Preterm birth interrupts the normal development of the respiratory system. Taken together with the lung injury that can occur antenatally such as from chorioamnionitis or postnatally by interventions such as mechanical ventilation and oxygen therapy, survivors are at risk of developing long term deficits of their respiratory system. Decrements of lung spirometry have been regularly reported in those born preterm across all gestational ages. Those who develop chronic lung disease of prematurity (also called bronchopulmonary dysplasia) are the most affected, but lung function decrements are also seen in those born at later gestation of between 33 and 36 weeks, a population that generally does not require respiratory support in the neonatal period. Besides spirometry, many other techniques have been used to assess the status of the respiratory system including measurement of static lung volumes, airway resistance and compliance, bronchial hyper-responsiveness, diffusing capacity, exhaled nitric oxide and newer imaging techniques including hyperpolarised 3-helium magnetic resonance imaging. Discussed in this review are the findings from such methods to delineate the respiratory outcomes that occur after preterm birth.
El nacimiento prematuro interrumpe el desarrollo normal del aparato respiratorio. Los sobrevivientes tienen riesgo de desarrollar déficit en su función, debido a la injuria prenatal por corioamnionitis y postnatal por ventilación mecánica y oxigenoterapia. Consistentemente se ha reportado la disminución de valores espirométricos en prematuros nacidos a cualquier edad gestacional, siendo los más afectados aquellos con enfermedad pulmonar crónica del prematuro o displasia broncopulmonar. Esta alteración se observa inclusive en aquellos nacidos entre las 33 y 36 semanas de edad gestacional, una población que generalmente no requiere de soporte respiratorio en el período neonatal. Existen otras formas de evaluación de la función pulmonar además de la espirometría, tales como la medición de volúmenes pulmonares, resistencia y reactancia de la vía aérea, hiperreactividad bronquial, capacidad de difusión, óxido nítrico exhalado y nuevas técnicas de imágenes tales como la resonancia magnética con gases hiperpolarizados con 3-helio. En esta revisión se discuten los hallazgos de estos métodos para evaluar el impacto del nacimiento prematuro en el aparato respiratorio.
ABBREVIATIONS:
| ADC | Apparent diffusion coefficient |
| BPD | Bronchopulmonary dysplasia |
| BHR | Bronchial hyper-responsiveness |
| CLD | Chronic lung disease of prematurity |
| DLCO | Carbon monoxide diffusing capacity |
| EIB | Exercise induced bronchoconstriction |
| ERV | Expiratory reserve volume |
| FeNO | Fractional exhaled nitric oxide |
| FEV0.5/1 | Forced expired volume in 0.5/1 second(s) |
| FOT | Forced oscillation technique |
| FRC | Functional residual capacity |
| FVC | Forced vital capacity |
| Gaw | Airway conductance |
| IC | Inspiratory capacity |
| IRV | Inspiratory reserve volume |
| IVC | Inspiratory vital capacity |
| LCI | Lung clearance index |
| Raw | Airway resistance |
| Rrs | Respiratory resistance |
| RV | Residual volume |
| TLC | Total lung capacity |
| VT | Tidal volume |
| Xrs | Respiratory reactance |
Preterm (defined as less than 37 weeks’ gestation) birth, a common occurrence, is associated with morbidity and mortality both in the neonatal period and beyond (1). Important long-term outcomes include neurodevelopment and respiratory health. The latter can be assessed in a variety of ways from outlining symptomology to health care utilisation to formal lung function testing. While spirometry is the most common respiratory assessment in follow-up of preterm children, increasingly more detailed testing is being used for preterm-born survivors. While previous focus has been on the extremes of prematurity, in particular those who develop chronic lung disease of prematurity (CLD), there is increasing evidence that even delivery at later gestations, including early term delivery, is associated with lung dysfunction in childhood (2). CLD is often also called bronchopulmonary dysplasia (BPD) and the terms are often used interchangeably. This review will discuss the impact of risk factors on the developing lung, including those which lead to the development of CLD, and the longer term respiratory outcomes of preterm birth with a particular focus beyond basic spirometry, the outcomes of which have been a frequent subject of recent reviews (3,4).
EPIDEMIOLOGYPreterm birth accounts for approximately 11% of all live births worldwide, with increasing rates in many countries (5). It is a leading worldwide cause of death in the under 5-year age group (6), accounting for over 1 million deaths in 2015. However, neonatal mortality as a result of being born preterm has decreased in both the developed and developing world, with the overall death rate decreasing from 10.5 per 1,000 live births 2000 to 7.6 in 2015 (7). Many of the deaths are due to respiratory diseases, which affect 2-3% of all newborn infants (8,9); the rates of respiratory illness increase with decreasing gestational age. Significant progress has occurred in the management of preterm neonates including the use of maternal antenatal corticosteroid administration, exogeneous surfactant and gentler forms of mechanical ventilation (10). However, as survival improves, the longer-term outcomes including good quality of life for the survivors become increasingly important (11).
