The ultrastructure of the human mature metaphase-II oocyte before and after the cortical reaction has been previously described. However, we found a new vesicular aggregate associated with smooth endoplasmic reticulum tubular aggregates (aSERT) and new observations regarding the cortical reaction.
ObjectivesTo describe at the ultrastructural level a novel vesicle aggregate associated with aSERT and new observations regarding the cortical reaction.
Materials and methodsDonated, surplus mature oocytes were processed for transmission electron microscopy immediately after recover. Calcium detection was performed using the pyroantimonate technique. The cortical reaction was artificially induced by ionophore treatment.
ResultsWe observed an accumulation of small vesicles at the periphery of aSERT. At high magnification these were composed by small pale vesicles coated by tiny vesicles, with associated dense materials, giving a rosette-like appearance. Adjacent to these there was another group of small dense vesicles incompletely coated by similar tiny vesicles. Using calcium detection at the ultrastructural level, antimonate deposits were observed in the tubules of aSERT and in the surrounding mitochondria but not in the rosette-like structures. Regarding cortical vesicles, although most previous studies described their contents as homogeneous dense, others referred the presence of another type of cortical vesicles whose contents were moderate dense. Using ionophore oocyte activation, we observed that moderate dense cortical vesicles may correspond to a progressive swelling of the dense cortical vesicles prior to exocytosis.
ConclusionsWe describe a novel vesicle aggregate associated to aSERT and new observations on the remodeling of cortical vesicles before exocytosis.
La ultraestructura del ovocito humano maduro en metafase-II humana antes y después de la reacción cortical se ha descrito previamente. Sin embargo, encontramos un nuevo agregado vesicular asociado a los agregados tubulares de retículo endoplásmico liso (aSERT) y nuevas observaciones con respecto a la reacción cortical.
ObjetivosDescribir a nivel ultraestructural un nuevo agregado vesicular asociado a los agregados tubulares de retículo endoplásmico liso y nuevas observaciones con respecto a la reacción cortical.
Material y métodosSe procesaron ovocitos maduros excedentes de ciclos de donante para microscopia electrónica de transmisión inmediatamente después de recuperarse. La detección de calcio se realizó mediante la técnica de piroantimoniato. La reacción cortical fue inducida artificialmente mediante tratamiento ionóforo.
ResultadosSe observó una acumulación de vesículas pequeñas en la periferia de aSERT. A mayor aumento, estas estaban compuestas por pequeñas vesículas pálidas recubiertas por diminutas vesículas, con materiales densos asociados, dando una apariencia de roseta. Junto a ellas había otro grupo de pequeñas vesículas densas, incompletamente recubiertas por similares vesículas diminutas. Con el uso de la detección de calcio a nivel ultraestructural se observaron depósitos de antimoniato en los túbulos de aSERT y en las mitocondrias de los alrededores, pero no en las estructuras de roseta. En cuanto a las vesículas corticales, aunque la mayoría de los estudios anteriores describen su contenido como denso homogéneo, se refirió la presencia de otro tipo de vesículas corticales cuyo contenido era denso moderado. Con el uso de la activación de ovocitos con ionóforo, se observó que las vesículas corticales de densidad densa moderada pueden corresponder a una hinchazón progresiva de las vesículas corticales densas antes de la exocitosis.
ConclusionesDescribimos una nuevo agregado vesicular asociado a aSERT y nuevas observaciones sobre la remodelación de vesículas corticales antes de la exocitosis.
The mature metaphase-II oocyte (MII) is surrounded by a fine fibrilar glycoprotein coat named zona pellucida (ZP). The oocyte surface presents short microvilli and is separated from the ZP by a narrow perivitelline space (PVS).1–8 The cytoplasm of the MII oocyte is very rich in mitochondria and smooth endoplasmic reticulum (SER). Due to the specific concentration of distinct SER vesicles and tubules, the oocyte cytoplasm can be subdivided into three cytoplasmic regions, cortex, subcortex and inner (central) cytoplasm. The cortex presents a submembranar layer of microfilaments,4,8,9 one or several layers of dense cortical vesicles and SER tiny vesicles. The subcortex contains SER vesicles surrounded by mitochondria (MV complexes) and SER aggregates of tubules (aSERT), also surrounded by mitochondria. The inner cytoplasm presents MV complexes, isolated mitochondria and a few secondary lysosomes.2,4,8,10–14 After fertilization, the periphery of the oocyte is transformed. The cortex and subcortex regions present a few isolated SER vesicles and mitochondria, whereas the central region displays a high concentration of organelles, with MV complexes, isolated SER vesicles and mitochondria appearing accumulated around pronuclei.14,15
Cortical vesicles are secretory vesicles originated from the Golgi complex. They are non-uniformly distributed, as some regions have only a few cortical vesicles, whereas others present more than one row of these vesicles.2,4,8,10,12,13 Their content is homogeneously dense and contains several types of carbohydrates, basic mucopolysacharides, acid mucopolysacharides, acidic proteins, acid phosphatase, alkaline phosphatase, glycosidases, peroxidases and proteases.5,16–20 The exocytosis of cortical vesicles (cortical reaction) occurs immediately after oocyte membrane depolarization and intracellular calcium release, both triggered by fusion between sperm and oocyte membranes.21–26 After fusing with the oocyte membrane, these secretory vesicles release several kinds of enzymes into the PVS, where they act over the ZP rendering it resistant to further sperm entry.20,23,26–28 Additionally, the carbohydrates released from cortical vesicles coat the oocyte membrane, which serve to protect the blastomere during embryo development, and may also display functional roles in blastomere signaling and interaction with the endometrium at implantation.5,20
During morphological ultrastructural studies of donor MII oocytes, with and without artificial activation, we observed a novel vesicular aggregate associated with aSERT and new aspects on cortical vesicle remodeling.
Materials and methodsEthical considerationsEthical guidelines were followed in the conduct of research, with written informed consent having been obtained before the beginning of the present work. This work did not involve human or animal experiments. An approval by an Ethics Committee and the provisions of the Declaration of Helsinky as revised in Tokyo 2004 on human experimentation does not apply to this kind of work. According to the Portuguese National Law on Medically Assisted Procreation (Law 32/2006) and the Portuguese National Council on Medically Assisted Procreation guidelines (CNPMA, 2015), donated, surplus oocytes, were analyzed without the need of further ethical obligations.
