Finding new agents for prevention and/or treatment of benign prostatic hyperplasia (BPH) especially from natural sources is a demanding field.
ObjectivesThis study aimed to evaluate the effect of black mulberry (BM) (Morus nigra) fruit hydroalcoholic extract on the establishment of BPH in rats.
Materials and methodsForty-nine adult male rats were randomly assigned into 7 equal groups: I: Sham control (SC), a sham surgery was performed. II: positive control (PC), rats were castrated and received testosterone propionate, at 10mg/kg/day S.C. for BPH induction. III: comparative control (CC), BPH was induced and the rats received finasteride at 5mg/kg/day P.O. IV–VII: (T1–T4): BPH was induced and the rats received BM extract at 25, 50, 100 and 200mg/kg/day P.O. for 4 consecutive weeks.
ResultsFinasteride and/or BM extract especially at the two higher dosages, significantly affected prostate weight, prostatic index, percent of inhibition, serum and prostatic levels of dihydrotestosterone (DHT), serum prostate-specific antigen (PSA), antioxidant parameters of prostatic tissue as well as histopathological and histomorphometric parameters (epithelial thickness and acinar area) of prostate.
ConclusionsBM extract has protective effects against experimentally-induced BPH in rats with regard to histopathological and biochemical parameters which may be related to its antioxidant as well as DHT reducing properties in prostatic tissue.
El hallazgo de nuevos agentes para prevenir y/o tratar la hiperplasia prostática benigna (HBP), procedentes especialmente de fuentes naturales, es un campo exigente.
ObjetivosEl objetivo de este estudio fue evaluar el efecto del extracto hidroalcohólico de las moras (Morus nigra) sobre el establecimiento de HBP en ratas.
Materiales y métodosSe asignaron aleatoriamente 49 ratas adultas macho en 7 grupos iguales: I: grupo control (GC), en el que se practicó cirugía de control; II: control positivo (CP), en el que se castró a las ratas y se les administró propionato de testosterona, a una dosis de 10mg/kg/día sc para inducción de BPH. III: control comparativo (CC), en el que se indujo BPH y se administró a las ratas finasterida a una dosis de 5mg/kg/día po; IV-VII: (T1-T4), en el que se indujo BPH y se administró a las ratas extracto de moras a una dosis de 25, 50, 100 y 200mg/kg/día po durante 4 días consecutivos.
ResultadosLa finasterida y/o el extracto de moras, especialmente en 2 dosis elevadas, afectaron significativamente al peso prostático, al índice prostático, al porcentaje de inhibición, a los niveles séricos y prostáticos de dihidrotestosterona (DHT), al antígeno prostático específico sérico (PSA), a los parámetros antioxidantes del tejido prostático, así como a los parámetros histopatológicos e histomorfométricos (espesor epitelial y área acinar) de la próstata.
ConclusionesEl extracto de mora tiene efectos protectores frente a HPB experimentalmente inducido en ratas, con respecto a sus parámetros histopatológicos y bioquímicos, y que pueden guardar relación con sus propiedades antioxidantes y reductoras de DHT en el tejido prostático.
Benign prostatic hyperplasia (BPH) is a common and vexing condition encountered in men of middle and old ages. The clinical condition is associated with lower urinary tract filling/irritative and obstructive symptoms.1
The etiology of BPH has not been completely clarified and a host of different factors including inflammatory mediators, hormones, dietary factors, inflammatory genes, and oxidative stress may have a role in the development of BPH.2
Generally, androgenic stimulation and increased adrenergic tone are considered as the main culprits and this constructs the logical basis for administration of anti-androgenic drugs (mainly prostate 5α-reductase inhibitors) and anti-adrenergic drugs (mainly α1-adrenergic receptors (α1-AR) antagonists) to patients with PBH.3,4
As can be expected, use of these agents is not without adverse effects and myopathies due to anti-androgenic drugs4 as well as ejaculatory or erectile dysfunction and decreased libido5 are important concerns. Moreover, lifetime use of these drugs can negatively affect patients’ compliance.
Finding agents with better safety profile and with the ability to target multiple and/or novel aspects of the pathogenesis of the disease is a challenging subject and can be helpful in management of BPH.