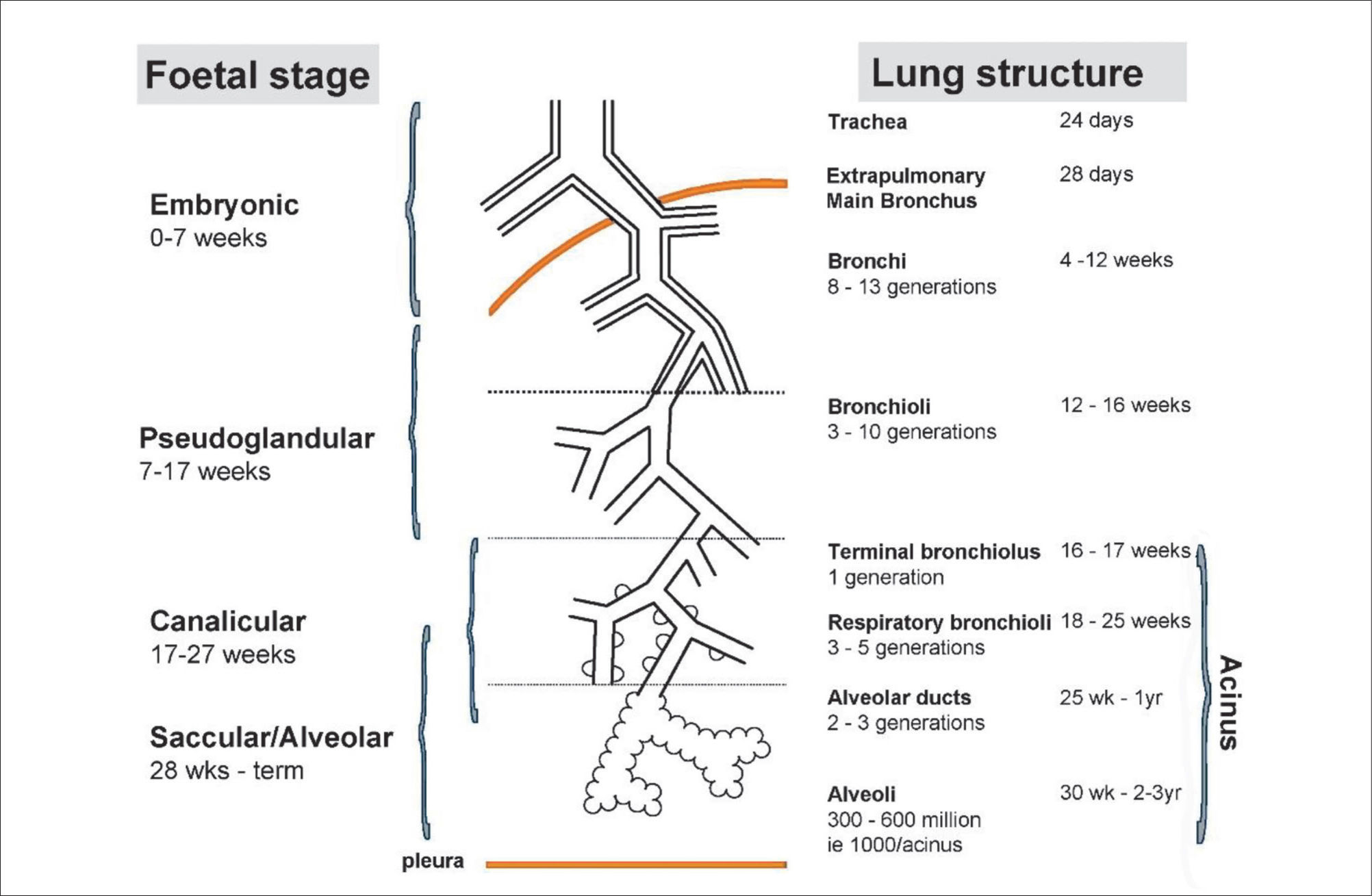
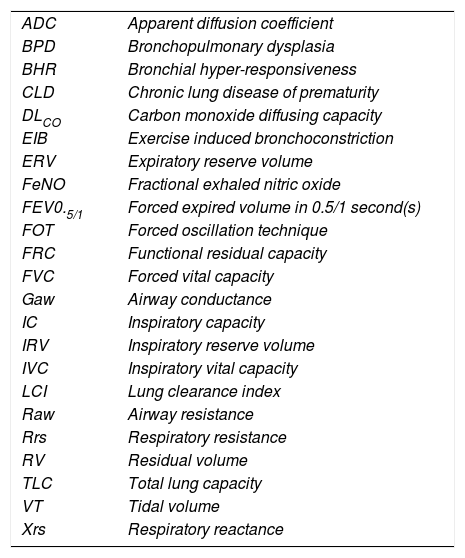
LUNG DEVELOPMENTAn understanding of lung development is key to understand why preterm infants may develop future respiratory deficits (12,13). Normal lung development has been classified into 5 phases (14) (see Figure 1(15)):
- •
Embryonic gestational age 0-7 weeks.
- •
Pseudoglandular weeks 7-17.
- •
Canalicular weeks 17-27.
- •
Saccular weeks 28-36 and
- •
Alveolar weeks 36 to at least 2 years post natal age.
Stages of normal lung development
Reprinted with permission (15).
In extremely preterm-born infants, especially those born at <26 weeks’ gestation, alveolar development has not yet commenced, thus the infant relies on respiratory saccules for their gaseous exchange. In addition, failure to produce adequate antioxidant, enzymes and surfactant, which reduces surface tension at the gas-exchanging units, often results in the development of neonatal respiratory distress syndrome (RDS) (16). The insults of extrauterine life on the immature and fragile lungs occur at a crucial phase of lung development, resulting in subsequent abnormal alveolarisation and aberrant pulmonary vascular development (15).
RISK FACTORS FOR LONG-TERM LUNG DISEASEEvolution of lung disease in the preterm population is a multi-factorial process, a combination of genetic susceptibility and environmental factors. The interventions that some preterm infants receive are particularly important, especially in those who develop CLD. Reviewed below are the important risk factors that potentially lead to the dysfunction of the respiratory system.