PatientsIt was not our intention to describe the ultrastructural morphology of the human mature MII oocyte or of the cortical reaction, as these have been previously well documented. Also, as it was not our purpose to quantify oocyte organelles, no morphometric analysis was performed. Our aim was to present new observations found during an ultrastructural morphological study of donor oocytes. For this reason, in this report we processed for transmission electron microscopy only six MII oocytes, which are enough to describe our new observations. For obvious ethical reasons we used fresh morphological normal mature surplus donor oocytes donated from assisted reproduction treatment cycles.
Six women aged 22–25 years (the young age avoids the ovary aging factor)8 were submitted to controlled ovarian hyperstimulation during a donor oocyte program. From each patient, one surplus fresh mature MII oocyte, without extra-cytoplasmic or intracytoplasmic dimorphisms, was donated for research (from women whose cycles had at least 10 mature MII oocytes). Oocytes were immediately processed for experiments to avoid in vitro aging.8 Two of them were processed as controls, two were treated for calcium detection and two were activated with ionophore. All oocytes were processed for transmission electron microscopy (TEM).
Stimulation protocol and oocyte retrievalWomen underwent controlled ovarian hyperstimulation using a short protocol with a gonadotrophin-releasing hormone antagonist (cetrorelix: Merck Serono, Geneve, Switzerland; ganirelix: Organon, Oss, Netherlands). For ovary stimulation, a recombinant follicle-stimulating hormone was used (Puregon: Organon; Gonal-F: Merck Serono). About 36h before oocyte recovery, human chorionic-gonadotrophin (HCG) was administered (Pregnyl; Organon). Estradiol serum levels were assayed at the day of HCG or one day before.29,30
Oocyte retrieval of large ovarian follicles was performed by ultrasonically-guided transvaginal follicular aspiration. The cumulus-oocyte complexes were washed in G-MOPS-Plus (Vitrolife, Kungsbacka, Sweden) or in Sperm Preparation Medium (SPM) (Medicult Origio, Jyllinge, Denmark), allocated in the central well of a tissue culture plate in G-IVF-PLUS (Vitrolife) or Universal IVF medium (Medicult), and incubated at 37°C, 6% CO2, 5% O2, 89% N2 (Sanyo, Tokyo, Japan).
For denudation, cumulus-oocyte complexes were incubated with recombinant hyaluronidase (ICSI Cumulase: Medicult; Hyase-10X: Vitrolife) for 30s, followed by mechanically dissociation in SPM or G-MOPS-Plus, with an oocyte denudation micropipette (Swemed, Billdal, Frolunda, Sweden). Denudation was performed under paraffin oil (Liquid Paraffin: Medicult; Ovoil-100: Vitrolife). Denuded oocytes were then incubated in 4-well plastic tissue culture dishes in in vitro fertilization medium (Universal IVF Medium; G-IVF-PLUS) for 1h until treatment or fixation.
Ionophore treatmentTo study the cortical reaction, oocytes need to be activated. For obvious ethical reasons we did not use fertilized oocytes. Instead, we artificially induced oocyte activation with ionophore as previously described, a technique that mimics natural activation and is generally used for rescue ICSI.31,32 For this, oocytes were incubated with 10μM ionophore A23187 (Sigma-Aldrich, St. Louis, USA) for 15min, then washed and further incubated for 1h before fixation for TEM.23
Oocyte processing for transmission electron microscopyFor TEM, oocytes were fixed with Karnovsky (2.5% glutaraldehyde, 4% paraphormaldehyde, 0.15M sodium cacodylate buffer, pH 7.3) (Sigma; Merck, Darnstradt, Germany) for 30min at room temperature (RT) and then for 2h at 4°C, washed in 0.15M sodium cacodylate buffer, pH 7.3 (Merck), post-fixed with 2% osmium tetraoxide (Merck) in buffer containing 0.8% potassium ferricyanide (Merck) for 2h at 4°C, washed in buffer (10min), serially dehydrated in ethanol (50, 75, 90, 95, 2× 100%, 30min each) (Panreac, Barcelone, Spain), equilibrated with propylene oxide (2× 15min) (Merck) and embedded in Epon (4h at RT and 3 days at 60°C) (Sigma). Semithin and ultrathin sections were prepared with a diamond knife (Diatome, Hatfield, Switzerland) in a LKB ultramicrotome (Leica Microssystems, Weltzlar, Germany). Serial sections of 1μm (5/5μm) were performed until the central region of the oocyte was observed. Semithin sections were stained with aqueous azure II (Merck) and methylene blue (Merck) (1:1) and observed by light microscopy. Ultrathin sections were collected on 200 mesh formvar carbon-coated copper grids (Taab, Berks, England), contrasted with 3% aqueous uranyl acetate (20min) (BDH, Poole, England) and Reynolds lead citrate (10min) (Merck), and observed in a JEOL 100CXII transmission electron microscope (JEOL, Tokyo, Japan) operated at 60kV.2,13
Ultrastructural visualization of calciumFor ultrastructural visualization of loosely bound calcium,15,33 oocytes were washed in calcium-magnesium free Dulbeco phosphate-buffered saline (Sigma) with 0.002% EDTA (Sigma) supplemented with 1% bovine serum albumin (Sigma) to chelate extracellular calcium. Oocytes were then fixed with 2% glutaraldehyde (Merck) in 0.01N potassium acetate (Merck) with 2% potassium pyroantimonate (Merck), pH 7.8, at 4°C, for 1h. After washing in 0.01N potassium acetate, pH 7.8, at 4°C, for 8h, oocytes were post-fixed with 1% osmium tetraoxide and 2% potassium pyroantimonate in 0.01N potassium acetate, pH 7.8, at 4°C, for 1h. After washing as above, with 0.05N potassium acetate, oocytes were processed for TEM. Ultrathin sections were not counterstained. As controls, some ultrathin sections were incubated with 6mM aqueous EDTA, pH8.0, at 60°C, for 1h, to remove calcium from the sections.
