Individuals with steeply sloping high frequency hearing loss can often hear speech but fail to understand it, and conventional treatments, including frequency transposition hearing aids, are usually ineffective when the hearing loss is severe or profound. Electro-acoustic stimulation (EAS) is a relatively new treatment option for this population, in which mid-high frequency information is provided by a cochlear implant (CI) inserted into the basal turn of the cochlea, supplemented by low frequency acoustic amplification. New atraumatic CI electrode arrays and surgical techniques have been shown to facilitate low frequency hearing preservation sufficiently to allow the use of EAS in the majority of suitable candidates. Clinical studies have consistently demonstrated synergistic combination of mid-high frequency information delivered electrically by a CI with low frequencies delivered acoustically, providing superior performance to that obtained from a CI alone.
Conventional hearing aids (HAs) represent the standard of care for the majority of individuals with sensorineural hearing loss, and are particularly effective when auditory thresholds are within the moderate-to-severe range. For very severe losses, however, restoration of speech recognition by HAs has been found to be limited, even when adequate gain can be provided according to prescriptive fitting algorithms. This is particularly true for mid-high frequency amplification, which may even be detrimental to speech understanding when auditory thresholds are poorer than around 60-70dB HL1,2. This is likely related to the fact that more severe thresholds are associated with damage to inner hair cells in addition to loss of the fine tuning function of the outer hair cells, and in extreme cases there may be total loss of inner hair cells over regions of the cochlea, i.e. so-called “dead regions”3.
In many cases of sensorineural hearing loss, auditory thresholds are better for low than for high frequencies. In certain individuals this threshold difference can be very large - sometimes with normal or near normal hearing in the low frequencies and severe-to-profound hearing loss in the high frequencies. In such cases, high frequency amplification may not provide substantial benefit. These individuals are often able to hear speech but not understand it as the important mid-high frequency information is not audible.
Treatment options for this population are limited. It is often difficult to provide the large variation in gain required at different frequencies using a conventional HA and some individuals may not even require any amplification in the low frequencies. Frequency transposition hearing aids, which compress a wide input frequency range into the (audible) low frequencies, might be expected to provide substantial benefit in this population, but to date clinical outcomes with currently available devices have been disappointing4,5.
Cochlear implantation has become a routine treatment for severe – profound hearing loss over the past 30 years and listening performance has consistently been shown to be improved in individuals with no preoperative hearing or those who cannot benefit significantly from conventional HAs. However, while high levels of performance are often reported in favourable listening situations, CI users typically have substantial difficulty in segregating competing speakers or in background noise conditions. This difficulty is believed to be largely due to relatively poor representation of the low frequency “fine structure” of the acoustic signal (i.e. the voicing fundamental frequency range) by electrical stimulation6,7.
Candidacy criteria for cochlear implantation typically involve specified levels of preoperative speech understanding (using HAs where appropriate) and unaided audiometric thresholds8. Individuals with severely sloping high frequency hearing losses often fall within these criteria, but may be reluctant to proceed with implantation due to fears of losing their residual natural hearing. During the early years of cochlear implantation it was assumed that any residual hearing would be lost following surgery, but later experience has shown that hearing loss is not inevitable, particularly when “soft surgery” techniques are employed.
Individuals with steeply sloping hearing loss have represented a particularly interesting population in the field of cochlear implantation in recent years. As a CI electrode is usually inserted via the basal (high frequency) region of the cochlea it was postulated that combined electrical and acoustic stimulation might provide a feasible treatment option for this population. In principle, a CI electrode inserted into the basal region of the cochlea could provide high frequency information by electrical stimulation and possibly preserve the residual apical (low frequency) cochlear function which could be provided with acoustic amplification if required. Such a combination might be more effective than either acoustic or electrical stimulation in isolation. In this article, we aim to provide an overview of existing clinical experience relating to “electro-acoustic stimulation” (EAS) together with hardware options available from Cochlear Ltd and with an update of recent clinical outcomes.
PRINCIPLES AND IMPLEMENTATION OF EASMuch of the early work on EAS included animal studies into the physiology of combined electrical and acoustic stimulation9, in an attempt to clarify whether the two modalities could provide effective synergistic stimulation of the spiral ganglion cells. This was considered important as the firing patterns produced by electrical and acoustic stimulation differ considerably. However, progress with the clinical application of EAS has arguably been more directly influenced by parallel clinical studies. As outcomes from CI have generally improved over the years, individuals with greater levels of residual hearing have been implanted. When there is some level of useful (aidable) hearing, individuals are often implanted in the poorer ear in order to avoid any risk of poorer outcomes post-implantation. Many studies have reported that such CI recipients can benefit from the combination of electrical stimulation in the implanted ear and acoustic input on the opposite side, i.e. “bimodal stimulation”10,11. This demonstrates that the central auditory system is able to effectively combine the neural responses to electrical and acoustic stimulation.
Many of the early trials with combined electrical and acoustic stimulation in the same ear used relatively short electrode arrays in the anticipation that these would facilitate better preservation of low frequency acoustic hearing than conventional full length arrays. Cochlear Ltd produced two commercial devices based around the CI24RE Freedom implantable cochlear stimulator. The Hybrid S8 device used a 10mm electrode array with 6 active electrode contacts. A multicentre trial in the US reported useable preserved low frequency hearing in 80% of subjects after 1 year, and significant improvement in speech understanding from the addition of acoustic input was demonstrated in 82.5% of subjects12. However, a minority of subjects appear to lose residual hearing at surgery or some time later13, and in this situation a very short electrode array usually provides less hearing benefit than a conventional array14. For this reason, an alternative array, the “Hybrid L24” was subsequently produced by Cochlear. The Hybrid L24 has 22 contacts spaced over 17mm, and typically extends to around 270o from the round window, i.e. to the 2000 Hz region of the cochlea15. High levels of hearing preservation have also been reported for this device, and CI-alone performance was shown to be comparable to that achieved by conventional recipients16. These outcomes are reviewed in more detail below.