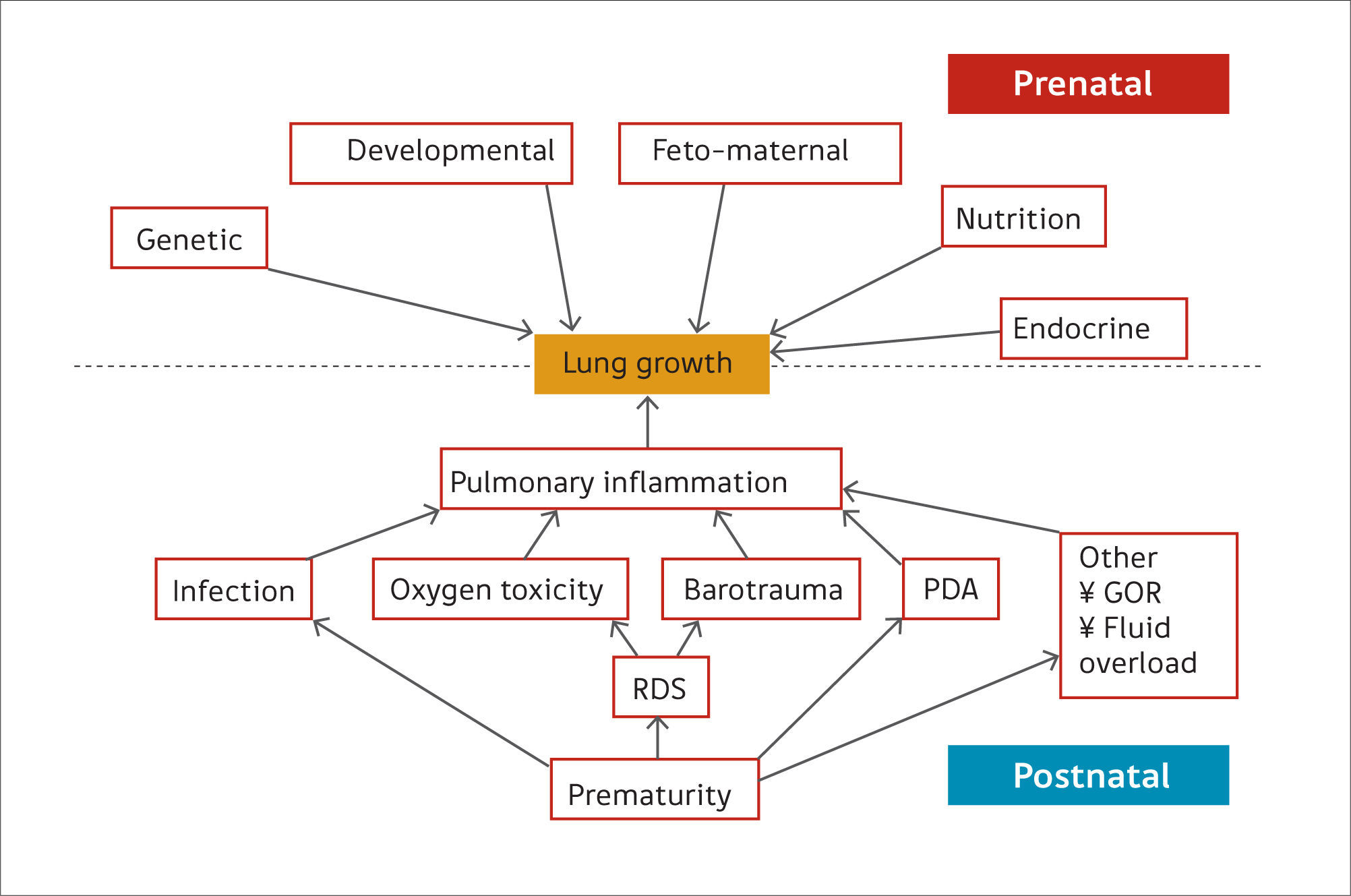
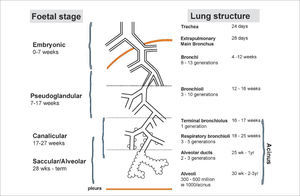
Preterm birthPreterm birth occurs at an earlier stage of lung development, thus, it is not surprising that concerns are raised regarding lung development after birth of preterm infants (17). Many of the ante- and post-natal factors that may affect lung growth in the preterm infant are shown in Figure 2(14). The common pathway for many of these risk factors affecting lung growth may be via pulmonary inflammation (15) but recent data suggest that even late preterm-born infants (of between 33 and 34 weeks’ gestation) or even early term-born children (at 37 – 38 weeks’ gestation) may have longer term decrements of their lung function (2,18,19). In one study of healthy preterm infants who had not received any respiratory support during the neonatal period, increased dead space ventilation, lower functional residual capacity and impaired gas exchange at 40 weeks’ postmenstrual age were seen when compared to term born controls. This suggests that preterm delivery per se affects postnatal lung growth (20). Similar findings have been reported by others (21,22).
Ante- and post-natal risk factors that can affect lung growth directly or via pulmonary inflammation
Reprint with permission (14).