Minciullo et al.2 presented an up-to-date systematic review about the association of oxidative stress and BPH. Obvious oxidative stress has been shown in patients with BPH,6 and restoring the antioxidant status has improved the clinical outcomes.7
Fruits and vegetables with red, purple, and blue colors are a valuable source of anthocyanins. These phenolic compounds are well known for their multiple beneficial effects in different disease conditions such as cardiovascular diseases, diabetes mellitus and obesity as well as their anticancer and antioxidant properties.8
Black mulberry (BM) (Morus nigra) is one of the most commonly known species in the Morus genus from the family Moraceae which is native to Southwestern Asia. Apart from their fantastic flavor and high nutritional values, the fruits are rich in anthocyanins9 and in a study,10 BM showed higher antioxidant activity and amounts of phenolic compounds than white mulberry. These fruits not only have a history of use in folk medicine, modern pharmacological studies have also shown their positive health effects, for instance, in a previous study,11 administration of ethanolic extract of BM to rats suppressed experimental atherosclerosis development by regulating lipid metabolism and antioxidant activities attributed to anthocyanins or the cooperative action of anthocyanins, polyphenols and flavonoids.
Taken together, in the present study we evaluated the possible protective effect of BM fruit hydroalcoholic extract on the establishment of the disease in a rat model of BPH with regard to histological, biochemical and oxidative stress status parameters.
Materials and methodsPlant material and preparation of the extractRipe BM fruits were collected in June 2017 from Torqabeh city, Mashhad, Iran (coordinates: 36.3115° N, 59.3829° E). Genus and species were authenticated by a botanist. Fruits were gently washed with water and then were dried in a clean and dust-free environment away from direct sun-light. Preparation of hydroalcoholic extract from fruits was performed by cold maceration method using 70% ethanol as described by Chen et al.12 with modifications. Briefly, 100g of BM powder was macerated in 1000mL of 70% ethanol (plant/solvent ratio of 1:10, w/v) and incubated in the dark at room temperature for 24h. Mixture was filtered by using Whatman filter (No. 1) paper and then was concentrated by using a rotary evaporator at 50°C. Then the extract was lyophilized under 50mmHg pressure, the powder was kept at −20°C until use and dissolved in distilled water at the time of administration.
AnimalsA total of 49 male Sprague Dawley rats, aged 3 months and weighed 200–250g were supplied by the animal lab of Shiraz University of Medical Sciences (Shiraz, Iran). The rats were kept in standard cages in a controlled temperature-environment (c. 22°C) with a 12/12h light/dark cycle, with free access to commercial pelleted food and tap water.
Study designAfter a week of adaptation period, rats were randomly assigned into 7 equal groups as follows: I: Sham control (SC) group, rats were subjected to a sham surgery. II: positive control (PC) group, rats were castrated and received testosterone propionate (Aburaihan Pharmaceutical Co., Iran), at 10mg/kg/day by SC injections for BPH induction.13 III: comparative control (CC) group, BPH was induced and also rats received finasteride (Sigma, Germany) at 5mg/kg/day by oral gavages.13 IV: treatment one (T1) group: BPH was induced and rats received BM extract at 25mg/kg/day by oral gavage. V: treatment two (T2) group: BPH was induced and rats received BM extract at 50mg/kg/day by oral gavage. VI: treatment three (T3) group: BPH was induced and rats received BM extract at 100mg/kg/day by oral gavage. VII: treatment four (T4) group: BPH was induced and rats received BM extract at 200mg/kg/day by oral gavage. Administration of all drugs was initiated 7 days post surgery and continued for 4 consecutive weeks.
All procedures used in the present study were approved by institutional ethical committee and were in accordance with EU guidelines on animal experiments.
Blood and tissue samplingAt the end of the experiment, and after an overnight fasting, all animals were weighed and blood samples were collected under anesthesia by cardiocentesis. Samples were taken from 9 to 11am.
All animals were sacrificed by deepening anesthesia and ventral prostatic lobes were dissected and weighed to calculate the prostatic index (PI) by dividing prostate weight to body weight of each animal. Percentage of inhibition for PI was also calculated for by using the formula: 100−[(T−SC)/(PC−SC)∗100], where SC, PC, and T were the values of the sham control, positive control and treatment groups, respectively.14
Half of the prostate was fixed in 10% neutral-buffered formalin. The other half was kept at −80°C for biochemical tests.
Histopathological evaluationAfter routine histological procedures, 5μm-thick sections were made from paraffin blocks prepared from ventral lobe and stained with hematoxylin and eosin (H&E). All slides were evaluated under a light microscope for histopathological changes, moreover the thickness of epithelium and acinar area were assayed in photomicrographs by using Digimizer image analysis software V4.1.1 as described by Ali et al.14
Preparation of prostatic tissue homogenateThe remaining part of the prostatic tissue was mechanically homogenized in 1/10 (w/v) PBS 100mM (pH 7.4) and then sonicated on ice 2 times (each time for 20s) with 20-s intervals, followed by centrifugation at 5000g for 15min at 4°C. The supernatant was used for further analysis.
Determination of oxidative stress parameters in prostate tissueOxidative stress status parameters including malon dialdehyde (MDA) and total antioxidant capacity (TAC) were assayed in prostate tissue by using commercial kits prepared by Zell bio, Germany. Glutathione (GSH) level was assayed by a kit prepared by Sunlong Biotech, China.