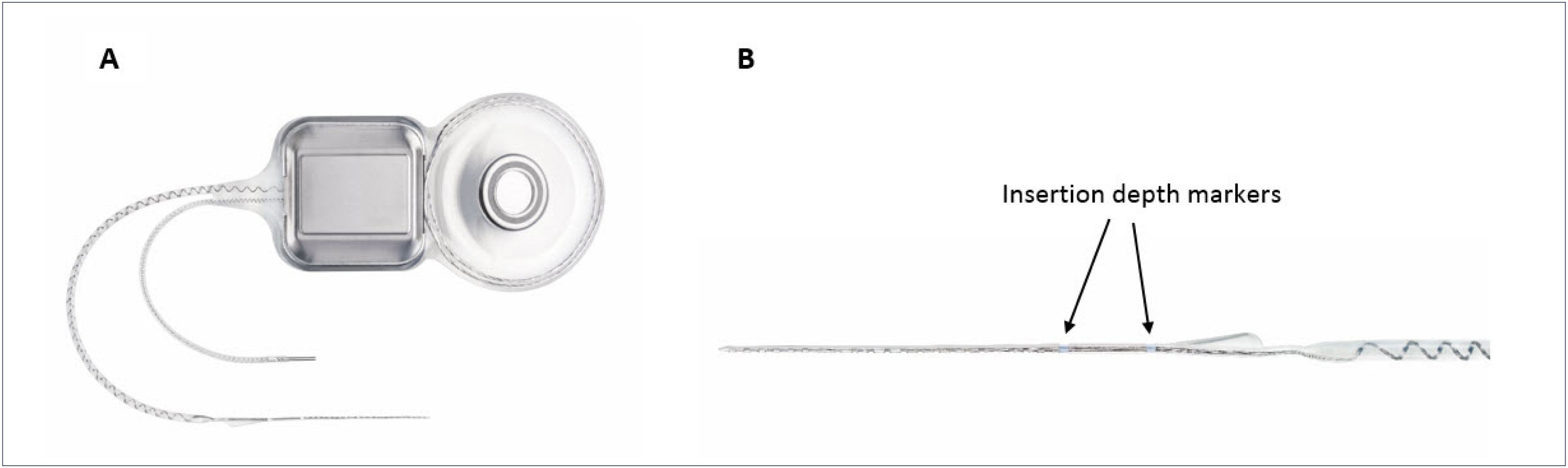
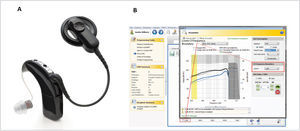
The most recent EAS hardware from Cochlear Ltd includes a new atraumatic electrode, the “Slim Straight” (SS) array, which has been coupled to the CI24RE and CI500 Profile implant packages to form the Nucleus CI422 and CI522 devices respectively (Figure 1). The SS array is a thin, flexible straight electrode array that may be inserted through either a cochleostomy or the round window and takes up a position along the lateral wall of the cochlea. The electrode carrier supports half-banded electrode contacts, which gives the array a smooth side which significantly reduces insertion forces and may reduce trauma when moved along the lateral wall of the scala tympani (ST). The 22 electrode contacts are distributed over 20mm and the array has two markers, designed to indicate insertion depths of 20 or 25mm. An insertion depth of 20mm is recommended when hearing preservation is an issue, such as with potential users of EAS.
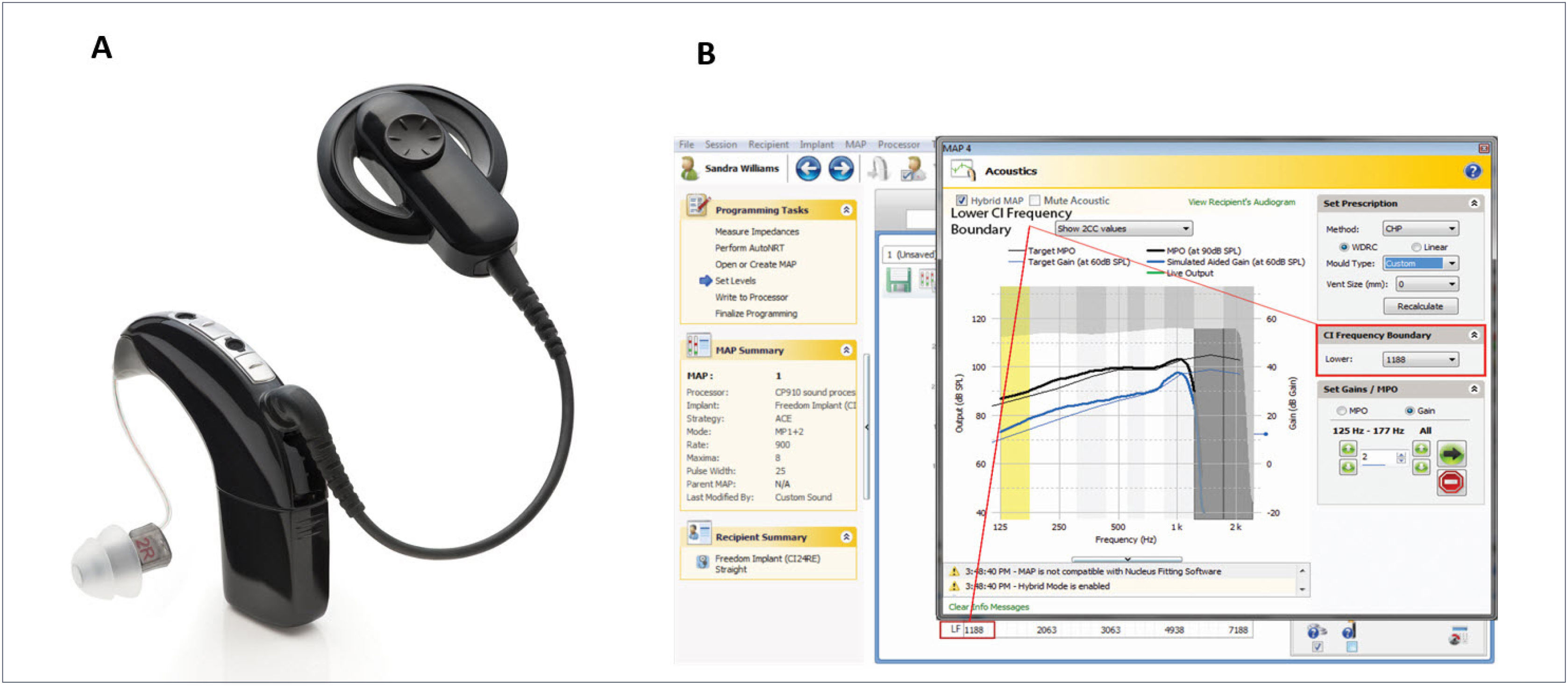
The CP900 series Sound Processors (CP910 and CP920) may optionally be fitted with the “acoustic component” (ACO) unit, which replaces the standard earhook and incorporates an acoustic transducer (receiver) which delivers its output directly to the ear canal. This development enables the use of a single integrated device, rather than the need for separate electrical and acoustic stimulation units (Figure 2A). A further advantage of an integrated unit is that electrical and acoustic output adjustments can easily be made within a single fitting system (Custom Sound software), allowing optimal fitting of the EAS system (Figure 2B).