CLD, often also called bronchopulmonary dysplasia (BPD), is a major respiratory complication of preterm birth affecting up to 75% of surviving infants at the extremes of gestation (<26 weeks’ gestation) (23). Histologically, CLD was originally associated with marked pulmonary fibrosis, varying hyperinflation and atelectasis, severe epithelial lesions and decreased alveolarisation, leading to reduced surface area for gas exchange (24). Since the introduction of surfactant, newer ventilation strategies and improved nutrition for preterm infants, the histology shows less fibrosis, less heterogeneity of lung disease and larger, fewer alveoli than observed in old BPD (17), often termed “new BPD/CLD”. It is likely that CLD represents the culmination of delivery at an immature stage of lung growth and exposure to factors that are well-known for causing lung injury. The risk factors for CLD have been previously reviewed (14,15) but include antenatal factors such as maternal health (e.g. pregnancy associated hypertensive conditions), placental function (e.g. chorioamnionitis) (25) and fetal health (e.g. preterm delivery and antenatal infection) (26). Postnatal risk factors that may lead to lung injury include supplemental oxygen therapy, mechanical ventilation (especially invasive mechanical ventilators), respiratory infections (27), patent ductus arteriosus (PDA) and fluid overload.
OTHER RISK FACTORSMany of the risk factors discussed above are associated with the epidemiologically small group of extremely preterm infants of <28 weeks’ gestation (<1% of overall birth) especially those who require respiratory support. Many more-mature preterm-born infants of >32 weeks’ gestation will also be exposed to antenatal risk factors. These include intrauterine growth retardation (IUGR), inadequate maternal nutrition, maternal disease such as diabetes mellitus, pregnancy-associated conditions such as hypertension, antenatal maternal smoking, postnatal smoking exposure from mothers and carers, and social deprivation, which could lead to future risk of developing lung disease. Some of these factors are known to be associated with preterm delivery that clearly has long-term consequences. Placental insufficiency may prevent adequate lung development. In mice, chronic hypoxia during the stage of lung development corresponding to the human 3rd trimester impairs alveolar and pulmonary artery development by upregulating transforming growth factor β (28). Preterm infants born with IUGR have a higher risk of developing CLD in comparison to those with appropriate birth weight for gestation (29). Similarly, term-born infants with IUGR may have long-term respiratory function deficits but robust studies for late preterm infants of>32 weeks’ gestation with IUGR are lacking. Interestingly, catch up growth in term-born infants may lead to some improvement in lung function (30). However, accelerated weight gain in infancy in both term -and preterm- born children may lead to increased respiratory symptoms (31) perhaps due to dysanapsis, i.e. the weight gain suggests somatic growth is occurring but lung growth may be lagging behind for a period of time with the mismatch resulting in respiratory symptoms.
Association between antenatal smoking and preterm birth is well recognised with a recent study showing improvement in preterm labour rates after legislation prohibiting smoking in public areas was introduced in several countries (32). Nicotine and its metabolite cotinine cross the placenta and are found in higher concentrations in the foetal serum, amniotic fluid and placenta than in maternal serum (33). Animal models have shown the specific effects of maternal smoking on fetal lung growth and development including smaller lung volumes and structural changes, including fewer but larger saccules, decreased elastic tissue length and decreased internal surface area in foetal rat lungs (34). Antenatal maternal smoking can impact on lung function in infancy, with those exposed having 7% lower FEV0.5 (forced expiratory volume in 0.5 seconds) (35) and reduced time to peak tidal expiratory flow (36,37). Preterm-born infants with an average gestational age of 33 weeks at birth, exposed to antenatal maternal smoking and assessed prior to hospital discharge, had reductions in expiratory flow parameters, in particular a 13% slower time to peak tidal expiratory flow as a proportion of total expiratory time, compared to those not exposed (38). In addition, a relationship between antenatal smoking and wheeze has been noted, including in infancy (39), childhood (40), and with evidence suggesting that this persists into adulthood (41).
Socioeconomic class is associated with preterm birth. In an epidemiological study of over 800,000 births between 1994 and 2004 in Australia, preterm birth was linked to lower socioeconomic status. Birth at decreasing gestational age was linked to increasing deprivation, with an odds ratio of 1.45 for being born extremely preterm for those in the lowest quartile of socioeconomic disadvantage when compared to the highest quartile (42). Additionally, lung dysfunction has been linked to lower socioeconomic status, with reductions of FEV1 (forced expiratory volume in 1 second) and FVC (forced vital capacity) by ∼17% in boys and ∼14% in girls of children from low-income groups in comparison to high income groups (43).
IMPACT OF PRETERM BIRTH ON RESPIRATORY HEALTHSymptoms and health care utilisationPreterm birth and the associated complications have a significant impact on future respiratory health. For instance, during infancy, those born preterm are at higher risk of respiratory diseases. A population-based study of over 300,000 children noted that decreasing gestational age was associated with increasing respiratory morbidity. Almost a quarter of all infants born at <33 weeks’ gestation required an admission for respiratory illness before the age of 1 year compared to 7.8% of those born at 40-42 weeks’ gestation, with a fourfold increase in actual number of admissions (41.5 vs 9.8 per 100 child-years respectively). With every week of decreasing gestation from 40 to 33 weeks, there was an increase in both proportion of infants admitted and number of admissions. Even those born late preterm at 35-36 weeks’ gestation had almost double the proportion requiring admission and number of admissions (13.5% and 18.8 admissions per 100 child-years respectively) compared to the 40-42 weeks’ gestation group. Additionally, the lower the gestation of the infant, the earlier in life they were likely to be admitted. This finding was not confined to the infant population, with the 1-5 years age group showing a similar but less marked trend (44).