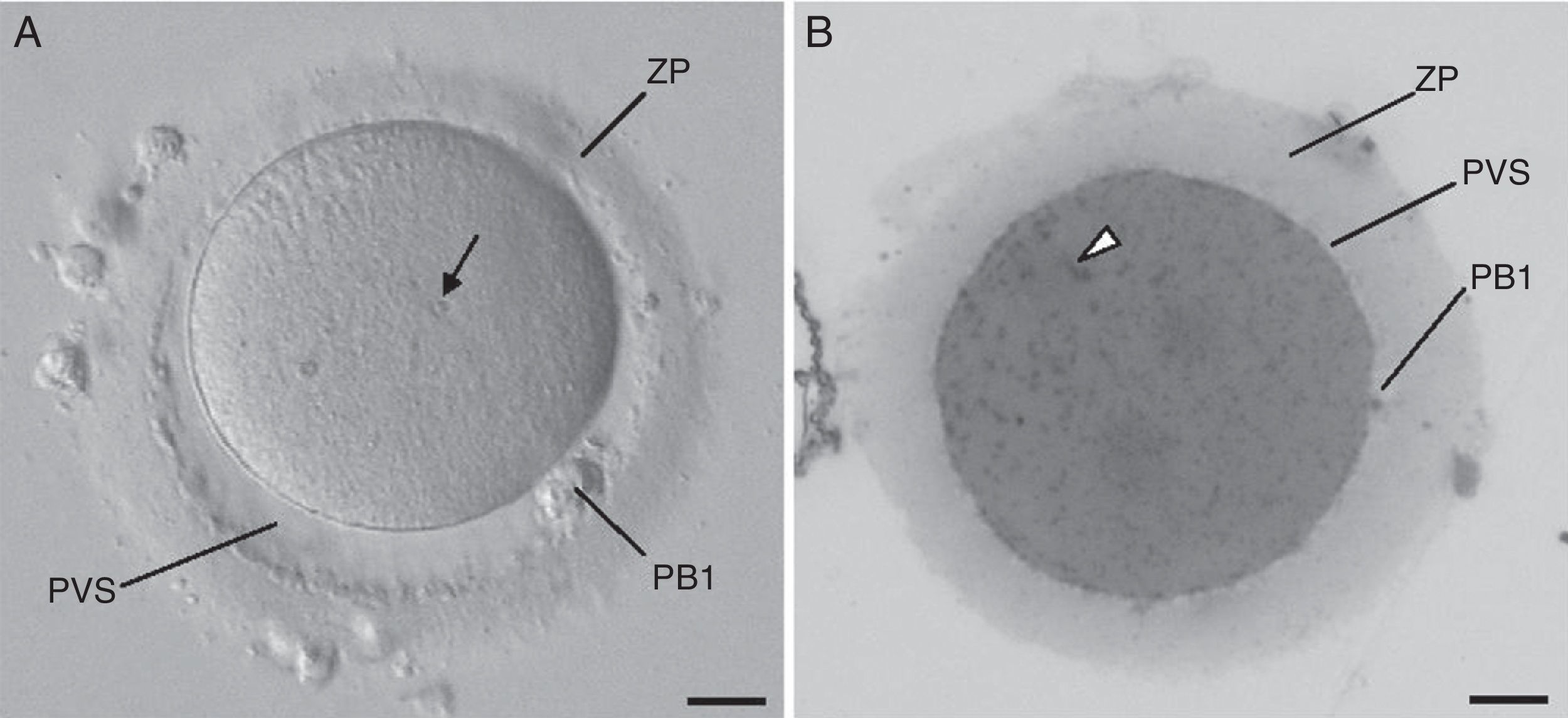
ResultsAt the inverted microscope (Fig. 1A), the mature MII oocyte appears as a round cell separated from the surrounding zona pellucida by a narrow perivitelline space. The oocyte cytoplasm presents a homogeneous fine granular texture, with a few small inclusions (known as refractile bodies, lipofuscin bodies or secondary lysosomes). At semi-thin sections (Fig. 1B), the oocyte cytoplasm displays a homogeneous appearance and is filled with numerous very small dense inclusions that correspond to mitochondria. At the ultrastructural level (Fig. 2A), the oocyte cortex shows several dense cortical vesicles and tiny SER vesicles, whereas the subcortex is filled with small SER vesicles, either isolated or associated with mitochondria, MV complexes, aSERT, isolated SER tubules and mitochondria.
(A) Live morphological normal human mature metaphase II oocyte observed at the inverted microscope. (B) The same oocyte observed in semithin section after processed for transmission electron microscopy. Zona pellucida (ZP), perivitelline space (PVS), first polar body (PB1), refractile body (arrow), and mitochondria (arrowhead). Bars: 20μm.