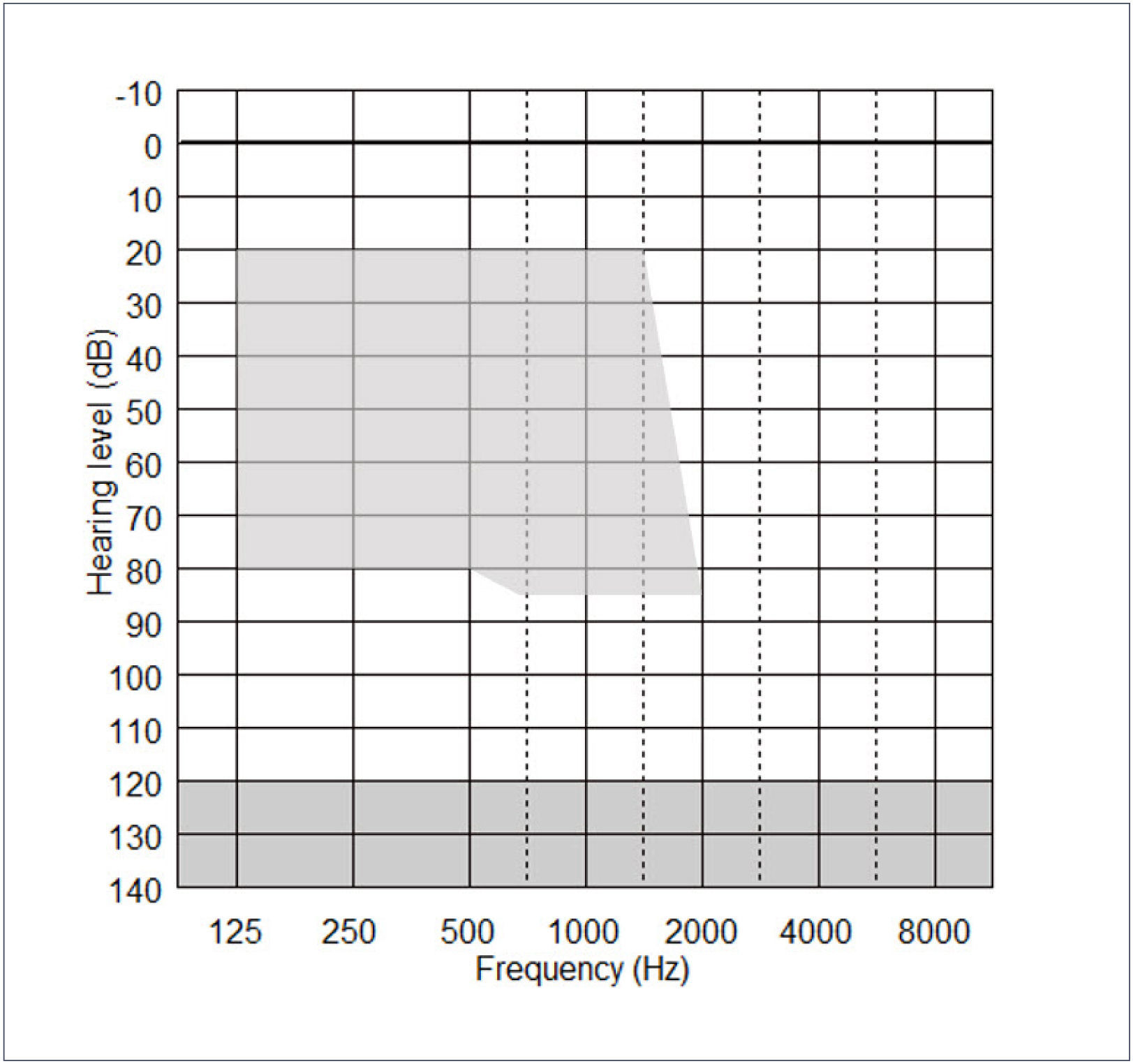
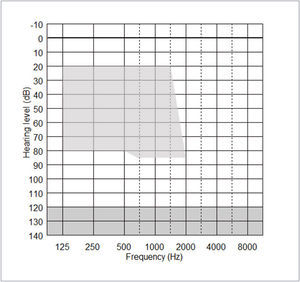
The acoustic component (ACO) of the CP900 processor may be fitted or removed at any time, but its effective use depends on the degree of residual postoperative acoustic hearing, which cannot be reliably predicted before implantation. Indications for use of the ACO are provided by the fitting range shown in Figure 3 below. Post-operative thresholds falling within the shaded range indicate frequencies that can be amplified by the CP910/920 in EAS mode.