Respiratory symptoms in preterm-born children are not restricted to those born at extremes of prematurity. A questionnaire-based study of preterm- and term-born children from Wales received over 7000 replies, with respondents classified into preschool aged (<5 years) and school aged (≥5 years) children. In the younger age group, those born very preterm (≤32 weeks’ gestation) were 2.8 times more likely to have wheeze-ever compared to the term controls. Furthermore, those born moderately (33-34 weeks’ gestation) and late (35-36 weeks’ gestation) preterm also had greater rates of wheeze-ever (odds ratios of 1.9 and 1.5) when compared to the term group. Similarly, the school aged children also had greater odds ratios for wheeze-ever (respectively 3.29, 1.84, and 1.58 for the <32, 33–34 and 35–36 weeks’ gestation groups) when compared to term controls. Similar trends were seen for both age groups for recent wheeze, recent hospital admissions for respiratory illness and inhaled medication use (45). Even children born at early term (37-38 weeks’ gestation) from both age groups had increased respiratory symptoms and medication use than later term-born subjects (39-42 weeks’ gestation) (18), a finding recently confirmed with spirometry (19). Similar findings were reported in another study of over 14000 UK born children by face-to-face interviews showing an increasing gradient of respiratory symptoms with decreasing gestational age (46). A systematic review of 30 studies including over 1.5 million children showed that those born preterm were 1.7 times more likely to be affected by wheeze, in particular those born at <32 weeks’ gestation who were 3 times more likely to have wheezing disorders (47).
CLD in particular is associated with poorer respiratory outcomes. In the follow-up of the UK EPICure population of extreme preterms born in 1995 at <26 weeks’ gestation, an association was noted between CLD and prevalence of respiratory symptoms and medication use when compared to term-born controls. Rate of treatment with either bronchodilator therapy or inhaled steroids during the first year of life was approximately 20% higher in those with a history of CLD compared to those without. By 6 years of age, significantly more children with a history of CLD had suffered from wheeze in the previous year, wheeze or cough at night, or had received bronchodilators or inhaled steroids within the past 12 months when compared to term controls (48). At 11 years of age, the preterm group had 9-14% higher rates of an asthma diagnosis, requiring asthma medication, or having suffered from nocturnal cough or exercise-induced wheeze within the past 12 months than term-born children. Those with a history of CLD had a 13% higher rate of wheeze within the past 12 months (49).
Respiratory disease following preterm birth can persist into adulthood. Follow-up of a cohort of preterm-born adults at age 21 revealed higher rates (over a quarter of the subjects) of cough, wheeze or asthma compared to term controls (50). Other studies have reported higher rates of ongoing respiratory symptoms in populations of preterm-born adults with a history of CLD. Wong et al revealed respiratory symptoms were experienced by 71% of their study population of 17-33 year olds within the past 12 months (51).
Impact of preterm birth on lung function testingIn addition to subject-reported respiratory symptoms, objective data on the impact of preterm birth on the respiratory system can be obtained from lung function testing. Spirometry can reveal obstructive and restrictive lung abnormalities and is widely available with equipment that can be reliably used within community settings as well as in specialist lung physiology labs (52). However, there are also many other respiratory investigations that have been used to understand lung dysfunction following preterm birth.
SpirometryAbnormalities on spirometry testing have been consistently noted in preterm populations during childhood, with the highest levels of dysfunction observed in those with a previous history of CLD. Synthesised data from a meta-analysis show that preterm infants, excluding those with a history of CLD, have a deficit of 7% of percent predicted FEV1 when compared to term controls. Populations with a previous diagnosis of CLD of supplemental oxygen dependency at either 28 days of age or beyond 36 weeks’ post-menstrual age have deficits of 16% and 19% respectively (53). In addition to respiratory symptoms, lung function abnormalities are not confined to those born very or extremely preterm. Those born at 33-34 weeks’ gestation also have spirometry deficits during mid-childhood, of between 0.35 and 0.49 for adjusted mean z-scores for FEV1, FVC, and expiratory flow rates when compared to term-born children. Similar deficits were seen in children born at earlier gestations (2). Since lung function is thought to “track” throughout life, of particular concern is the premature development of chronic obstructive pulmonary disease in children, especially if preterm-born children are exposed to additional insults such as environmental pollution (54).
As well as having baseline spirometry deficits, preterm-born children, especially those who had CLD, appear to have increased bronchial hyper-responsiveness (BHR) in some studies (55). BHR may persist in adulthood, with increased response to methacholine shown in one population of extremely preterm-born adults (56) and across all preterm groups in comparison to term controls in another (57). However, not all studies have shown increased prevalence of BHR in preterm-born children (58). Exercise-induced bronchoconstriction (EIB) is also seen in preterm populations, particularly CLD groups, as shown by Joshi et al, with a maximal average FEV1 decrease of 11% at approximately 20 minutes post-exercise (59).
