 | 0.079 |
| Linearity | 0.057 | 0.589 |
| PM | 0.547 | <0.001* |
VSL, straight-line velocity; VCL, curvilinear velocity; mALH, mean amplitude of lateral head displacement; BCF, beat-cross frequency; PM, progressive motility.
The scatterplots of the relationship between SMI and each semen parameter using the Spearman correlation coefficient. There was significant correlation between SMI and motility (ρ=0.641), concentration (ρ=0.538), straight-line velocity (VSL) (ρ=0.352), curvilinear velocity (VCL) (ρ=0.360), mean amplitude of lateral head displacement (mALH) (ρ=0.60), and progressive motility (PM) (ρ=0.578), respectively (p≤0.001).
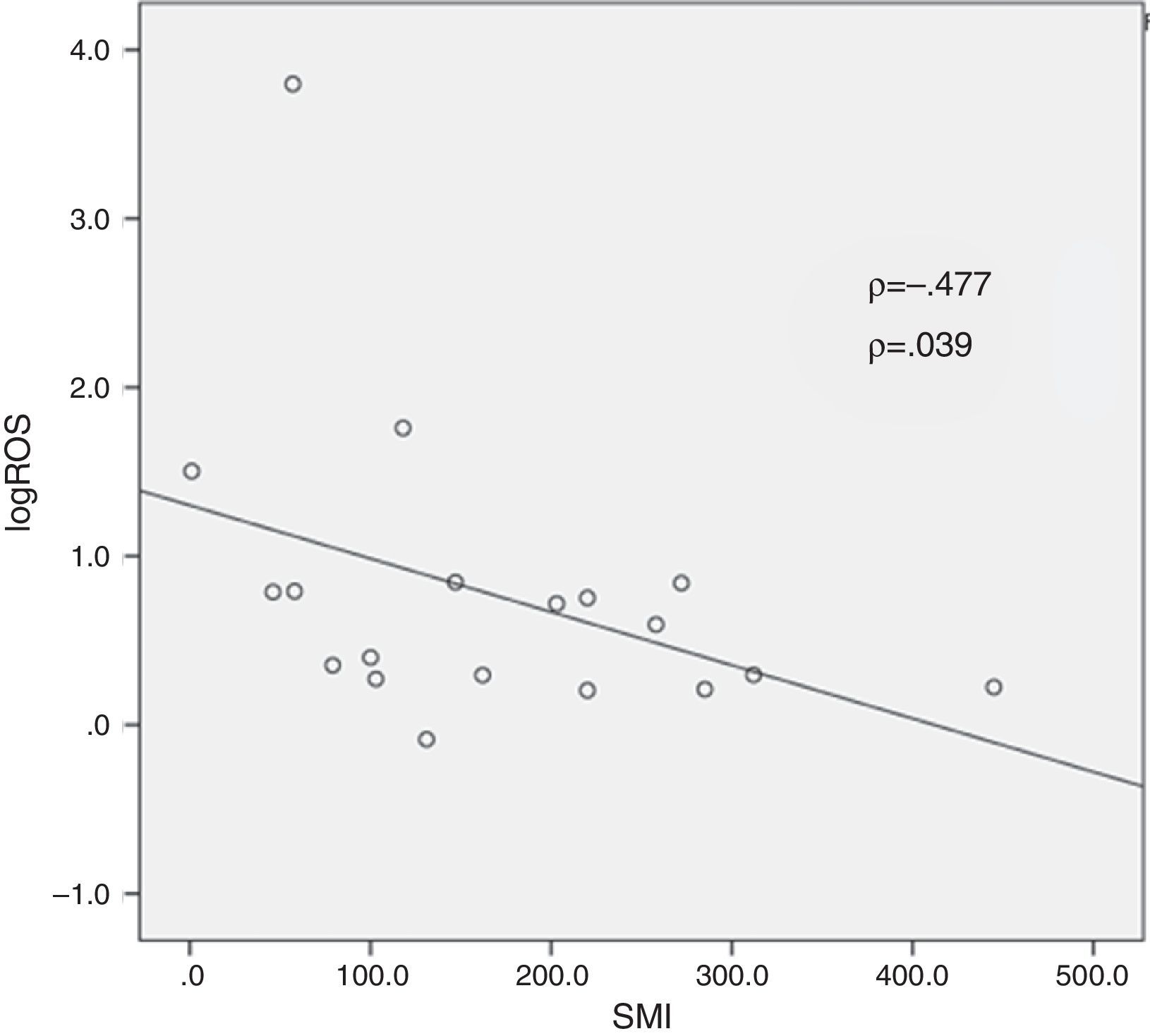
In the ROS-positive group, log ROS level and SMI were significantly negatively correlated (Fig. 3; ρ=−0.477, p=0.039). As for other semen samples, there was negative correlation between log ROS level and semen concentration (ρ=−0.482, p=0.036) although there was no significant correlation with the remaining 7 parameters.