Biochemical assaysSera were separated by centrifugation at 3000×g for 15min and stored at −80°C. Serum BUN and creatinine levels were determined by using commercial kits (Pars azmoon Co., Iran).
Serum and prostatic tissue dihydrotestosterone (DHT) levels were assayed by rat DHT ELISA kit prepared by Cusabio®, China based on competitive inhibition enzyme immunoassay technique and was accomplished as described by the manufacturer. Inter- and intra-assay coefficient of variations (CVs) of the kit were both <15%.
Serum prostate-specific antigen (PSA) was assayed by rat PSA ELISA Kit based on quantitative sandwich enzyme immunoassay technique (Cusabio®, China), with both inter and intra assay CVs of <10%.
Statistical analysisData were expressed as mean±SD. Statistical analysis was performed by one-way ANOVA followed by Tukey's multiple comparison tests using Graph pad Prism 6 software. p<0.05 was considered as significant level.
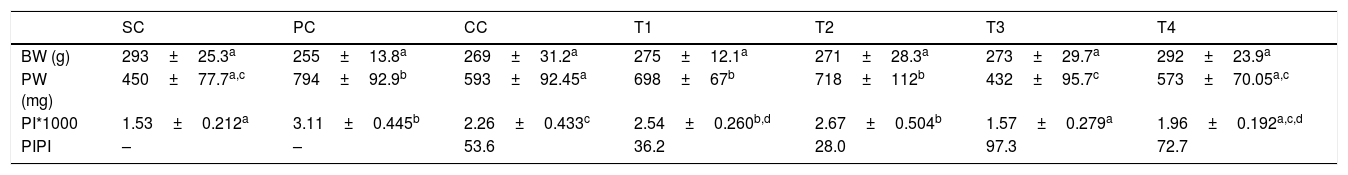
ResultsBody and prostate weight, prostatic index (PI) and percentage of inhibitionNo significant difference was observed in body weight among groups (p>0.05). Induction of BPH was associated with a significant increase in prostate weight of rats in PC group as compared to SC rats (p<0.0001). Finasteride administration was associated with a significant reduction in prostatic weight as compared to PC group (p<0.01), moreover; BM extract at two higher dosages (T3 and T4) significantly decreased this parameter as compared to PC group (p>0.0001 and p<0.01, respectively).
A significant increase in prostatic index was observed in rats of PC group as compared to SC rats (p<0.0001). Administration of finasteride significantly reduced this parameter as compared to PC group (p<0.01). Prostatic index of rats in T3 and T4 groups was significantly decreased as compared to PC rats (p<0.0001 for both comparisons).
The highest percentage of inhibition for PI was observed in T3 group, followed by T4, CC, T1 and T2 groups. Data are summarized in Table 1.
Body and prostate weight, prostatic index (mean±SD) and percentage of inhibition of rats in different groups.
| SC | PC | CC | T1 | T2 | T3 | T4 | |
|---|---|---|---|---|---|---|---|
| BW (g) | 293±25.3a | 255±13.8a | 269±31.2a | 275±12.1a | 271±28.3a | 273±29.7a | 292±23.9a |
| PW (mg) | 450±77.7a,c | 794±92.9b | 593±92.45a | 698±67b | 718±112b | 432±95.7c | 573±70.05a,c |
| PI*1000 | 1.53±0.212a | 3.11±0.445b | 2.26±0.433c | 2.54±0.260b,d | 2.67±0.504b | 1.57±0.279a | 1.96±0.192a,c,d |
| PIPI | – | – | 53.6 | 36.2 | 28.0 | 97.3 | 72.7 |
SC: sham control, PC: positive control with benign prostatic hyperplasia, CC: comparative control, rats with benign prostatic hyperplasia that received finasteride at 5mg/kg/day by oral gavages and T1–T4: rats with benign prostatic hyperplasia that received black mulberry extract at 25, 50, 100 and 200mg/kg/day by oral gavages for 4 consecutive weeks. BW: body weight, PW: prostate weight, PI: prostatic index and PIPI: percentage of inhibition for prostatic index.
Values without one common letter within a row are significantly different (p<0.05). For PIPI statistical analysis was not applicable.