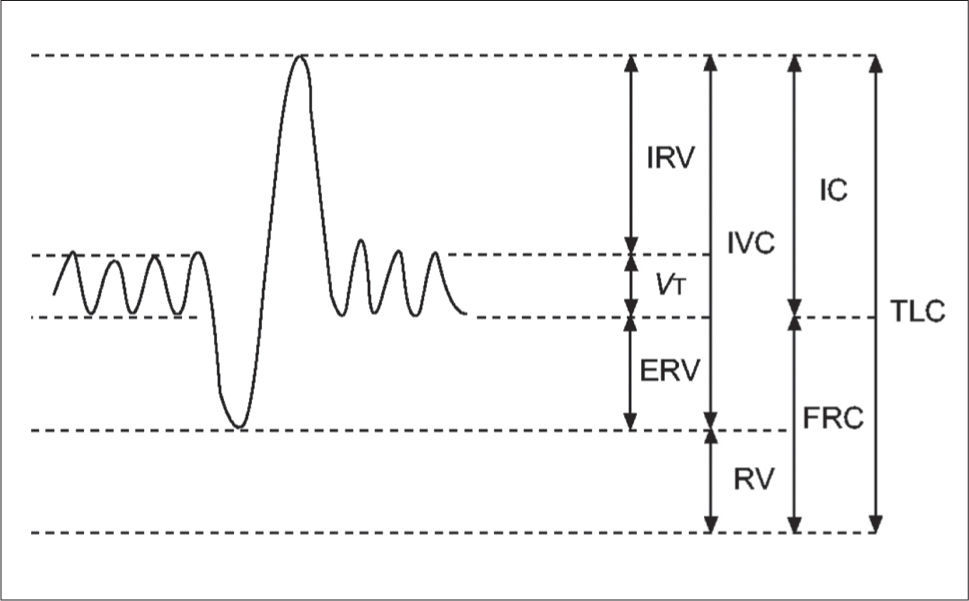
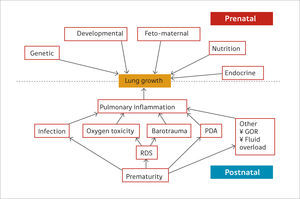
Static lung volumesAlthough spirometry is the most common method for assessing lung function, it has limitations in the paediatric population. Measuring dynamic lung volumes requires cooperation and coordination on the part of the participant performing the procedure. It may, therefore, be difficult to obtain accurate results, especially in younger age groups (60). Body plethysmography allows measurement of static lung volumes, providing structural information about the lungs. These include total lung capacity (TLC), functional residual capacity (FRC), residual volume (RV) and vital capacity (VC). These lung volumes can be represented schematically as seen in Figure 3(61).
A schematic representation of subdivisions of lung volume
IRV: inspiratory reserve volume; VT: tidal volume; ERV: expiratory reserve volume; IVC: inspiratory vital capacity; RV: residual volume; IC: inspiratory capacity, FRC: functional residual capacity; TLC total lung capacity. Reprinted with permission (61).
This method is useful to identify lung hyperinflation from increased RV or FRC. Additionally, increased RV/TLC ratio can also suggest hyperinflation, although this finding is less specific as it may result from normal RV but decreased TLC (62). Hyperinflation is associated with obstructive lung disease or structural abnormalities associated with preterm birth (63). Static lung volumes have been investigated in a number of studies of preterm-born children and young adults. In general, RV and RV/TLC are increased in preterm-born subjects with the greatest increases seen in those with CLD when compared to term-born equivalent controls (59,64-69). These findings suggest that the injuries occurring in infancy persist and, despite remodelling, there is continuing evidence of air-trapping likely to be related to the airway obstruction consistently observed in preterm-born children. A possible association between hyperinflation and bronchial hyper-reactivity (70) suggests the mechanism of air-trapping may be that of a ball-valve, with air being able to enter the lungs but, due to either floppy or constrictive airways, unable to escape. Whether these improve or deteriorate with ageing will be important to assess in longitudinal studies as well as whether they have any response to, or indeed worsening after, bronchodilator treatment which has not yet been accurately assessed.
Airway resistance and respiratory mechanicsRespiratory mechanics, which include measurements of airway resistance and compliance, can give valuable insight into the underlying disease processes. Airway resistance in particular can be measured using different techniques including body plethysmography and forced oscillation technique (FOT). A number of studies have used body plethysmography to compare respiratory mechanics in preterm groups against term controls. Increased Raw or decreased airway conductance (Gaw, reciprocal of Raw) are commonly reported in preterm groups (69,71,72). In addition, among preterm-born children with a history of CLD, Raw is increased (1.05 kPa·L·s-1) compared to preterm controls without CLD (0.75 kPa·L·s-1) (73).
FOT involves assessing the respiratory response to soundwaves superimposed on tidal breathing at varying frequencies. The lower frequency waves travel further into the lungs to give information about the distal airways whereas the higher frequencies report on the proximal airways. Both of these procedures have advantages for the paediatric population as they rely on tidal breathing (74,75). Correlation has been noted between airway resistance (Raw) obtained by plethysmography and resistance measured at 5 Hz during FOT measurements (73). Reactance at 5 Hz has been shown to correlate with spirometry (73,76), suggesting that FOT may be a useful alternative for assessing lung disease in young children born preterm.
The study by Malmberg et al of preterm-born children (aged 8 years and born at <30 weeks’ gestation) had significantly higher resistance and lower reactance (Xrs), at the lower frequencies (5 and 10 Hz) compared to their term counterparts. The findings suggest that smaller, distal airways are predominantly affected. This may be due to altered elastic properties of the lungs resulting from airway obstruction associated with preterm birth (73). A subgroup of 16 children from this study underwent pre- and post-bronchodilator measurements of Rrs at 5 Hz (peripheral airways). The preterm children had a 10% greater decrease in resistance values following bronchodilator administration compared to the term control group, suggesting a greater degree of reversible airways disease as a result of an obstructive picture (77).
