The integration and coordination of the musculature of the pelvic floor and the anal sphincters is critical to two important physiological functions: defecation and continence. Consequently, disorders affecting the pelvic floor muscles, the anal sphincters, their innervation or their precise coordination will, depending on their nature, result either in obstructed defecation or fecal incontinence. Both of these disorders are much more common in females and the latter, in particular, is linked with parity. While the symptomatology, presentation and optimal mode of investigation of fecal incontinence are well standardized, considerable debate and controversy continues to surround the contributions of pelvic floor and anal sphincter dysfunction to chronic constipation and the optimal clinical approach to their investigation remains to be defined. In appropriately chosen cases surgical intervention may provide the best outcome for sufferers from incontinence; biofeedback approaches may be of value in both incontinence and obstructed defecation and surgery has little role to play in the latter.
The pelvic floor refers to all of the structures supporting the abdominal wall and pelvic cavity and, in the female, includes those organs and tissues that are contained between the perineum and the vulvar skin: the peritoneum, the pelvic viscera and endopelvic fascia, the perineal membrane, the levator ani muscles [comprising the pubovisceral (which, in turn, is composed of puborectalis and pubococcygeus portions) and the iliococcygeus muscles], and the external genital muscles.
Support for the pelvic floor comes from its connections to the bony pelvis and its attached muscles. In the female, the pelvic floor is conveniently divided into anterior and posterior components by the genital tract, injury to the anterior pelvic foor resulting primarily in urinary incontinence and to the posterior floor in problems with anal continence and the act of defecation (1).
In their primary functions of facilitating and controlling defecation and maintaining continence, the pelvic floor, the intrinsic neuromuscular apparatus of the colon and rectum and the anal sphincters act in a highly coordinated and integrated manner. This integration is illustrated by even a cursory examination of the anatomy of the region:
- 1.
The internal anal sphincter and its innervation represent an extension of the circular muscle layer and the enteric nervous system of the rectum.
- 2.
The external anal sphincter muscle is intimately associated with the muscles of the pelvic floor, such as the pubo-rectalis,
- 3.
Fibers of important pelvic floor muscles, such as the puborectalis, interdigitate with the longitudinal muscle layer of the rectum and anal canal.
These interrelationships, which extend to the neural control of these muscle groups, are critical to the coordination of a process as complex as defecation, which includes the following steps:
- 1.
Transfer of stool to the rectum, through the propulsive forces generated by giant migrating contractions in the colon (2).
- 2.
Sensing of the arrival of stool in the rectum with activation of the recto-anal inhibitory and sampling reflexes which allow stool to enter the anal canal and to be distinguished from flatus.