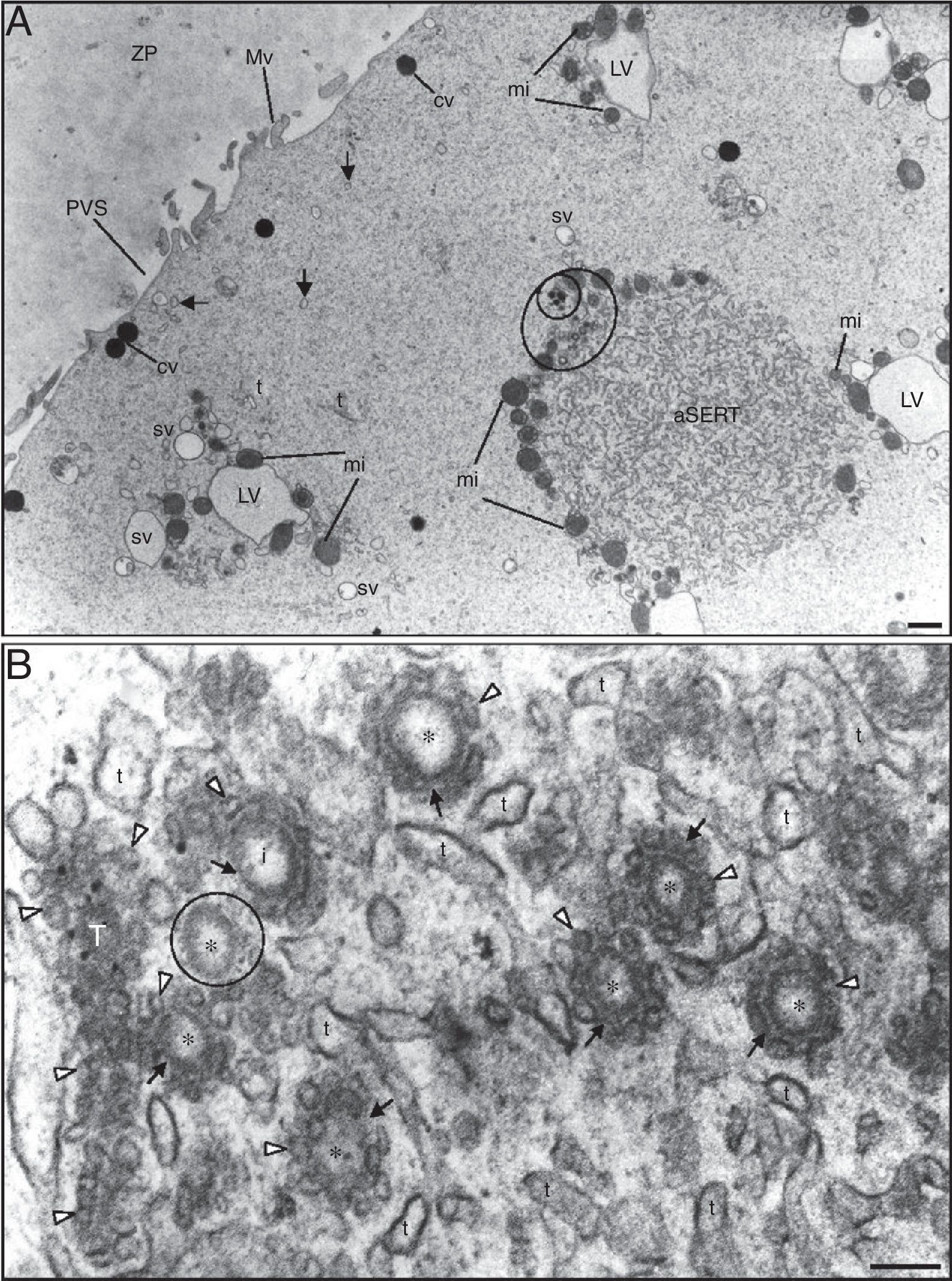
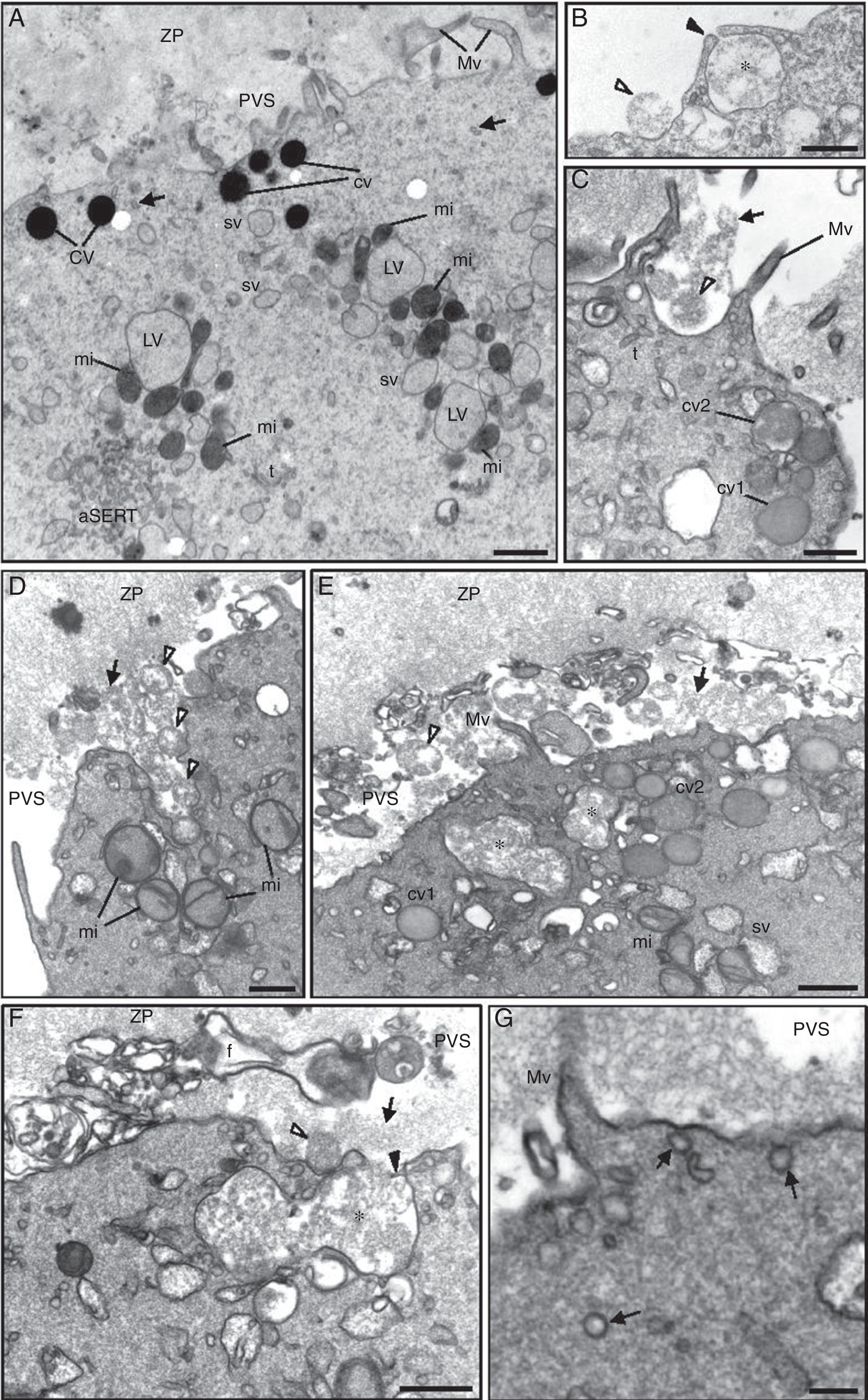
Ultrastructure of a morphological normal human mature metaphase-II oocyte. (A) Note the zona pellucida (ZP) coat, the narrow perivitelline space (PVS) and the short microvilli (Mv). In the cortex it can be observed dense cortical vesicles (cv) and tiny smooth endoplasmic reticulum (SER) vesicles (arrows). In the subcortex note the small SER vesicles (sv) (either isolated or surrounded by mitochondria) and large SER vesicles (LV) surrounded by mitochondria (MV complexes), the aggregate of SER tubules (aSERT), isolated SER tubules (t) and mitochondria (mi). At the periphery of the aggregate of SER tubules there is an aggregate of small vesicles (large circle), light and dense (small circle). (B) Small light vesicles (*) reside inside the mesh of SER tubules (t) of aSERT and are coated by tiny vesicles (white arrowheads) associated with a dense material (arrows). Note incompletely coated (i) and uncoated (small circle) small light vesicles. Tubular structure (T) of dense material impregnated with tiny vesicles. Bars: (A) 1μm; (B) 0.1μm.