In another study, Vrijlandt et al compared preterm-born children aged 3–5 years with and without CLD with term controls. Both the preterm groups had similar resistance values at 6 Hz which were significantly higher compared to controls; lower reactance values at 6 Hz were noted in the CLD group compared to the non-CLD preterm group. The resonant frequency, the frequency at which the lower frequency elastic properties and the higher frequency inertial properties cancel each other out, was also highest in the CLD group (26.8 Hz) compared to the other groups (22.7 Hz) (78). Udomittipong and colleagues obtained similar findings assessing 64 children aged 3–6 years and diagnosed with CLD. Resistance and reactance values at 6, 8 and 10 Hz were measured and compared to a previous healthy control cohort. The z-scores were increased for resistance (between 1.03 and 1.28) and decreased for reactance (between -1 and -1.41) when compared to the control group (79). Bronchodilators improved both Rrs and Xrs at 6, 8 and 10 Hz, with decreases of between 18.2 and 23.3% from baseline values for the former and increases of between 31.9 and 38.8% for the latter (80). More recent studies (76,81) also report increased resistance and decreased reactance particularly in those with a history of (severe) CLD.
Interestingly, Er et al reported increased resistance values at 5 and 10 Hz in late preterm children aged 3–7 years with gestation at birth of 34-36 weeks when compared to term-born controls, further suggesting that peripheral airway disease may be a consequent of delivery at an early stage of lung development (82).
Overall findings from FOT consistently show increased resistance and decreased reactance, particularly at lower frequencies, in preterm-born children with the worst values observed in those with a history of CLD. The observations are most likely to be due to altered structure resulting after birth at an early stage of lung development.
Diffusing capacityCarbon monoxide diffusing capacity (DLCO) of the lungs is a well-described technique whereby the ability of oxygen to transfer from the alveoli into the lungs’ capillary network is assessed using carbon monoxide thus giving information about the alveolar-capillary interface. There are a number of methods to measure DLCO but the single breath technique is most commonly used. It is determined from concurrent measurements of carbon monoxide uptake from alveolar gas and the volume of the alveoli. There are several factors that can affect this uptake, including structural abnormalities (e.g. lung volumes, area of capillary-alveolar interface) and functional issues (e.g. ventilation-perfusion mismatch, gas tensions within the blood) (83). In several studies, preterm-born children with CLD had similar DLCO values to preterm control groups (68,84). A more recent study of 126 preterm children and 34 term born children showed DLCO was lower in controls (86.3 percent predicted) than in preterm subjects (92.1 percent predicted) suggesting that preterm-born children are unlikely to have any significant gas exchange abnormalities (69).
In contrast, Hakulinen et al assessed 31 preterm-born children with and without CLD compared to healthy term controls. The preterm groups had significantly lower percent predicted DLCO compared to the term group (91.1% and 89.5% in the preterm groups, and 100.7% in the controls, p=0.021) (71). Data from a Finnish cohort showed similar results in their cohort of 55 preterm-born, very low birth weight (<1500g) children, both with and without CLD. Both these groups had significantly lower DLCO, including when corrected for alveolar volume, with percent predicted values of 91% and 94% in the CLD and no-CLD preterm groups respectively, compared to 102% in the term control group (72). Cazzato and colleagues noted that DLCO in a group of very low birth weight (≤1500g), very preterm (≤32 weeks’ gestation) children was approximately 1 z-score lower when compared to term controls (85). A similar deficit in z-score was noted in the EPICure cohort of extreme preterm children when compared to term controls (66).
In other studies, subjects with a history of CLD have been shown to have decreased diffusion capacity. Balinotti et al studied infants with CLD aged between 4 and 18 months after birth at 24-29 weeks’ gestation, reporting lower DLCO of 2.7 versus 3.4ml/min/mmHg adjusted for lung volume in the CLD group compared to term controls (86). For Joshi et al, a CLD group in mid-childhood had lower predicted DLCO values compared to a preterm-born group without CLD (74.6% versus 89.7%) (59).
In general, there appears to be observations of impaired alveolar-capillary interface after preterm birth especially in those who develop CLD, although not all studies report differences.
Exhaled nitric oxide testingFractional exhaled nitric oxide (FeNO) has been used as a marker of (eosinophilic) inflammation in respiratory diseases. While higher concentrations have been reported in asthma (87), a label often inappropiately given to preterm-born children with airway obstruction, there is little evidence of increased FeNO in preterm-born children including those who had CLD in infancy. In infancy, nitric oxide output (exhaled NO accounting for expiratory flow) appears to be lower in CLD populations compared to healthy preterm- and term-born children (after adjusting for sex, atopy, passive smoke exposure and caffeine consumption) with 10% and 17% lower values respectively (88). Lower concentrations of exhaled nitric oxide were noted in 31 preterm-born school-aged children with a diagnosis of CLD in comparison to healthy preterm and term controls (7.7, 9.9 and 10.7 parts per billion (ppb) respectively) (89). Results remain similar in adulthood, with median FeNO of 19 ppb in preterm-born children with and without CLD and 27 ppb in term-born controls (p=ns) (90). The data suggest that there is unlikely to be eosinophilic inflammation occurring in preterm-born children with airway obstruction.