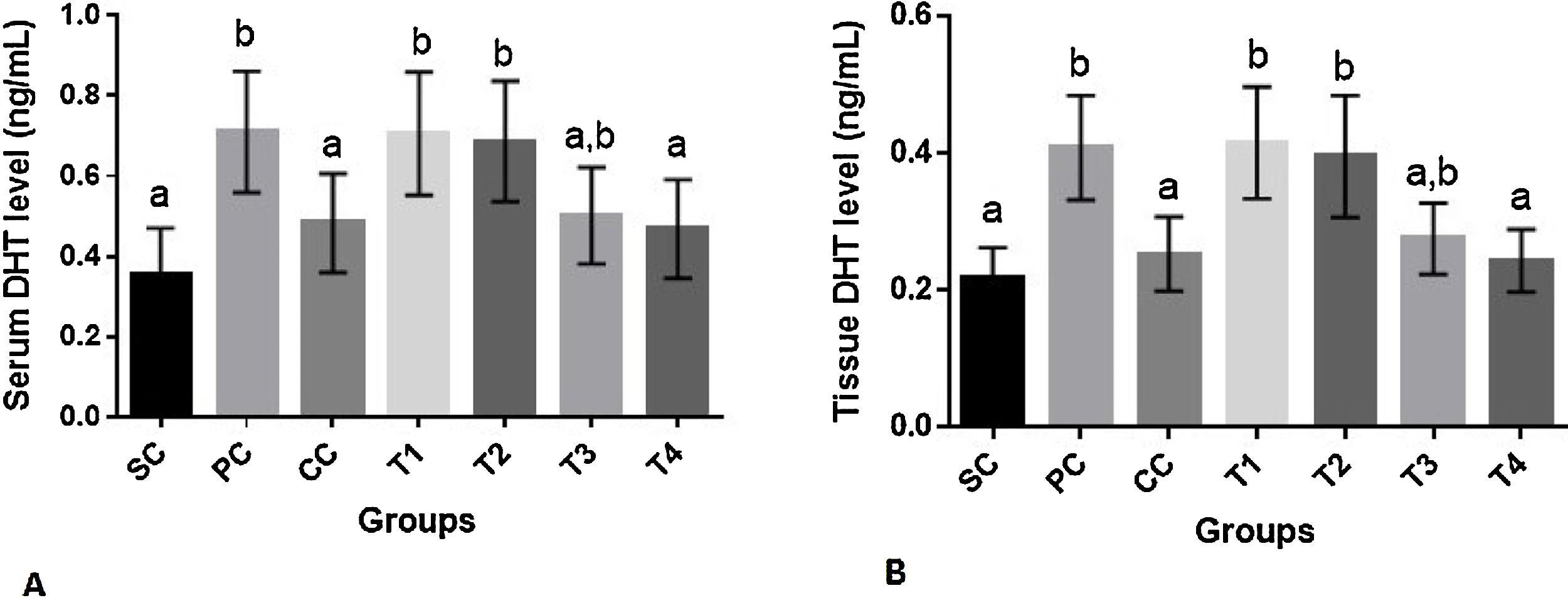
No significant difference was observed in serum BUN and creatinine levels among groups (p>0.05) (data not shown). As demonstrated in Fig. 1A serum level of DHT was significantly increased in PC group as compared to SC rats (p=0.001). Finasteride and the highest dose of BM extract appreciably reduced this parameter as compared to PC group (p<0.05 for both comparisons). The level of serum DHT in these groups was statistically similar to that of SC group (p>0.05).
Serum (A) and prostate tissue (B) dihydrotestosterone (DHT) levels (mean±SD) of rats in different groups. SC: sham control, PC: positive control with benign prostatic hyperplasia, CC: comparative control, rats with benign prostatic hyperplasia that received finasteride at 5mg/kg/day by oral gavages and T1–T4: rats with benign prostatic hyperplasia that received black mulberry extract at 25, 50, 100 and 200mg/kg/day by oral gavages for 4 consecutive weeks. Columns without the presence of a common letter have significantly different values (p<0.05).
The results of the effect of finasteride and BM extract on DHT levels of prostate tissue was similar to that of serum where finasteride and BM extract at its highest dose could reverse DHT concentration to its value in SC group (p>0.05). The level of this hormone in prostate tissue of rats in T4 and CC groups was significantly lower than rats in PC group (p<0.01 for both comparisons) (Fig. 3B).