- 3.
A voluntary decision to proceed, and, finally.
- 4.
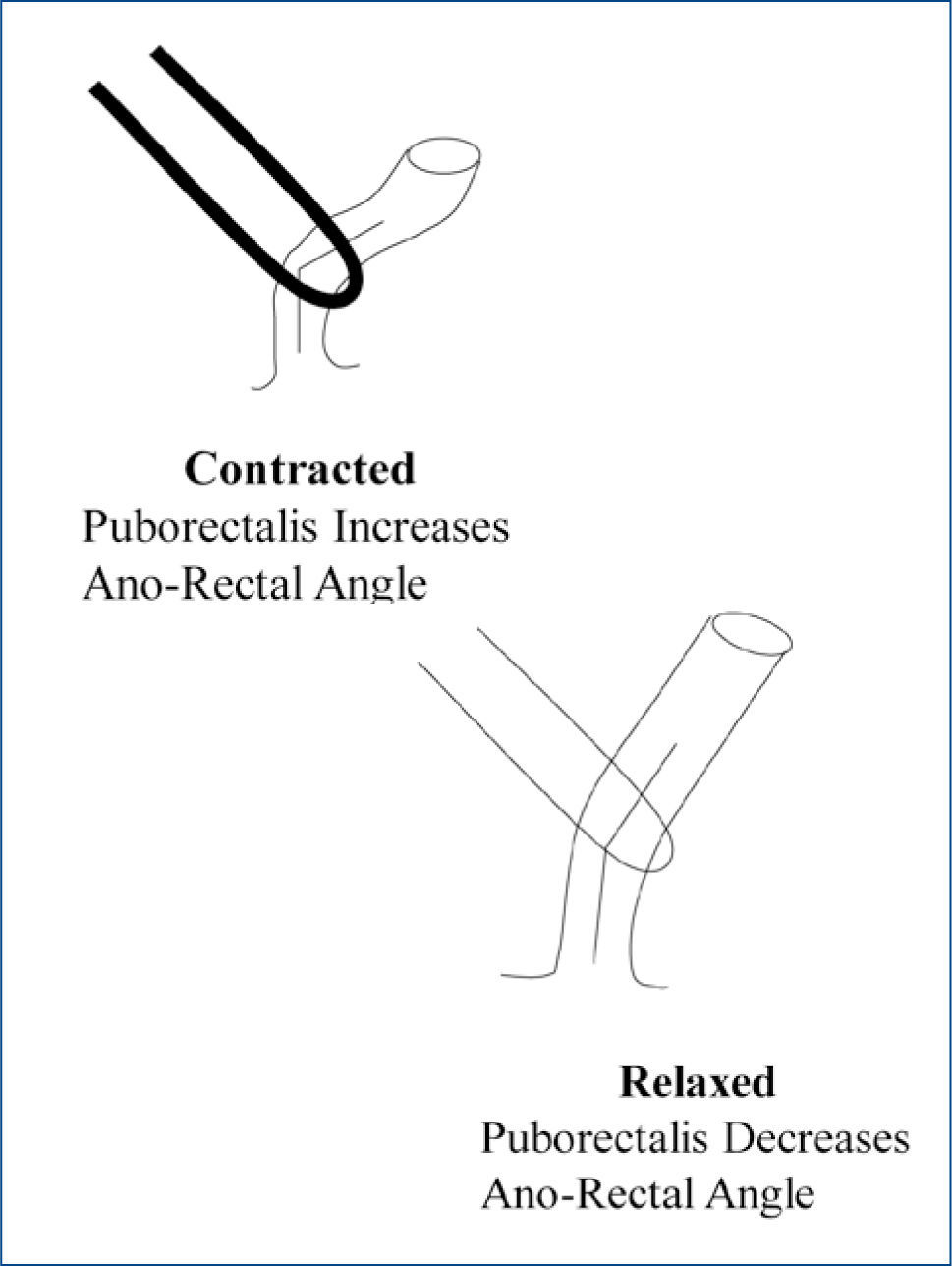
The act of defecation itself which involves the integrated, and appropriately timed, actions of, firstly, the pelvic floor musculature, and the puborectalis, in particular, which relaxes to straighten out the anal canal and facilitate defecation (Fig 1), secondly, the external sphincter, which relaxes, thirdly, the internal sphincter, which relaxes, and, finally, the diaphragm and abdominal wall muscles, which contract, increase intra-abdominal pressure and generate the pressure gradient between the rectum and anal canal that propels stool out of the body.
Figure 1.The role of the pelvic floor in the maintenance of continence and the facilitation of defecation
Diagrammatic representation of the manner in which the puborectalis muscle influences continence (when contracted) and defecation (when relaxed), through its effects on the ano-rectal angle.
(0.09MB).
The maintenance of continence is a similarly complex and coordinated process involving:
- 1.
The anal canal high pressure zone which is, in turn, generated by the internal and external anal sphincters, the latter being intimately related to the musculature of the pelvic floor,
- 2.
The pelvic floor; contraction of the puborectalis, for example, increases the ano-rectal angle and promotes the retention of stool in the rectum, and,
- 3.
Ano-rectal sensation and reflexes.
One can appreciate how susceptible many of these parameters may be to alteration and injury during pregnancy and parturition and also the difficulties that may be encountered in determining the precise pathophysiology of incontinence or constipation in a particular instance (3).
The process of defecation can be summarized as follows. When colonic contents reach the rectum, a sensation of rectal fullness is generated by rectal afferents, probably arising from activation of stretch receptors in the mesentery or pelvic floor muscles. In response to this, a “sampling” reflex, also known as the rectoanal inhibitory or rectosphincteric reflex, is generated and leads to internal anal sphincter relaxation and external sphincter contractions. At this stage, the individual can decide to postpone o r, if it is considered socially acceptable, proceed with defecation. To facilitate the process, the puborectalis muscle and external anal sphincter relax, thereby straightening the rectoanal angle and opening the anal canal. The propulsive force for defecation is then generated by contractions of the diaphragm and the muscles of the abdominal wall which now propel the rectal contents through the open sphincter. The internal anal sphincter is a continuation of the smooth muscle of the rectum and is under sympathetic control. It provides approximately 80% of normal resting anal tone. The external anal sphincter and pelvic floor muscles are striated muscles, innervated, respectively, by sacral roots 3 and 4 and the pudendal nerve. The anorectum represents, therefore, a site of convergence of the somatic and autonomic nervous systems and is susceptible to disorders of both striated and smooth muscle, as well as to diseases of the central, peripheral and autonomic nervous systems.