Several clinical studies have addressed the indications for hybrid stimulation in terms of pre-implant hearing levels. Initially, only subjects with low frequency thresholds poorer than around 65dB HL were implanted17, but encouraging early outcomes resulted in relaxation of such limits. Recent studies have suggested that acoustic amplification can be effectively provided for low frequency hearing thresholds down to about 70dB HL at 250Hz. When thresholds are poorer than this, subjects tend to prefer CI (electrical) alone stimulation18. At higher frequencies, thresholds should be below about 80dB HL (i.e. in line with indications for conventional CI), as acoustic hearing aids cannot provide useful listening benefit for such high frequency hearing losses. However, although these general audiometric threshold guidelines are relatively clear, there is considerable heterogeneity in recipient types. Some individuals have very good low frequency hearing so that they do not require amplification of low frequencies19. Many have substantial hearing levels in the non-implanted ear and are able to benefit from binaural low frequency hearing in addition to the mid-high frequency information provided by the CI.
THE INFLUENCES OF DEVICE DESIGN AND SURGICAL TECHNIQUE ON HEARING PRESERVATIONAs preservation of low frequency hearing is a pre-requisite of successful use of EAS, this pivotal topic continues to receive much attention through both basic research and clinical studies. Findings from these studies have shown that hearing preservation is dependent on several distinct factors, particularly: (i) CI electrode design, (ii) surgical technique, and (iii) patient factors such as degree of residual hearing.
The concept of “soft surgery” was first introduced by Lehnhardt20, and incorporated a range of guidelines intended to minimize cochlear trauma, including opening the ST as late as possible, avoiding suction of perilymph, use of lubricants such as Healon® and slow insertion of the electrode array. The original aim of soft surgery was to minimize damage to the cochlea in general and the neural substrate in particular, in the anticipation of achieving more effective electrical stimulation. However, most of these principles are equally valid for hearing (hair cell) preservation. In addition, surgeons attempting hearing preservation will often try to avoid drilling into the endeosteum and the use of intravenous and/or oral pre- and perioperative steroids is now commonplace21.
When using soft surgery, some level of hearing preservation has been reported in conventional CI recipients with measureable hearing for the majority of currently available electrode types, demonstrating that loss of residual hearing is not an inevitable consequence of cochlear implantation. For example, Fraysse et al.22 reported a median low frequency threshold deterioration of 23dB after one month in 12 recipients of the Nucleus CI24 device with Contour Advance electrode, when using a soft surgery protocol. Obholzer & Gibson23 reported preservation of residual hearing in 58 of 81 patients implanted with the Nucleus CI24 device (straight banded and Contour electrode arrays) after 6 months follow-up. The mean deterioration of 500Hz thresholds was 15dB in those with preserved hearing.
Hearing preservation reported for specific electrode typesWhen hearing preservation is a specific aim, as in candidates for EAS, then most clinical studies have used electrode arrays designed specifically for hearing preservation. These arrays are relatively thin, flexible straight arrays designed to lie along the outer margin of the ST. A substantial number of studies have quantified the degree of hearing preservation achieved with specific electrode types, but comparison among studies is difficult due to a wide range of reporting methods. Some studies have reported the proportion of subjects with postoperative low frequency thresholds within 10dB of pre-implant levels (often referred to as “complete hearing preservation”), or within 20 or 30dB (“partial hearing preservation”). Other studies report the mean or median low frequency threshold change post-implantation. Furthermore, outcomes have been reported at a wide range of follow-up times, further precluding comparison. Finally, hearing preservation outcomes with specific devices may be influenced by variations in surgical technique and/or patient characteristics.
Evidence for the Cochlear Hybrid devices comes primarily from a series of multicentre clinical studies. The shorter Hybrid S8 device was evaluated in a US trial reported by Gantz et al.12,24. 87 adults were implanted with the 10mm electrode array in their poorer ear, with preoperative low frequency (125–500Hz) thresholds of 60dB HL or better. At initial activation, two subjects (1.3%) had lost all hearing, and in the remaining subjects the low frequency average thresholds decreased by a mean of 14.8dB. Over the subsequent 12 months a further 14 subjects lost functional hearing (low frequency thresholds >90dB HL), but there was little change in thresholds for the remaining subjects. At 12 months post-activation, 80% of the subjects retained functional low frequency hearing and were able to utilize EAS.
Lenarz et al.16 reported on the European Hybrid L24 multicentre study, which included 66 adults with severe-profound high frequency hearing loss and with thresholds <60dB HL at frequencies below 500Hz. At initial activation, 89% of subjects showed low frequency hearing preservation within 30dB of preoperative levels, and in 61% of subjects thresholds dropped by less than 10dB. By 12 months these proportions were 74% and 43% respectively, indicating further hearing loss in some subjects. 88% of subjects retained sufficient hearing to use EAS at the 12 month interval. Outcomes from the US multicentre clinical trial were reported by Roland et al.25, which assessed 50 adults with similar preoperative characteristics to those in the European study. 66% of subjects retained functional acoustic hearing after 6 months, and the proportions of subjects with thresholds changes of <10dB and <30dB were 25% and 56% respectively.