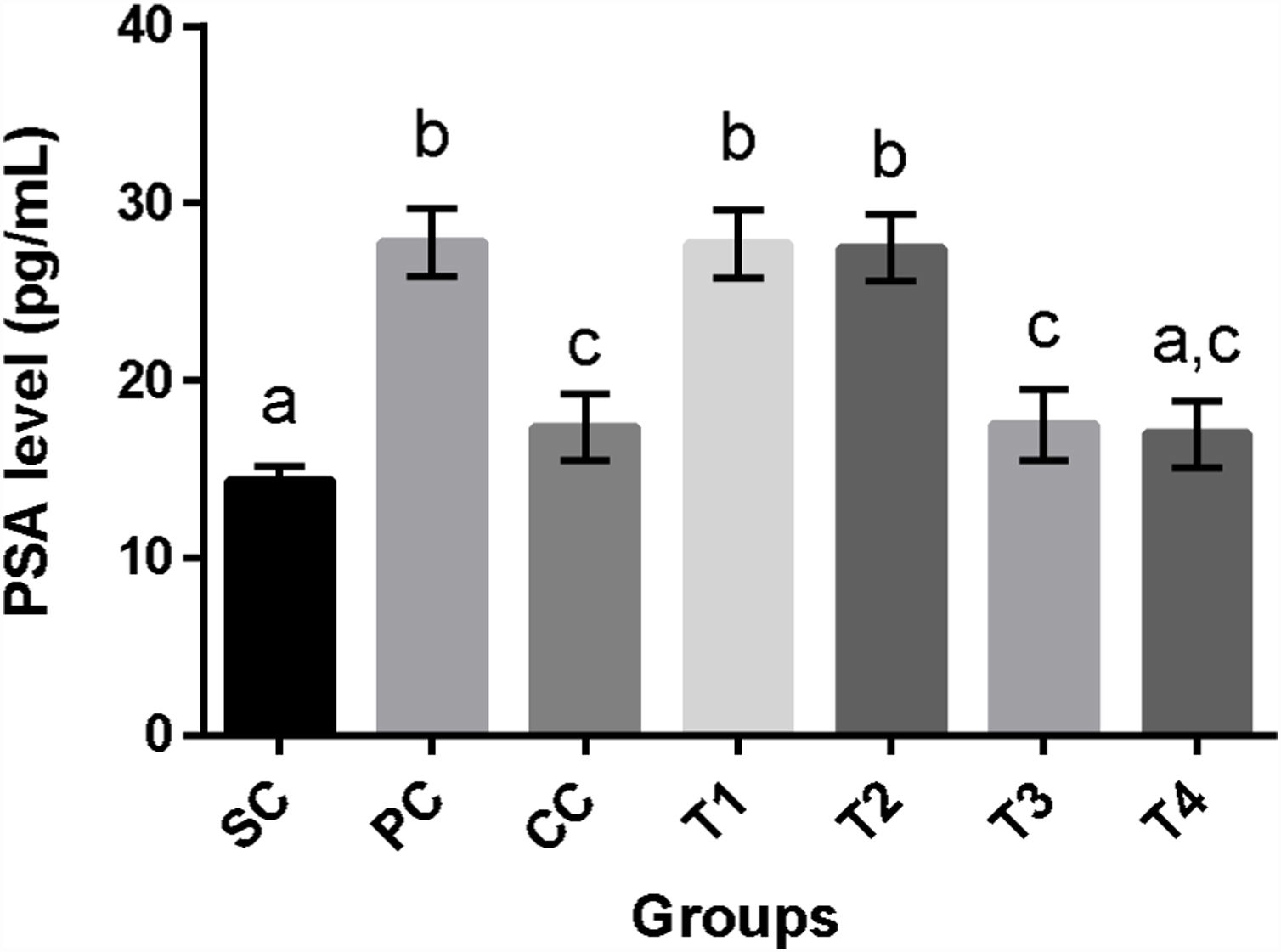
Prostate-specific antigen levelsInduction of BPH in rats of PC group was associated with a significant increase in PSA levels as compared to SC rats (p<0.0001). Although finasteride and two higher doses of BM extract appreciably reduced this parameter as compared to PC group (p<0.0001 for all comparisons), only T4 rats showed statistically the same values for PSA in comparison with SC rats (p>0.05) (Fig. 2).
Serum prostate-specific antigen (PSA) levels (mean±SD) of rats in different groups. SC: sham control, PC: positive control with benign prostatic hyperplasia, CC: comparative control, rats with benign prostatic hyperplasia that received finasteride at 5mg/kg/day by oral gavages and T1–T4: rats with benign prostatic hyperplasia that received black mulberry extract at 25, 50, 100 and 200mg/kg/day by oral gavages for 4 consecutive weeks. Columns without the presence of a common letter have significantly different values (p<0.05).