The clinical assessment of disorders of the pelvic floor and anal sphinctersThe two clinical syndromes that may arise from disordered or disrupted anatomy or function of the pelvic floor and anal sphincters are fecal incontinence and obstructed defecation (also referred to as anismus).
The clinical recognition of fecal incontinence would appear, at first sight, to be relatively straightforward but one must recognize that for many individuals suffering from fecal soilage it may prove too embarrassing to admit to and words like “diarrhea” may be employed instead (4). The clinician must, therefore, be alert to this possibility and must ask directly about the presence or absence of incontinence. If incontinence is present, more details must be sought: occurrence with liquid stool only or with solid stool, is there a warning (e.g. urgency) or not, can the individual differentiate between gas and stool? While certain risk factors (e.g. vaginal delivery, anal sphincter surgery) can be identified in certain individuals these will not be identified in the majority and it must also be remembered that incontinence is commonly associated with such “benign” disorders as irritable bowel syndrome and obesity (5). The key to uncovering fecal incontinence, therefore, is to seek it out.
The clinical definition of obstructed defecation is much more problematic (6). Traditionally constipation has been subdivided according to pathophysiology into two basic subtypes: slow transit constipation (or colonic inertia) and obstructed defecation (or anismus); the supposition being that the former was primarily a disorder of colonic motor function and would, therefore, be responsive to approaches that stimulated motility whereas the origins of the latter lay in dysfunction in, or lack of coordination between, the pelvic floor and sphincter muscles. Accordingly, infrequent defecation and hard stools were regarded as the classical symptoms of slow transit constipation and straining, sensations of incomplete evacuation and anal blockage and the use of manual maneuvers to facilitate defecation were looked upon as indicative of obstructed defecation. Critical analysis of the literature on this topic has, unfortunately, failed to support this neat distinction between slow transit and obstructed defecation subtypes of functional constipation to the extent that a recent systematic review concluded that “the medical history could not distinguish among the different subtypes of chronic constipation” (7). Not only are these clinical definitions problematic but slow transit and obstructed defecation commonly coexist. These observations have profound implications for the validation of tests for the evaluation of constipation as well as for the evaluation of therapeutic strategies (8). Furthermore, distinctions between constipation and irritable bowel syndrome, so neat and tidy in consensus criteria are much more difficult to make in real life (9). It is no wonder that this has proven to be such a difficult and problematic area for the clinician and the clinical investigator.
Functional testing of the pelvic floor and ano-rectumIn the constipated patient, defects in the defecatory process are especially challenging to define and manage and, as the affected individual may require a somewhat different therapeutic approach to that of the patient with slow transit constipation or colonic inertia, considerable effort has been expended in developing reliable and clinically useful tests for the assessment of ano-rectal and pelvic floor function. Symptoms alone have not proven to be especially useful in differentiating between the two main categories of constipation. Furthermore, the identification of abnormalities in ano-rectal or pelvic floor function is regarded as a contra-indication to colectomy in the patient who, on the basis of symptoms or other tests, appears to have colonic inertia.
These same anatomical structures also contribute to the maintenance of fecal continence and a somewhat similar array of tests may also be applied to the evaluation of their function in the patient with fecal incontinence.
In contrast to the relative paucity of tests available of the assessment of small intestinal or colonic motility, a relative plethora of approaches has been applied to the study of ano-rectal and pelvic floor function. Most experts would advocate the application of a number of tests, each assessing somewhat different parameters, to the assessment of the patient with constipation or diarrhea.
1AnatomyThough not strictly speaking a “motility” test, approaches that evaluate the integrity of the various structures that comprise the pelvic floor and anal sphincters are of considerable value in the evaluation of the patient with fecal incontinence (10). Both endoanal ultrasound and endoanal magnetic resonance imaging (MRI) are widely employed to define anatomical (usually obstetric or post-surgical) defects in the internal and external anal sphincters with ultrasound being the preferred modality for the former and MRI for the latter (11). MRI has also gained favor as the preferred method for the dynamic assessment of pelvic floor anatomy and function (12). Static images of the anorectal angle can be obtained during defecography (whether performed using fluoroscopy or MRI), a procedure employed to describe the movements of the pelvic floor musculature in relation to the anorectum during various maneuvers and which is described below.