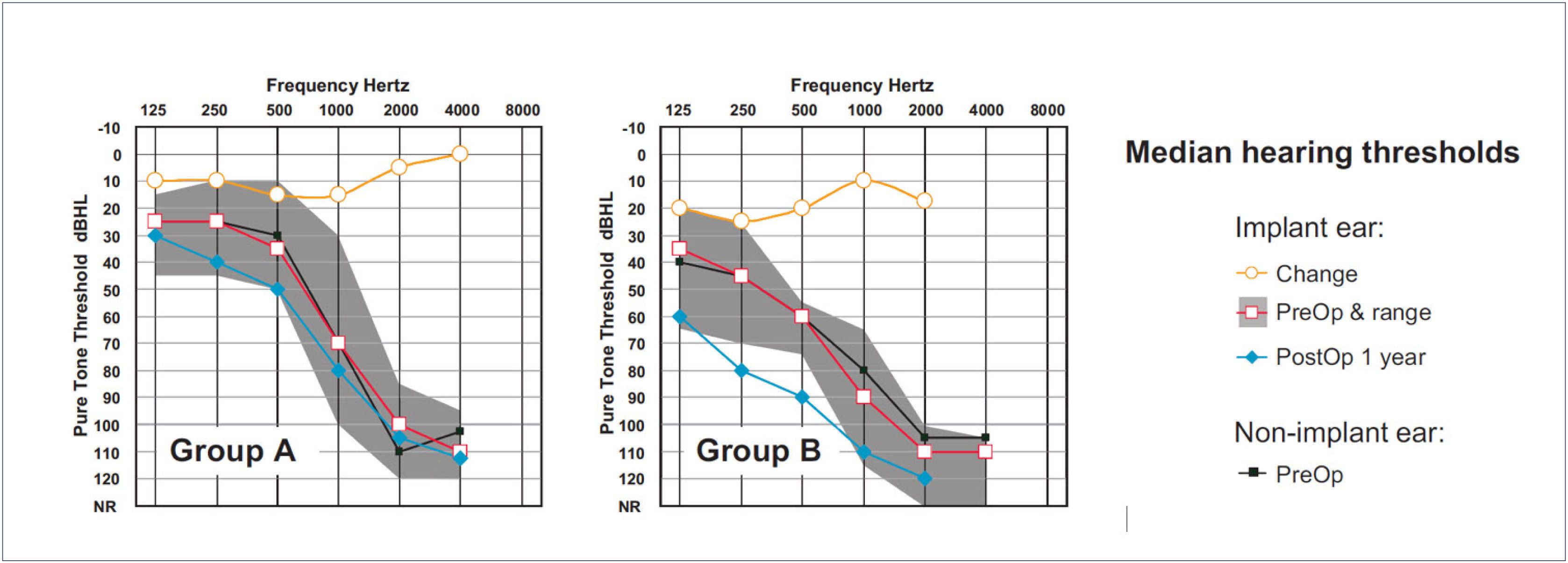
The largest cohort of recipients of the relatively new Cochlear Slim Straight (SS) electrode array has been implanted by Skarzynski and colleagues. Skarzynski et al.26 reported on hearing preservation outcomes in 35 adults separated into three groups according to preoperative 500Hz thresholds. Groups A, B and C had 500Hz thresholds of <50dB HL, 50-80dB HL and >80dB HL respectively. For the total cohort, median threshold increase for low frequencies was 10dB and 15dB at 1 month and 1 year postoperative respectively, with three subjects (9%) losing all residual hearing. At the one year interval 38% of subjects had 500Hz thresholds within 10dB of preoperative levels and 79% had thresholds within 30dB. Figure 4 shows the median preoperative and 12 month postoperative thresholds for Groups A and B (i.e. subjects who were typical candidates for EAS). In each panel the upper trace shows the difference between pre- and postoperative thresholds (Figure 4).
MEDIAN PRE- AND POSTOPERATIVE THRESHOLDS FOR TWO GROUPS OF COCHLEAR SLIM STRAIGHT ELECTRODE RECIPIENTS
N was 11 and 13 in groups A and B respectively. Redrawn from Skarzynski et al.26.
The same group later reported on outcomes from 19 children implanted with the Nucleus CI42227. In this study, hearing preservation was expressed as an overall percentage (for all audiometric frequencies combined) comparing pre- and postoperative thresholds and taking into account audiometer output limits (for example, pre- and post-implant thresholds of 60 and 75dB HL respectively, with a 120dB HL output limit, would indicate 75% hearing preservation). The authors suggested that preservation of >75% may be considered “complete” in terms of practical implementation of EAS. The mean hearing preservation recorded was 75% and 67% at 1 and 2 years postoperative respectively (compared with 97% and 94% in the non-implant ears). All subjects retained sufficient hearing to use EAS at 24 months apart from four subjects who had very poor preoperative hearing levels.
Several other studies have reported on hearing preservation with the SS electrode in smaller cohorts, generally with similar findings. Lenarz28 reported preservation within 15dB of preoperative thresholds in 48% of 29 subjects implanted with the CI422 device after 6 months. Mean preoperative low frequency thresholds were 63dB HL and the median pre-postoperative increase in thresholds was 15dB at 250Hz and 25dB at 500Hz. Friedmann et al.21 reported a 21.5dB increase in low frequency thresholds at initial activation in a group of twelve CI422 recipients, with a further 8.5dB deterioration by 12 months.
These studies have included a variety of surgical techniques, patient types and follow-up times, but it can be concluded that long-term low frequency hearing is typically preserved within 15–25dB of preoperative levels when using the SS array, with the large majority of recipients able to utilize EAS. Similar hearing preservation outcomes have been reported for the 24mm MED-EL FLEXEAS array29,30. However, use of the full length 31mm MED-EL FLEXSOFT array in subjects with functional preoperative hearing has been shown to be associated with poorer hearing preservation and a higher incidence of total hearing loss31,32.
Factors predictive of good hearing preservationStudies on specific electrode types have implanted a variety of recipient types and used a variety of surgical techniques, and it is likely that some of these factors have contributed towards the variability in observed outcomes. Several recent review articles have attempted to identify factors predictive of successful hearing preservation by analyzing outcomes from a large number of studies. However, while a few such factors have emerged, it is probably reasonable to state that the reported influence of many factors currently remains inconsistent.