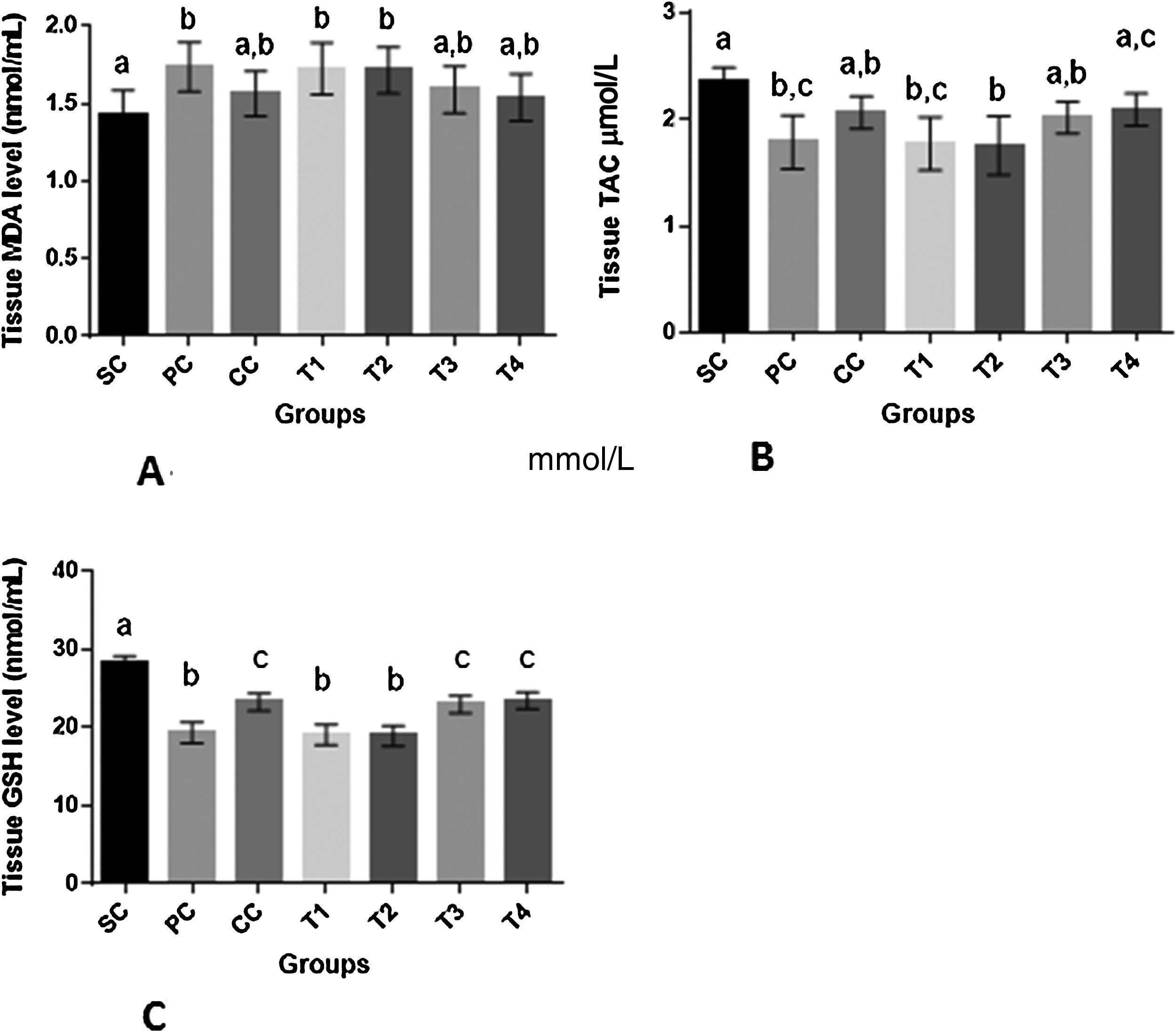
Overt oxidative stress was present in prostate tissue of rats in PC group as shown by decreased TAC and GSH associated with increased MDA level as compared to SC group (p<0.01 for all comparisons). Administration of finasteride to rats of CC group resulted in increased GSH level (p<0.001) as compared to PC group. The changes in TAC and MDA levels in CC rats were insignificant as compared to both PC and SC rats (p>0.05). Higher dosages of BM (T3 and T4) resulted in significant increase of GSH as compared to PC group (p<0.0001). In rats of T3 and T4 groups changes in MDA and TAC was insignificant as compared to both SC and PC groups (p>0.05) (Fig. 3).
Oxidative stress parameters (A–C) (mean±SD) of prostatic tissue of rats in different groups. SC: sham control, PC: positive control with benign prostatic hyperplasia, CC: comparative control, rats with benign prostatic hyperplasia that received finasteride at 5mg/kg/day by oral gavages and T1–T4: rats with benign prostatic hyperplasia that received black mulberry extract at 25, 50, 100 and 200mg/kg/day by oral gavages for 4 consecutive weeks. Columns without the presence of a common letter have significantly different values (p<0.05).