2TransitTransit of feces (or more usually, a simulated stool) is typically assessed by means of defecography using standard contrast imaging, scintigraphy or MRI. The first two involve radiation exposure and the use of a customized “throne” on which the patient sits and performs various maneuvers following the insertion of a material to simulate the consistency of feces into the rectum. In this manner, the behavior of the pelvic floor musculature can be recorded as the patient attempts to retain or expel stool. Magnetic resonance imaging offers many advantages over barium defecography but for a truly physiological test, requires a dedicated “open” system, a facility that is available at only a few highly specialized centers (11).
The balloon expulsion test has been developed and validated by some centers as a simple method to assess defecatory function. A balloon is placed in the rectum and inflated with 50 cc of air; the ability of the subject to expel the balloon either unaided or with the addition of external weights is then, assessed (13).
3ManometryAnorectal manometry has been used for decades to assess the integrity of the internal and external sphincters and is a well-established technique for the identification of Hirschsprung’s disease and the definition of poor sphincter tone in patients with incontinence (14). In the latter context, the clinician can go on, to employ manometry as the basis for bio-feedback approaches to improving sphincter function.
A variety of manometric assemblies have been employed; multiple balloon, perfused catheter, solid-state and high-resolution. The most widely used assembly incorporates an inflatable balloon at its tip (used to test sensation and elicit the recto-anal inhibitory reflex) and a radially arranged array of closely spaced sensors (either perfused side holes or miniaturized solid state sensors) which record pressure transients in the sphincters.
4ElectromyographyElectromyographic approaches have been employed to study both the integrity and responsiveness of the anal sphincters (typically using an intraluminal electrode assembly incorporated in a manometric assembly) and the innervation of the external sphincter and the pelvic floor musculature (using concentric needle, fine needle or single fiber techniques). While the former is quite commonly employed in some centers as an aid to biofeedback, the latter approaches are employed in some centers to define neurogenic incontinence (15, 16). Approaches involving relatively large bore needles have been criticized on the basis of procedure-related artifact. Formerly, pudendal nerve terminal motor latency (measured by a customized device which incorporated both stimulating and recording electrodes fixed 3 cm apart on a rubber finger stall and mounted on the index finger which was then inserted into rectum) was advocated as a valuable technique for identifying injury or neuropathy of the pudendal nerve (17) but has fallen out of favor because of poor reproducibility in some hands.
5BarostatWhile rectal sensation, compliance and capacity can be estimated using the inflatable balloon mounted on a typical manometric assembly, these parameters can be most accurately and objectively measured using a barostat system (18). As has been the case elsewhere in the gastrointestinal tract, barostat balloon systems, with electronic control of inflation and deflation, have been widely employed in research studies of the colon and ano-rectum but their clinical application has been restricted. Nevertheless, whether assessed by a simple balloon or by the barostat, abnormalities of rectal sensation, both hypo- and hyper-sensation, have been well documented and considered of pathophysiological importance among patients with both constipation and incontinence
Management of disorders of the pelvic floor and anal sphinctersFrom the perspective of the gastroenterologist two clinical issues may involve disrupted anatomy or disordered function of the pelvic floor and anal sphincters: fecal incontinence and obstructed defecation (anismus). This is not to dismiss the various urogenital problems that may relate to the pelvic floor but to state that these are beyond the scope of this review. Furthermore, a detailed discussion of the many options that may be employed in the management of fecal incontinence and constipation will not be presented but rather some aspects that are especially relevant to the gastroenterologist will be emphasized and some new approaches introduced.
Fecal incontinenceThe management of the individual with fecal incontinence will be governed by many factors, including, but not limited to: the nature of the anatomical defect, the severity of the symptoms, the presence of co-morbid gastrointestinal disorders (for example, the resolution or control of an underlying diarrheal disorder may resolve the problem), the general health and cognitive status of the patient and the etiology of the incontinence. In some instances, such as total disruption of the anal sphincter as a consequence of birth injury or surgical trauma, surgical intervention, if timely, may be the most appropriate option; in other situations such as in the patient with advanced Alzheimer’s disease a more conservative approach will be preferred.
In the cooperative patient, biofeedback and/or pelvic floor exercises are often the preferred option. While a number of studies have been published attesting to the value of biofeedback therapy in fecal incontinence, a very recent Cochrane Database systematic review concluded that “the limited number of identified trials together with methodological weaknesses of many do not allow a definitive assessment of the role of anal sphincter exercises and biofeedback therapy in the management of people with fecal incontinence” (19). Nevertheless, this approach is widely advocated by experts in the field and seems to be a valuable option (20). One new option that deserves mention is sacral nerve stimulation (21). The Cochrane review suggested that “biofeedback and electrical stimulation may enhance the outcome of treatment compared to electrical stimulation alone or exercises alone and that exercises appeared to be less effective than an implanted sacral nerve stimulator”. Sacral nerve stimulation appears to be generally safe.