Lung clearance indexThe lung clearance index (LCI), using multiple breath washout techniques, assesses ventilation inhomogeneity. In disorders such as cystic fibrosis, LCI is useful in detecting early lung disease (91). Given the continuing airway and parenchymal abnormalities observed in preterm survivors, especially those with a history of CLD, LCI may be useful in assessing ventilation inhomogeneity. Surprisingly, in general, studies have not consistently reported differences between preterm-born children with or without CLD and term-born children (92-95). Lum et al noted increased LCI which may be due to decreased gas mixing efficiency in the extremely preterm group of <26 weeks’ gestation which may reflect some parenchymal or small airway disease, but the LCI was not helpful in discriminating lung disease between preterm groups with and without CLD and term groups. Hülskamp and colleagues did not find differences for LCI between preterm and term groups but on multivariable analyses noted an association between ventilation inhomogeneity and duration of supplemental oxygen. It has been speculated that the lack of differentiation using LCI in children with established airway disease and changes on imaging such as CT scans (see below) may be due to fixed airway obstruction beyond which the study gas may not diffuse during testing, hence lack of inhomogeneity observed.
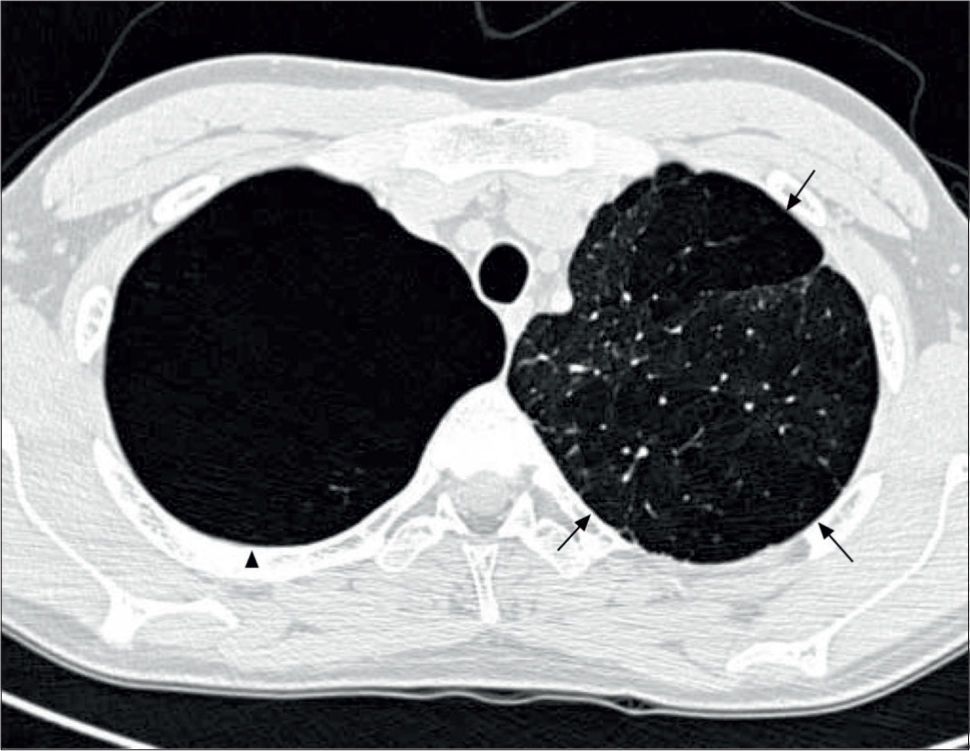
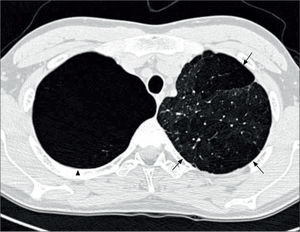
Lung imagingPreterm-born children, including those who had CLD in infancy, do not routinely undergo imaging with chest radiographs or CT scans unless there is an underlying clinical indication. Where they have been reported, chest radiographs show areas of collapse and hyperinflation in keeping with the reported air-trapping seen in preterm-born children with significant airway disease. The few studies reporting data on thoracic CT scans are, in general, of subjects from the pre-surfactant era with abnormalities such as opacification, bronchial and interlobal septa thickening, and air trapping, especially in those who had CLD in infancy (96-98). In the study by Wong et al, 84% of subjects with CLD showed evidence of emphysema, as demonstrated in Figure 4(51). More recently, hyperpolarised 3-helium MRI scanning has been used to assess alveolar dimensions using the apparent diffusion coefficient (ADC). One initial study shows that the ADC was remarkably similar in preterm-born children with and without CLD and term-born controls (99), thus suggesting that there is alveolar development occurring well beyond the currently accepted two years of age.
CT thorax of a 20 year old with a history of BPD
The arrows indicate emphysematous changes in the left lung field, while the arrowhead shows a large right upper lohe bulla. Reprinted with permission (51).
Lung dysfunction is associated with preterm birth and there are increasing concerns the deficits may progress to chronic lung disease in adulthood. The causes of the lung deficits associated with preterm birth are multifactorial but are largely due to the interruption of the normal in-utero lung growth and development, which can be subsequently compounded by (pulmonary) inflammatory processes triggered either in-utero (e.g. by chorioamnionitis) and/or by postnatal interventions such as mechanical ventilation and oxygen therapy. While previous emphasis has been on the respiratory outcomes of those born at the more extremes of preterm, in particular those with CLD, it is clear that even those born moderately preterm are at risk of future decrements of their respiratory function. There are a variety of investigative options to aid diagnosis of lung dysfunction, increase our understanding of the underlying mechanisms and to assess responses to treatment, which are currently lacking for preterm-born children (100). Several techniques offer promise of increasing knowledge of disease mechanisms, for instance, FOT that can be used in the younger, preschool-aged children where alternative modes of testing have difficulties. There is also the potential to assess if quality of life, including physical activity in preterm-born children (101,102), improves after treatment of the deficits in lung function observed in preterm-born children.
The authors declare no conflicts of interest in relation to this article.