In histopathological evaluation of prostatic tissue, rats in SC group showed normal appearance without any specific lesion. Although the luminal diameter of acini was diverse, only a single cellular layer was present in the wall of all acini. On the contrary, acinar walls showed considerably thickened appearance due to cellular hyperplasia in rats of PC group. Hyperplastic cells were arranged in papillary form structures that were projected in to the lumen of acini. In rats of CC group, most of the acini showed normal appearance although hyperplastic foci were still present in 70% of the samples. Papillary projections were not detected in this group. In rats of T1 and T2 groups, some normal acini were present, however some of the acini showed hyperplasia and occasionally papillary form projections. These changes were more prominent in T1 group. Epithelial thickening was also observed in these groups. In T3 group, 57% of samples had normal-like appearance of the acini. In the remaining samples, most of the acini were normal but a few had mild epithelial thickening. Signs of immune cells infiltration in interstitial tissue of prostate was observed in rats of this group. In T4 group like T3 group, 57% of samples showed normal appearance. In remaining samples, mild epithelial thickening was observed in a few number of acini (Fig. 4).
Representative photo micrographs of prostatic tissue of rats in different groups. SC: sham control, PC: positive control with benign prostatic hyperplasia, and T1–T4: rats with benign prostatic hyperplasia that received black mulberry extract at 25, 50, 100 and 200mg/kg/day by oral gavages for 4 consecutive weeks. Arrows show papillary projections or hyperplastic foci and asterisk signs are used to show hyperplastic cells. Bar=100μm.
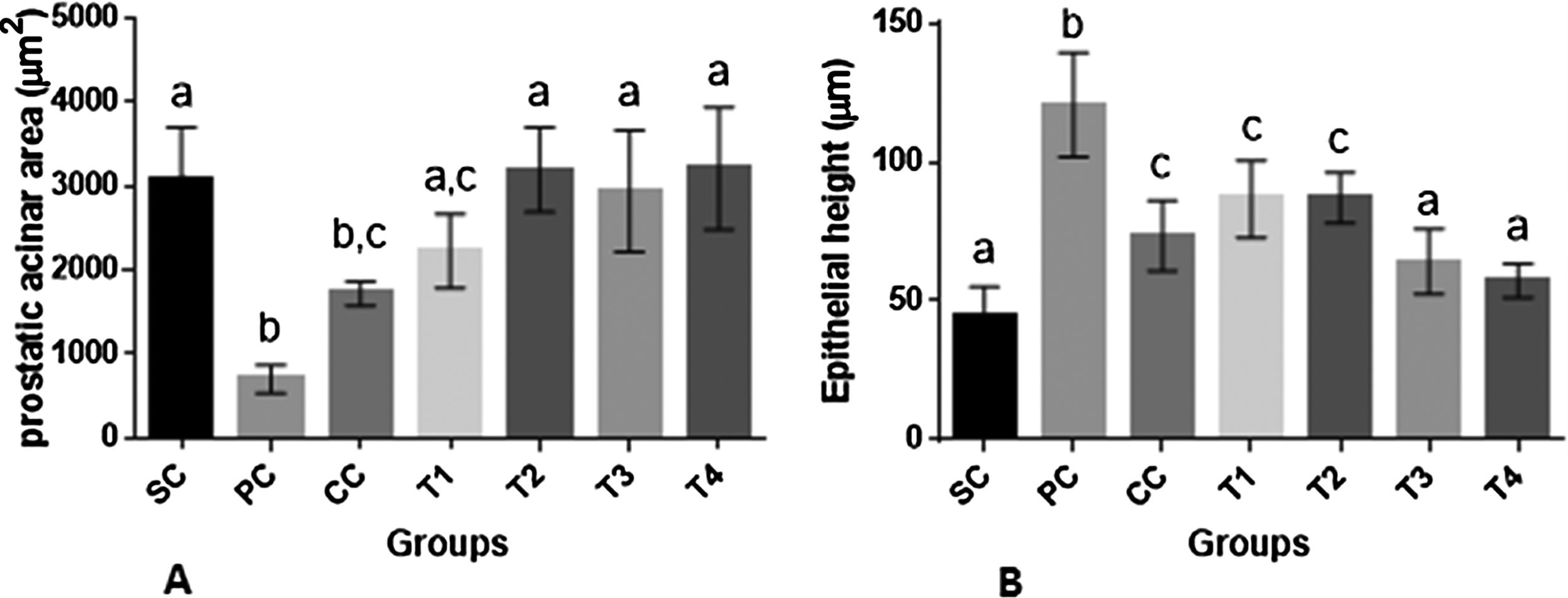
As shown in (Fig. 5A and B), induction of BPH was associated with a significant decrease in prostatic acinar area along with increased epithelial height in rats of PC group as compared to SC rats (p<0.0001 for both comparisons). Finasteride administration resulted in a significant decrease in epithelial height as compared to PC rats (p<0.0001). Administration of BM extract especially at higher dosages reversed the changes in both parameters to control levels of SC rats (p>0.05).