Obstructed/dyssynergic defecation (anismus)Of the various dietary and pharmacological approaches that have been employed in the management of constipation, in general, few have attempted to differentiate the patient populations involved in terms of constipation subtype. As older studies focused on stool frequency (and, at most consistency) as the only therapeutic outcome, little or no information is available on symptoms, such as straining or sensation of incomplete evacuation, that might (rightly or wrongly) be regarded as indicative of pelvic floor and/or anal sphincter dysfunction (22). More recent pharmacological approaches, such as lubiprostone (23), prucalopride (24) or linaclotide (25) have assessed these symptoms and have demonstrated efficacy for these agents, suggesting that approaches to the management of constipation, per se, should be tried in the patient in whom pelvic floor and/or anal sphincter pathology may be invoked.
Though seldom studied in a formal manner, both enemas and suppositories are widely used in the management of constipation in the elderly. Enemas play an important role in the management and, especially, the prevention of fecal impaction among those at risk. Suppositories can help to initiate and/or facilitate evacuation. For example, an approach which combined the daily administration of lactulose with a glycerine suppository and a once-weekly tap water enema was successful in achieving complete rectal emptying and preventing incontinence related to impaction in some institutionalized elderly patients (26). Similar success rates were obtained by a combination of a laxative and a suppository among stroke patients (27).
Biofeedback has also been employed in the management of dyssynergic defecation. With biofeedback, patients are trained to relax their pelvic floor muscles during straining and to correlate relaxation and pushing to achieve defecation. In one uncontrolled study, biofeedback provided long-term benefit for patients with intractable, slow and normal transit constipation (28). This study followed 100 patients over a 23 month period. Straining, need for digital manipulation, pain and bloating were all significantly reduced immediately after biofeedback and after 23 months follow up. More recently, two randomized controlled studies have provided convincing evidence for efficacy for biofeedback among patients with pelvic floor dyssynergia (29,30). There may be limitations to the application of this approach among some elderly individuals or those with cognitive impairment due to an ability to cooperate fully in the biofeedback program. Some preliminary data suggests a possible role for sacral nerve stimulation in the management of intractable constipation (31).
Several of the imaging techniques described above may reveal anatomical defects (rectocele, prolapsed, etc) which may prompt consideration of a surgical approach. Furthermore, it has been assumed that disruption of the anatomy of the pelvic floor during parturition is of fundamental importance to the subsequent development of perineal descent, rectoceles and pelvic floor prolapse and to lead to difficulty with defecation. However, while there is some evidence for an effect of pelvic floor prolapse on defecatory performance, the relationship has been far from perfect or consistent. Thus, while constipation and other bowel symptoms are certainly common among patients with perineal descent and vaginal prolapse (32), a cause and effect relationship has not been established, as exemplified by a failure to establish any correlation between the severity of prolapse and the prevalence of bowel dysfunction (33). In one study of 1004 women in the US, no association could be developed between the extent of vaginal wall or pelvic descent and constipation whether expressed as the passage of hard or lumpy stools, a sense of incomplete evacuation or infrequent bowel movements. Straining at stool was associated with more anterior vaginal wall and perineal descent (34). The perils of identifying correlations between prolapse and any symptom were dramatically illustrated by Klingele and colleagues who could demonstrate prolapse, of at least stage II, in 55% of their healthy control population (35). 42% of their patients with obstructed defecation had prolapse. Furthermore, there was no association between the severity of prolapse and the prevalence of obstructed defecation, though this symptom did relate to the presence of perineal descent. They concluded that, while a subset of subjects with defecatory disorders, and obstructed defecation, in particular, have evidence of perineal descent their findings overall, “argue against a major role for pelvic organ prolapse in defecatory disorders” (35). It stands to reason that great restraint must be exercised in the interpretation of such imaging findings and the temptation to surgically correct theses defects resisted.
Where investigations reveal a failure of the puborectalis muscle to relax, direct, ultrasonographically-guided, injections of Botulinum A toxin have been performed with good short-term results in uncontrolled studies (36); this seems a preferable approach to surgery given the likelihood of incontinence with the latter.
The author have no interest conflicts with this article.