DiscussionThe SMI is a measurement of optical density fluctuations caused by motile sperm cells. The SMI values are determined by introducing the diluted spermatozoa into a flat glass capillary tube and passing light through the tube; the SQA detects the variations in light caused by the moving spermatozoa.12 Therefore, the SMI is the figure that reflects the motile sperm's concentration and velocity. Considering the mechanism, it is easy to presume that SMI would correlate well with traditional semen parameters such as concentration and motility. As expected, motility, concentration, and most other semen parameters correlated with SMI significantly in our study. Previous studies have shown the clinical performance of SMI in screening and predicting infertility. Martinez et al. reported that SMI correlated with the concentration of progressively motile spermatozoa and concluded that SMI is useful to rule out oligozoospermia and asthenozoospermia.4 As for assisted reproductive technology (ART), a previous study showed that SMI could be a useful predictor of fertilization after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) through setting adequate cut-off values.13 Considering these findings, SMI appears to be a single value that can reliably predict sperm quality and activity.
On the other hand, some studies have identified an association between the presence of ROS in semen and male infertility and the negative outcome of fertility treatments. Agarwal et al. reported that ROS level could distinguish infertile males from normal controls through setting a cut-off level of 102.2 relative light unit (RLU)/s/10(6) sperm (sensitivity 76.4%, specificity 53.3%).14 Their group previously suggested that a scoring system composed of a combination of ROS level and total antioxidant capacity (ROS-TAC score) is a useful measurement of infertility and can predict idiopathic male infertility.10,11 In IVF/ICSI procedures, high ROS levels have shown a negative association with embryo development to the blastocyst stage.15 As for the outcome of fertility treatment, namely pregnancy, our group has previously reported that the ROS level in semen samples from the patients’ first visit was significantly higher in the nonpregnant group than in the pregnant group among the infertile couples who visited our clinic.16 Also, among the patients who underwent ICSI, this study showed that pregnancy was less common in the high-ROS group.17
Considering these results, it is important to determine the role of ROS in semen, because it is possible that reducing the level of ROS could be a treatment target for idiopathic male infertility. According to previous reviews, antioxidant therapy for infertile males has been effective for improving sperm function and motility in experimental and clinical reports. However, the overall effectiveness remains controversial mainly due to nonstandardization in measuring the level of ROS and assessing clinical outcomes, as well as incomplete understanding of the role of free radicals in normal and abnormal sperm function leading to male infertility.18,19 Our group measured ROS as an integrated level in nonprocessed semen so that we could better confirm the relationship between motile parameters. Similarly, Takeshima et al. reported that sperm concentration, motility, mALH, and progressive motility showed inverse correlations with the logarithmic transformed integrated reactive oxygen species levels.20 Measurement of ROS levels can be performed simply using nonprocessed semen samples. In the present study, we did not find the significant correlation between log ROS level except concentration. The cause is probably there were much less number of patients than in the previous report. Correlation between sperm concentration may suggest that ROS reflects the low quality of sperm.
This study showed a significant negative correlation between SMI and ROS levels. The SMI is a useful parameter to show the total quality of semen samples and predict fertilization rates in vitro. Our results indicate that the presence of ROS lowers the sperm quality as reflected by the SMI, suggesting that ROS levels themselves have the potential to be a parameter that predicts low quality of semen. Although it was not examined the correlation between ROS and results of treatment such as pregnancy rates in the present study, ROS would be a reliable biomarker of sperm quality if the cut-off value for each treatment such as IVF and ICSI are established. It allows us to predict pregnancy or fertilization rate, or predict the effect of treatment in ART through examining ROS level of cryopreserved or swim up sperm samples.
The primary limitation of this study is that it was a retrospective analysis undertaken at a single center. Prospective analysis involving a multicentre approach is required to confirm the results.
ConclusionThis is the first study to show a significant negative correlation between SMI and ROS level. Therefore, ROS level may have the potential to be a parameter that can predict low quality of semen or the probability of fertilization or pregnancy, just as SMI is used. Further investigation of the potential uses of measuring ROS levels is needed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThere is no conflict of interest to declare.