We observed that some of the aSERT contain at one of its pole an aggregate of small vesicles, light (internal) and dense (external) (Fig. 2A). At high magnification the inner aggregate showed to be formed by small light vesicles coated by tiny vesicles associated with a dense material, giving a rosette-like appearance (Fig. 2B). Some of these vesicles appeared incompletely coated and others were totally uncoated (Fig. 2B). In this region it was also observed tubular structures made of a row of tiny vesicles impregnated in a dense material (Fig. 2B). The components of this zone were immersed in the peripheral net of the aSERT tubules (Fig. 2B). Slightly away from this place, there was another vesicle aggregate made of small dense vesicles partially coated by tiny vesicles associated with a dense material (Fig. 3A). In this part it was also observed several aggregates of a dense material with impregnated tiny vesicles, either isolated or associated to the uncoated zone of the dense vesicles (Fig. 3A). These dense rosette-like vesicles were more peripherally located and thus were immersed on a few tubules of the aSERT (Fig. 3A).
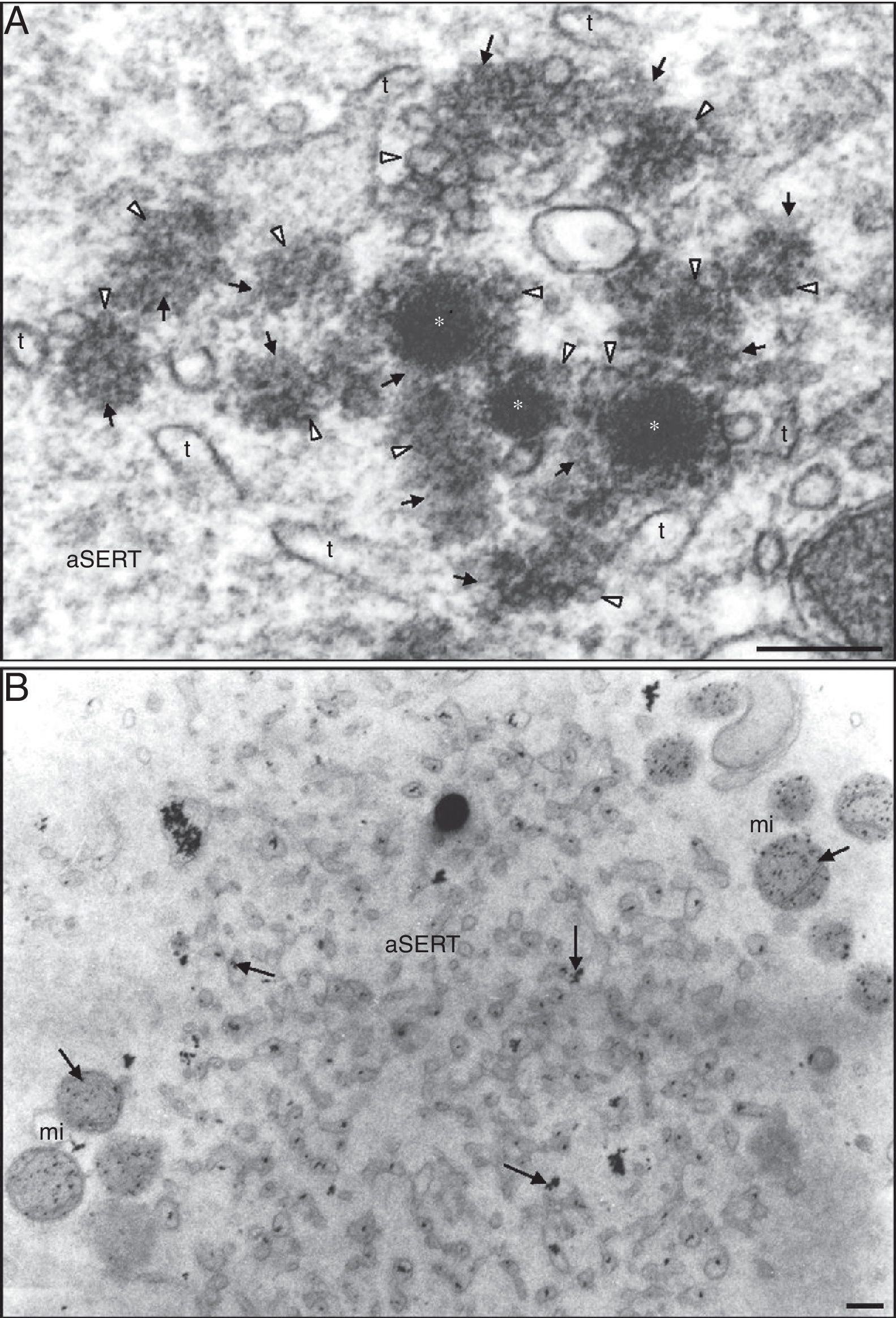
Ultrastructure of a morphological normal human mature metaphase-II oocyte. (A) Small dense vesicles at the periphery of the aggregate of smooth endoplasmic reticulum (SER) tubules (aSERT). The small dense vesicles (*) are partially coated by tiny vesicles (white arrowheads) associated with a dense material (arrows). Note the numerous isolated dense materials with impregnated tiny vesicles. There are only a few SER tubules (t). (B) Detection of calcium. Note the antimonate deposits (arrows) inside the tubules of aSERT and mitochondria (mi). Bars: (A) 0.1μm; (B) 0.2μm.