Santa Maria et al.33, Kopelovich et al.34 and Causon et al.35 each examined a wide range of recipient characteristics and surgical techniques and looked for correlations with hearing preservation by meta-analysis or multivariate regression analysis. The meta-analysis of Santa Maria et al.33 suggested better outcomes for (i) cochleostomy insertion (rather than RW), (ii) posterior tympanometry approach (rather than suprameatal), (iii) insertion time of >30s, and (iv) use of postoperative systemic steroids. Electrode parameters were not found to be predictive of outcomes. Causon et al.35 extracted low frequency average hearing preservation reported for 110 patients in 12 studies. This review identified the use of steroids, particularly when administered intraoperatively, to be predictive of better outcomes, but reported superior outcomes from RW insertions. In addition, pre-curved (perimodiolar) electrodes produced poorer outcomes and hearing preservation was inversely correlated with insertion angle. Regarding patient variables, stable hearing loss appeared to be predictive of better hearing preservation than progressive losses. Kopelovich et al.34 analysed patient variables from the Cochlear Hybrid S FDA trial (85 subjects). Low frequency hearing preservation 1 year post-implantation was clearly superior in subjects implanted at a younger age (within a range from 17 to 84 years) and was significantly superior in females. Patients with noise induced hearing loss were also found to suffer from greater hearing loss than those with other aetiologies, though aetiology was unknown in 31% of the subjects.
Administration of steroids has become common in hearing preservation surgery, but the route and timing of administration varies. The efficacy of pre-operative steroids (usually dexamethasone, prednisolone or triamcinolone) was recently reviewed by Kuthubutheen et al.36, who pointed out that although systemic administration at various time points between induction and cochleostomy has become commonplace, the efficacy of short duration steroid treatment is not well established from clinical studies, though the evidence from animal studies is more robust. The intracochlear steroid concentration may be increased by local application to the round window during surgery, and there is evidence that this can reduce surgically-related hearing loss37. There is also some evidence that extended exposure may be effective; Sweeney et al.38 reported better hearing preservation in patients who received a 2-week oral corticosteroid (prednisolone) taper commencing three days prior to surgery, though this study was retrospective and non-randomized. Studies on long term postoperative steroid application, using drug-eluting electrode arrays39 or via osmotic pumps40 show promise in reducing intracochlear fibrosis and hearing loss, though these approaches currently remain in the experimental stage.
FUNCTIONAL OUTCOMES FROM ELECTRO-ACOUSTIC STIMULATION (EAS)In order to satisfy general candidacy criteria (preoperative speech understanding) as well as the audiometric indications of EAS, potential EAS users typically have more or less symmetrical hearing losses. When there is a significant difference between ears, the poorer ear is usually selected for implantation in order to avoid the risk of losing the natural hearing in the better ear. EAS users can therefore usually utilize acoustic low frequency information from both ears as well as the electrical signal in the implanted ear. If hearing is lost in the implanted ear, then CI recipients may still be able to benefit from “bimodal” stimulation, as combination of electrical and acoustic stimulation has been found to be effective when the acoustic input is from either the implant or non-implant side.
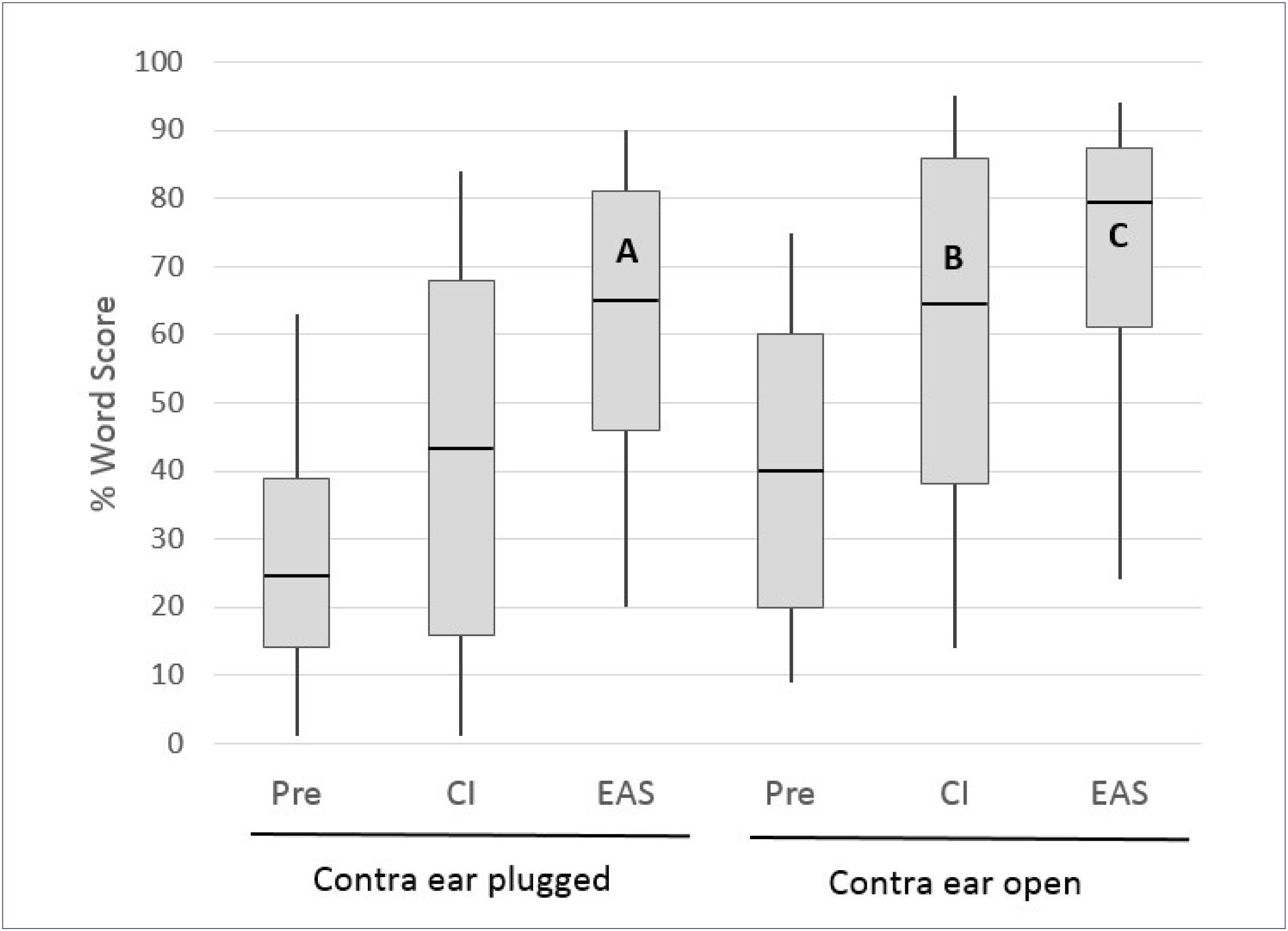
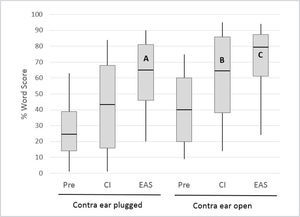
The multicentre European Hybrid L study, reported by Lenarz et al.16 provided a clear illustration of the individual contributions of these inputs, as many of the subjects were tested with the ears plugged individually. The 61 study participants were postlingually deafened adults with auditory thresholds of <60dBHL at frequencies below 500Hz and >80dBHL at frequencies above 1500Hz. At one year post-implantation 74% of the subjects retained 500Hz thresholds within 30dB of preoperative levels. The electrical signal from the CI was programmed in a “non- overlapping” fashion, with the lowest filter boundary set close to the frequency where auditory thresholds just exceeded 80dBHL41. Figure 5 shows monosyllable recognition performance at the 12 month postoperative interval for 54 subjects with complete data (including any with substantial loss of residual hearing).