Histomorphometric parameters (A and B) (mean±SD) of prostatic tissue of rats in different groups. SC: sham control, PC: positive control with benign prostatic hyperplasia, CC: comparative control, rats with benign prostatic hyperplasia that received finasteride at 5mg/kg/day by oral gavages and T1–T4: rats with benign prostatic hyperplasia that received black mulberry extract at 25, 50, 100 and 200mg/kg/day by oral gavages for 4 consecutive weeks. Columns without the presence of a common letter have significantly different values (p<0.05).
Benign prostatic hyperplasia, as one of the most common urological diseases in aging men, is usually managed by different approaches ranging from watchful waiting and pharmacological therapy to surgical procedures based on the severity of the disease. Phytomedicines are also commonly used in patients with mild to moderate symptoms and efficacy and proper safety profile of some of these herbs are shown in randomized clinical trials.15 Herbal preparations may also be helpful as adjunct therapy.
Black mulberry is an anthocyanin-rich fruit which has a history of use in traditional and folk medicine. Turan et al.16 showed antiproliferative and apoptotic effect of BM extract on human prostate adenocarcinoma cells. This motivated us to evaluate the possible protective effect of this fruit against BPH in an animal model of androgenic stimulation of prostatic hyperplasia which is commonly used for testing the effects of compounds on cellular proliferation in the prostate.17
In our study, overt signs of BPH was observed in the rats of PC group as shown by increased prostatic weight, prostatic index, PSA and DHT levels and histopathological changes which were in agreement with previous studies.18,19 These parameters were positively changed by different dosages of BM extract and the effect of BM extract was comparable to that of finasteride for some of the assayed parameters.
One of the interesting findings of our study was the ability of two higher dosages of BM extract in reversing DHT levels in both serum and prostatic tissue to normal values of SC rats which was comparable to that of finasteride. Dihydrotestosterone is synthesized from testosterone by the enzyme 5-α reductase in the target organs such as prostate and seminal vesicles. It has been clearly demonstrated that during first hours after castration in rats the concentrations of both testosterone and DHT decrease in a time-dependent manner, however the decrease in DHT concentrations is more dominant in prostate (drops to 3% of the normal levels in 72h), while in serum, testosterone shows a more profound decrease (drops to 4% of the normal levels in 3h). This controversy is possibly related to the fact that opposite to the serum, DHT is the dominant androgen in prostate and prostate has a high activity of 5-α reductase which is even higher than seminal vesicles.20
Therefore, regarding the time course of our study and the fact that rats were castrated, it seems logical to assume that the DHT which was assayed in the serum and prostate of rats in PC, CC and treatment groups was produced from the exogenous testosterone propionate by the activity of the enzyme 5-α reductase. Consequently, the reduction that was observed in DHT levels in prostate due to administration of BM extract may be related to the reduction of the activity of this enzyme in prostate that can subsequently affect serum DHT levels. Although this hypothesis needs to be further evaluated.
As another finding, we observed a decreasing effect of two higher dosages (T3 and T4 groups) of BM extract on PSA levels which was similar to that of finasteride. Prostate epithelium predominantly expresses PSA and its concentration is consistently correlated with clinical and pathologic parameters of BPH.21 The effect of herbal preparations on PSA levels in animal models of BPH has been previously shown, for instance, Mbaka et al.22 demonstrated that hydroalcoholic extract of Secamone afzelii can reduce PSA concentrations in rats with experimentally induced-BPH. In our study, the reduction in PSA was consistent with the histopathological findings of the positive effect of BM extract on BPH that confirms its protective effect.
Two higher dosages of BM extract also showed antioxidant properties on prostate tissue as demonstrated by increased GSH and TAC and decreased MDA levels.
Oxidative stress has been involved in BPH and prostate cancer development, progression and the response to therapy.7 It has been shown that oxidative DNA damage may be important in pathogenesis of BPH. Vital et al.23 evaluated levels of 8-OH deoxyguanosine (8-OH dG), as a marker of oxidative stress, in human prostate tissues from normal transition zone and BPH. These researchers observed that the levels of 8-OH dG were correlated with prostate weight.
The fruits of BM have higher antioxidant activity than the other Morus species.9,24 One of the limitations of our study is that we did not perform a phytochemical assessment on the BM extract. However, a previous study9 has shown that M. nigra has a rich amount of anthocyanin with an average of 571mg of cyanidin-3-glucoside equivalent in 1g of fresh weight. The average total phenolics contents of BM were 2737mg gallic acid equivalent in 1g of fresh weight basis. Therefore, the antioxidant properties of this fruit can be associated with its protective effect against BPH as shown in our study.
In conclusion, BM extract has protective effects against experimentally-induced BPH in rats with regard to histopathological and biochemical parameters and its effect may be related to its antioxidant properties in prostate tissue as well as DHT reducing effect.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe study was founded by Shiraz University, School of Veterinary Medicine according to grant number: 92GCU3M163773.
Conflicts of interestAuthors have no conflict of interest.