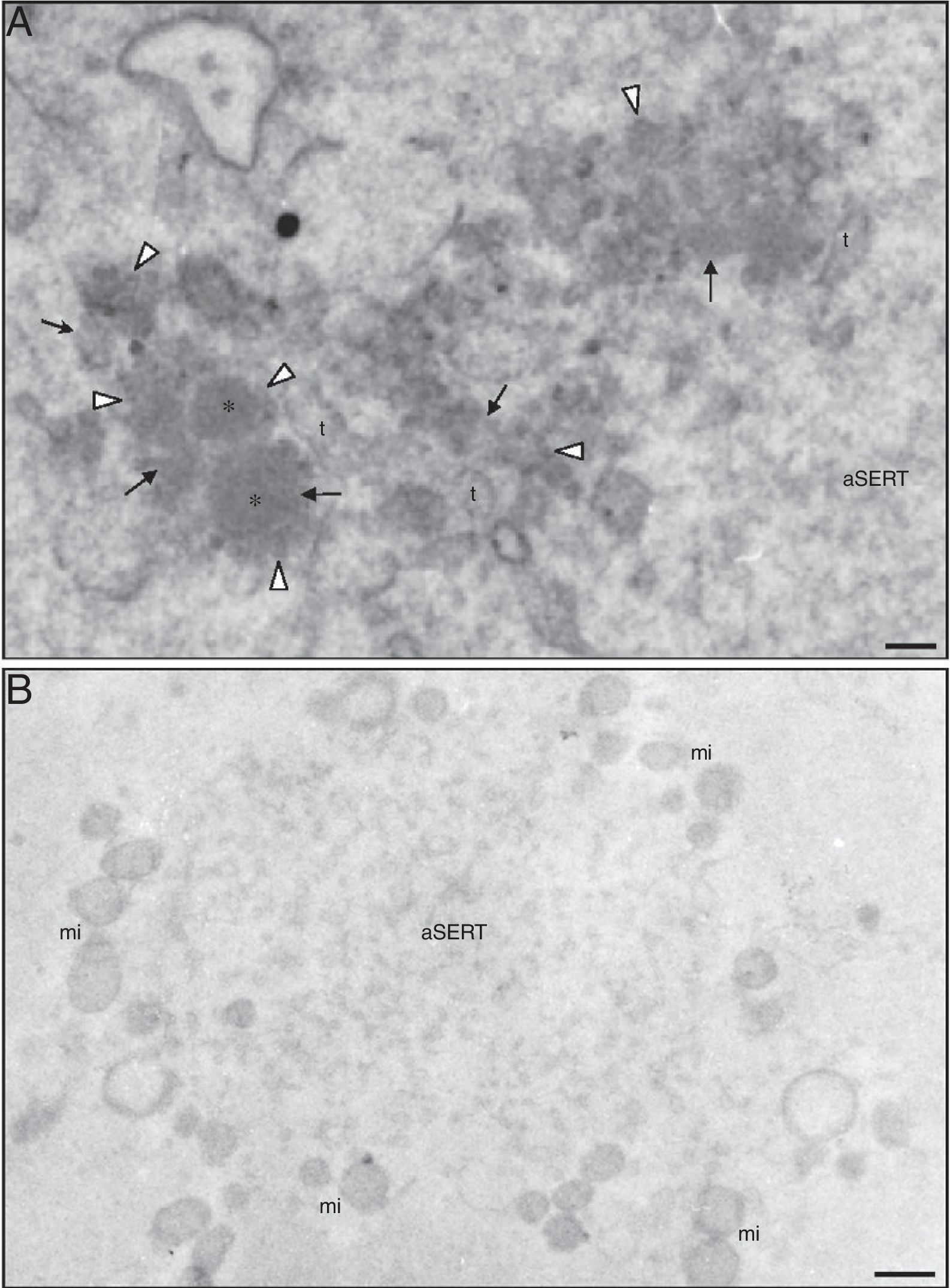
Antimonate deposits, indicating the presence of calcium, were evident in aSERT tubules and surrounding mitochondria (Fig. 3B), but not in the rosette-like vesicles, in the surrounding dense material or in the associated aSERT tubules (Fig. 4A). In controls, no antimonate deposits were observed (Fig. 4B).
Ultrastructure of a morphological normal human mature metaphase-II oocyte. (A) Detection of calcium. There are no antimonate deposits in small vesicles (*), in associated tiny vesicles (white arrowheads), in dense materials (arrows) either associated with small vesicles or in aggregates impregnated with tiny vesicles and in SER tubules (t). (B) Control of calcium detection. No antimonate deposits are observed in aSERT or in the associated mitochondria (mi). Bars: (A) 0.1μm; (B) 0.5μm.
Regarding the cortical reaction, before oocyte activation cortical vesicles evidenced homogeneous dense contents (Fig. 5A). After oocyte activation, the dense contents of cortical vesicles first acquired a moderate dense appearance and then a flocculent aspect just before fusion with the cytoplasmic oocyte membrane (Fig. 5B–D). Cortical vesicles were observed to individually fuse with the oocyte cytoplasmic membrane (Fig. 5B) or to fuse between each other before exocytosis (Fig. 5C and D). Sometimes, intracellular fusion between multiple cortical vesicles formed large vesicles with flocular contents (Fig. 5E). These then fused with the oocyte cytoplasmic membrane to deliver their contents into the PVS (Fig. 5F). The activated oocytes also showed signs of receptor mediated endocytosis as numerous coated vesicles were observed in the oocyte cortex under the oocyte membrane (Fig. 5G).