MONOSYLLABLE RECOGNITION IN QUIET IN A COHORT OF 54 HYBRID L USERS PREOPERATIVELY AND AT THE 12 MONTH INTERVAL FOR THE CI ALONE AND EAS CONDITIONS.
Boxes represent 25th/75th percentiles and whiskers represent 10th/90th percentiles; horizontal lines show median values. All implanted ear scores represent performance with the opposite ear plugged. Redrawn from Lenarz et al.16.
Several important observations can be made from the data in Figure 5. Firstly, for the implanted ear in isolation (the opposite ear was plugged) performance in Hybrid hearing (EAS) mode was significantly higher than that obtained using the electrical signal alone or the preoperative (acoustic alone) condition, demonstrating effective synergistic combination of acoustic and electrical inputs. Secondly, bars A and B show enhancement of the CI-alone performance when adding acoustic input from the ipsilateral or contralateral sides respectively; approximately equivalent performance is evident for the two conditions. It is also important to note here that B (implanted ear plugged) indicates the range of performance that would be achieved in any subjects who lose all residual hearing in the implanted ear. Thirdly, bar C demonstrates a possible further improvement in performance when acoustic input from both sides is available (though this was not statistically significant in this series). Lenarz et al.16 also provided data for listening in noise performance in a smaller subset of subjects, which showed very similar patterns to those demonstrated for monosyllables in quiet.
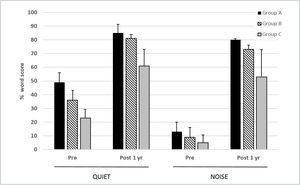
Skarzynski and colleagues have reported extensively on outcomes from implantation of subjects with a range of different levels of low frequency hearing19,26. They classified potential CI recipients according to the level of preoperative low frequency thresholds. Thus, “electric complement” subjects (Group A) had 500Hz thresholds better than 50dB HL and did not always require any acoustic amplification. “EAS” subjects (Group B) had 500Hz thresholds of 50–80dB HL and would be anticipated to utilize acoustic amplification and electrical stimulation in the implanted ear, and “electrical stimulation” subjects (Group C) had 500Hz thresholds outside the range that might benefit from amplification (>80dBHL), i.e. conventional CI candidates.
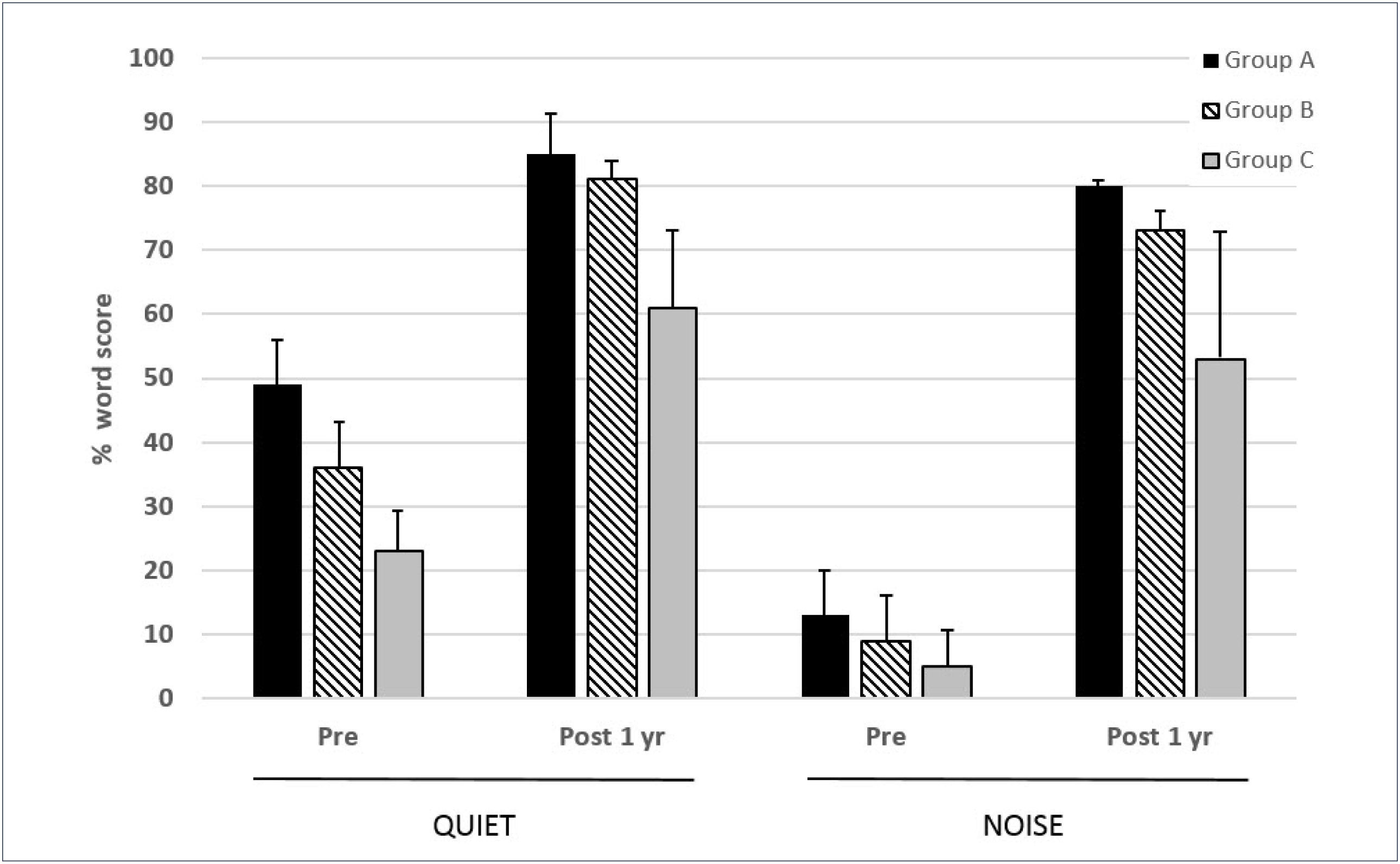
In a series of 35 subjects implanted with the Cochlear CI422 (with Slim Straight electrode array), this centre used a partial insertion (20–23mm) for Group A and B subjects and a full 25mm insertion for Group C subjects26. Outcomes for the Group C subjects (n=11) were comparable to those obtained in conventional CI candidates with other full length electrode arrays. Of the two groups of potential electro-acoustic stimulation users, there were 11 and 13 subjects in Groups A and B respectively. Figure 6 shows the 1 year scores for the three groups tested on monosyllabic words in quiet and in speech-shaped noise (10dB signal-noise ratio). The added benefit of acoustic input is evident from the significantly higher scores in Groups A and B relative to those of the Group C subjects. Greater post-implant improvement is evident in noise as compared with in quiet for all three groups.
MEAN 1 YEAR POST-IMPLANTATION MONOSYLLABLE SCORES IN QUIET AND 10DB SNR NOISE FOR THREE GROUPS OF SUBJECTS IMPLANTED WITH THE COCHLEAR CI422
Error bars show standard deviations. See text for further details. (from Skarzynski et al.26 with permission).
As outlined in the previous section, there is evidence of an increased risk of a major loss of residual hearing with longer electrode arrays, either at the time of surgery or over the subsequent months or years. The use of short arrays tends to result in slightly better hearing preservation, but a very short electrode may not provide equivalent CI-alone performance to longer arrays. When loss of functional hearing occurs, the CI user only has access to information delivered electrically to the implanted ear. There are reports of small numbers of recipients of the 10mm Cochlear Hybrid S device who lost residual hearing and received limited benefit in the CI-alone condition. Some of these subjects were subsequently re-implanted with standard Contour arrays, with improved outcomes in both the CI-alone and “best aided” conditions14,42.
Friedmann et al.21 assessed the functional effects of loss of residual hearing in a cohort of Cochlear CI422 and Hybrid L recipients. Median low frequency threshold loss after surgery was 21.5dB and 16.5dB in the CI422 and Hybrid L recipients respectively, increasing to 30dB and 22dB after 12 months. Seven CI422 users who lost residual hearing showed significantly superior CI alone and bimodal performance than that obtained by three Hybrid L users who also lost hearing. The authors therefore concluded that there is a degree of trade-off in the choice of electrode length in terms of likely hearing preservation and outcomes if residual hearing is lost. However, the studies by Lenarz et al.16 and Skarzynksi et al.26 both demonstrated that the bimodal condition for subjects using the CI422 together with residual hearing in the non-implanted ear (implanted ear blocked) provided substantially improved performance relative to the preoperative condition. These findings suggest that the Slim Straight array of the CI422 and CI522 devices provides an effective combination of hearing preservation and CI-alone performance in potential EAS users as well as offering an effective option for conventional CI candidates.
CONCLUSIONS- 1.
Individuals with steeply sloping high frequency hearing loss can usually hear speech but fail to understand it. Conventional or frequency compression acoustic hearing aids have been shown to be relatively ineffective in providing a useful auditory signal when mid-high frequency hearing loss is severe or profound.
- 2.
Prospective CI recipients with low frequency (up to 500Hz) audiometric thresholds better than around 70-80dBHL are candidates for electroacoustic stimulation (EAS), whereby a CI is implanted into the basal turn of the cochlea in order to provide mid-high frequency information and acoustic amplification is provided to stimulate residual low frequency hearing. Such candidates are usually implanted in the poorer ear when there is functional preoperative hearing.
- 3.
A range of thin, flexible CI electrode arrays have been shown to preserve residual hearing within levels commensurate with EAS in the majority of recipients. The Cochlear Slim-Straight array, as used in the CI422 and CI522 devices, provides good hearing preservation performance yet is long enough to provide high levels of CI-alone performance even if residual hearing is lost.
- 4.
Clinical studies have consistently demonstrated synergistic combination of mid-high frequency information delivered electrically by a CI with low frequencies delivered acoustically, providing superior performance to that obtained from a CI alone. The observed enhancement of CI-alone speech recognition by acoustic low frequencies has been shown to be effective when delivered to the side ipsilateral or contralateral to the CI.
- 5.
New integrated external sound processors, such as the Cochlear CP900 series, are able to deliver electrical and acoustic signals from a single unit, providing enhanced convenience for the user and better integrated fitting procedures.
Conflict of Interest.
Herbert Mauch is employee of Cochlear Latinoamerica, Paul Boyd is consultant for Cochlear Europe.