Ultrastructure of a morphological normal human mature metaphase-II oocyte. (A) Note the zona pellucida (ZP) coat, the narrow perivitelline space (PVS) and the short microvilli (Mv). In the cortex it can be observed dense cortical vesicles (cv) and tiny smooth endoplasmic reticulum (SER) vesicles (arrows). In the subcortex note the small SER vesicles (sv) (either isolated or surrounded by mitochondria) and large SER vesicles (LV) surrounded by mitochondria (MV complexes), the aggregate of SER tubules (aSERT), isolated SER tubules (t) and mitochondria (mi). (B) Exocytosis of a single cortical vesicle. Note the point of fusion with the oocyte cytoplasmic membrane (black-arrowhead). The flocular contents of the cortical vesicle (*) after expelled into the PVS show a round structure (white-arrowhead). (C and D) Simultaneous exocytosis of two or more cortical vesicles. The expelled contents retain a round structure (white-arrowheads) but thereafter are dispersed (arrow) in the PVS. In the oocyte cortex, the dense cortical vesicles are no longer observed. Instead, they show moderate dense contents (cv1) followed by a flocular appearance (cv2). (E) Intracellular fusion of cortical vesicles, forming very large vesicles with flocular contents (*). (F) Exocytosis of the very large vesicles. (G) Coated vesicles (arrows). Bars: (A) 1μm; (B–F) 0.5μm; (G) 0.2μm.
Once the continuity between gametes is established, the sperm delivers into the oocyte cytoplasm a sperm factor, which is supposed to be a phospholipase C-zeta (PLCzeta).34–36 Gamete receptor-mediated binding and/or fusion initiates a signal transduction through binding to a G-Protein or a protein tyrosine kinase that activates a phosphoinositide-specific phospholipase C (PLC). This PLC cleaves phosphatidylinositol bisphosphate (IP2) to inositol triphosphate (IP3) and diacylglicerol (DAG). The PLC-zeta also cleaves directly IP2 to IP3 and DAG. Whereas IP3 binds to the IP3 receptor (IP3R), which is a SER cation channel regulated by calcium, DAG activates a calcium and phospholipid-dependent protein serine kinase C (PKC).37–42 Binding of IP3 to SER and mitochondria calcium stores liberates calcium, and as a consequence a large increase in the cytosolic free calcium concentration is observed at the site of gamete fusion. This spike immediately spreads throughout the oocyte cytoplasm like a wave. This initial wave is then followed by a series of waves (calcium oscillations), always in the same direction (periphery-to-center).43,44 Calcium waves are dependent on calcium release from IP3-sensitive calcium stores at the oocyte periphery, followed by a calcium discharge from ryanodine-sensitive calcium stores at the oocyte center.14,15,43–47 These waves are also dependent on PKC, as it inhibits the opening of ryanodine stores when activated (facilitating the calcium spike at the periphery) and blocks it at the open state when at low activity (facilitating central calcium release).14,44,47
After the first calcium waves, the maintenance of calcium oscillations becomes dependent on external calcium. In this mechanism, external calcium is used to recharge intracellular calcium stores through the action of the stromal interacting molecule (STIM), a calcium-depletion sensor transmembrane protein of the SER that at fertilization moves to the cytoplasmic membrane to activate the calcium channel ORAI1, thus enabling calcium entry. In this system, PKC at high activity inhibits this channel and at low activity activates this calcium channel. The free calcium (liberated and exogenous) is then reabsorbed into the SER via transmembrane receptors of the calcium-ATPase family (SERCA).14,37–39,41,42,45,46 From the moment of pronuclei formation (zygote stage), calcium waves change to a central-to-periphery orientation and become of weaker intensity. This is accompanied by the movement of SER and PKC from the periphery to the center of the cell.14,47
During calcium oscillations, the calcium-binding protein calmodulin (CAM) binds calcium and, with the assistance of PKC, phosphorylates and activates a calmodulin-dependent protein kinase (CaMK). This kinase activates the Na+/H+ antiporter channel with subsequent H+ efflux, leading to cytoplasmic alkalinization. This is essential for oocyte meiotic resumption and metabolism activation, as well for cortical vesicle exocytosis and cortical actin rearrangements.21,37–39,41,42,48 Whereas increased calcium levels enable microvilli elongation, the elevated pH triggers the polymerization of the cortical submembranar actin into rigid bundles. On the contrary, actin filament depolimerization was observed to underlie cortical vesicle exocytosis and endocytosis.21,49 Besides microvilli elongation, endocytosis also helps in recycling the excess membrane caused by cortical vesicle exocytosis.50
Before fertilization, the ultrastructural analysis showed that calcium predominantly accumulates inside SER vesicles and tubules, as well as in mitochondria, in the oocyte cortex and subcortex.15 However, after fertilization, aSERT disaggregate and the large SER vesicles are no longer observed, with the cortex and subcortex oocyte regions becoming populated by only a few small SER vesicles and mitochondria, both with low calcium content. On the contrary, at the oocyte center and around pronuclei, calcium deposits became abundant in the innumerous SER vesicles and mitochondria. This redistribution of organelles and calcium follows the changes observed for calcium waves and PKC positioning during fertilization and at the pronuclear stage.15,47 In fact, the first calcium spike and the following waves observed at fertilization44 are supported by the presence of a high concentration of calcium in SER and mitochondria,15 with waves presenting a periphery-to-center direction.44 However, at the pronuclear stage, calcium waves change to a center-to-periphery direction,47 which is accompanied by an accumulation of calcium stores and calcium deposits in the oocyte center, with presence of only a few calcium stores and calcium deposits at the oocyte periphery.15
We here describe a novel component of aSERT. We observed a group of small vesicles at the periphery of aSERT. This region contained small pale vesicles coated by tiny vesicles, with associated dense materials, forming pale rosette-like structures. We also observed some small pale vesicles partially coated or totally uncoated. Additionally, in the same area, we observed elongated structures with dense materials impregnated with tiny vesicles. Adjacent to this region and at a slightly more distance from the aSERT periphery, it was also observed an aggregate of small dense vesicles incompletely coated with tiny vesicles, with associated dense materials, forming dense rosette-like structures. At the uncoated area these vesicles were associated with dense materials containing tiny vesicles.
We did not find any morphological evidence that could relate both types of rosette-like vesicles. We thus do not know if pale and dense small vesicles represent different entities or are somehow related, mainly because the dense vesicles are observed at a more external place of aSERT. Additionally, as some vesicles appeared incompletely coated and others were not coated, but were associated with the dense material, this raises the hypothesis that small pale and dense vesicles are first formed and then coated with dense materials containing the tiny vesicles. However, it is also possible that the coat is progressively lost, with liberation of tiny vesicles. After determination of calcium contents and as previously described,15 the tubules of aSERT and mitochondria were filled with antimonate deposits. As the components of the rosette-like vesicle regions did not present calcium deposits, to date we do not realize the function of these rosette-like structures. Large aSERT was described in abnormal oocytes,51 with no reference to these rosette-like vesicles, which may suggest that rosette-like vesicles are not a sign of pathology.
Cortical vesicles first translocate to the plasma membrane along the actin cytoskeleton with the aid of CaMK and a myosin light chain kinase (MLCK). In the oocyte cortex, under the cytoplasmic membrane, the polymerized actin network gel entraps cortical vesicles, inhibiting exocytosis, whereas when depolymerized actin facilitates docking and exocytosis of cortical vesicles.52,53 Soluble N-ethylmalameide-sensitive factor Attachment Protein Receptor proteins (SNARE) are transmembrane fusogenic proteins that act by interacting with membrane phospholipids. They mediate vesicle docking and membrane fusion. Proteins of the v-SNARE type include the vesicle-associated membrane protein (VAMP) and synaptotagmin (calcium sensor protein), which are present on membrane domains where vesicles will fuse. These proteins are coupled to small GTP-binding proteins (Rab). Proteins of the t-SNARE type include syntaxin and the synaptosome-associated protein (SNAP), which are found on the membrane of cortical vesicles. These proteins are associated with membrane vesicle complexin (stabilizes the SNARE complex and prevents the premature fusion of the interacting membranes). Docking occurs when t-SNARES bind to v-SNARES via complexin. Under calcium oscillations at fertilization, CAM binds calcium and the activated PKC phosphorylates and activates CaMK. After binding calcium, synaptotagmin is phosphorylated by PKC and CaMK, which triggers complexin release. This allows the association between the two types of SNARE, followed by merges between the two membranes. Cortical vesicles are coated not only by complexin but also by clathrin. When these coating proteins dissociate from the cortical vesicle membrane after vesicle fusion, endocytosis is triggered.24–26,28,50,54 The oocyte cortical microfilament network is involved in the cortical reaction, as actin remodeling molecules interact with synaptotagmin, which after phosphorylation triggers actin depolimerization, enabling exocytosis. In this process, syntaxin is dephosphorylated.24,52,53,55
The large majority of ultrastructural studies showed that cortical vesicles are observed as a homogeneous population of vesicles with dense contents.2–4,10,11,56,57 However, other studies described two morphologically different populations of cortical vesicles, the classical homogeneous dense vesicles and slightly larger vesicles with moderate dense granular contents.1,58 Additional suspicion for a second type of cortical vesicles came from a study where different lectin labeling indicated the presence of two types of cortical vesicles. However, in this study, labeling did not enable to uncover the morphology of vesicles. Thus it remains unknown if there are two morphologically distinct vesicles with different lectin contents or if there is only one morphological type of cortical vesicles although with two different types of lectin contents.5
We here first describe that after oocyte activation, the dense contents of cortical vesicles change to a moderate dense appearance, followed by a light appearance with granulo-fibrilar contents at the moment of membrane fusion. We place the hypothesis that this is caused by swelling of the cortical vesicles prior to exocytosis. Thus, it is possible that the two ultrastructural types of cortical vesicles previously observed (in premature cortical reaction of immature oocytes) correspond in fact to a unique type of cortical vesicles observed at distinct moments of the cortical reaction. Swelling of cortical vesicles immediately before exocytosis at fertilization has also been previously documented in echinoderms.16
On the oocyte surface coated vesicles and coated membrane invaginations were previously observed.1–4,57,58 In our observations, receptor mediated endocytosis was also evident after oocyte activation, which is accordance with the observed increase in endocytosis at fertilization.50
In conclusion, we describe a new vesicle component associated with aSERT and present new observations on the remodeling of cortical vesicles before exocytosis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was financed by the Institutions of the authors and in part by UMIB, which is funded by National Funds through FCT-Foundation for Science and Technology, under the Pest-OE/SAU/UI0215/2014.
Conflict of interestThe authors declare to have no conflict of interest.
We would like to acknowledge: Jorge Beires, MD, PhD, Gynecology and Obstretrics (Department of Gynecology and Obstetrics, Director of the Unit of Gynecology and Reproductive Medicine, Hospital de S. João, E.P.E, Porto, Portugal), and José Manuel Teixeira da Silva, MD, Gynecology and Obstretrics, for oocyte retrieval. José Correia, MD, Anesthesist (Department of Anesthesiology, Hospital de S. João, E.P.E, Porto, Portugal), for anesthesiology assistance during oocyte pick-up. Cristiano Oliveira, MD, Gynecology and Obstretrics, Subspecialization in Reproductive Medicine; and José Teixeira da Silva, MD, Gynecology and Obstretrics, Subspecialization in Reproductive Medicine (CGR), for patient evaluation and controlled ovarian hyperstimulation. Joaquina Silva, MD, Senior Clinical Embryologist-ESHRE, Mariana Cunha, BSc, Biologist, Clinical Embryologist-ESHRE, Paulo Viana BSc, Biologist, Clinical Embryologist-ESHRE, Ana Gonçalves, BSc, Biochemist, and Cláudia Osório, BSc, Biologist, for IVF laboratory work (CGR). Ângela Alves, teaching and research Technical assistant, for additional support on electron microscopy (ICBAS-UP).