We report the case of a preterm infant of 35 weeks gestation, who presents vomiting towards the end of the first week of life. Suspected diagnosis of hypertrophic pyloric stenosis by exclusion. The ultrasound confirms the diagnosis. Pyloromyotomy is performed with excellent evolution. We describe this case due to time of onset of the disease associated to a preterm infant.
Se presenta el caso de un recién nacido prematuro de 35 semanas de gestación, quien presenta vómitos de contenido gástrico hacia el final de la primera semana de vida. Se sospecha diagnóstico de estenosis hipertrófica de píloro por exclusión. El ultrasonido corrobora el diagnóstico. Se realiza piloromiotomía con excelente evolución. Se describe este caso por el tiempo de presentación del padecimiento asociado a un recién nacido prematuro.
Hypertrophic pyloric stenosis (HPS) is a disease which occurs in the second week of life, of unknown origin, which consists of the narrowing of the pylorus due to concentric muscular hypertrophy, causing gastric outlet obstruction with progressive vomiting that leads to malnutrition, dehydration and serious metabolic disorders.1
Prompt diagnosis prevents complications, reduces the morbidity rate and enables surgical treatment with an excellent prognosis.2
The origin is unknown, but the most accepted hypotheses suggest the use of concentrated baby formulas, lack or reduction of pyloric muscle innervation, elevation of gastrin and gastric somatostatin and even allergy.3,4
Repeated vomiting favours oedema of the pyloric mucous membrane, which exacerbates the symptoms, leading to loss of fluids, hydrogen ions and chlorine, all of which leads to hypochloraemic alkalosis. The lack of actual ingestion leads to malnutrition and greater sensitivity to metabolic, haemodynamic and infectious complications.2,5,6
Some patients present jaundice derived from elevated indirect bilirubin, from a not completely understood mechanism that reduces glucuronyl transferase and increases enterohepatic bilirubin circulation; this is corrected when the patient undergoes surgery.6,7
The symptoms do not usually appear before the second or third week of life, and earlier onset is exceptional.8–11
Case reportA male newborn (NB), from the second pregnancy of a 27-year-old mother. She denies drug addiction. Three normal obstetric ultrasounds. Discharge of clear transvaginal fluid for 30h. Baby born vaginally with Apgar 8-9, Capurro “B” 35 weeks, gestation, weight 2550g, height 48cm.
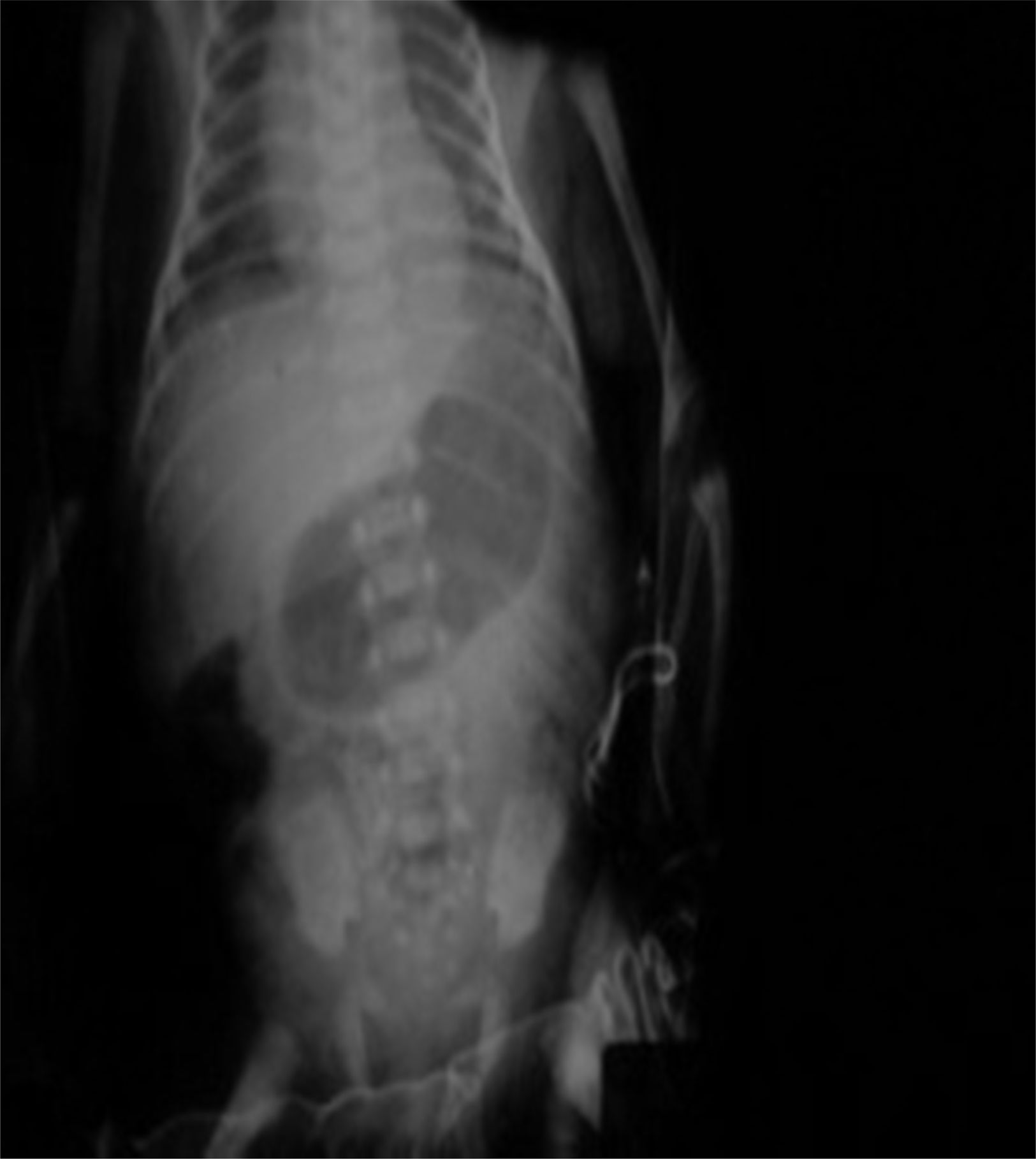
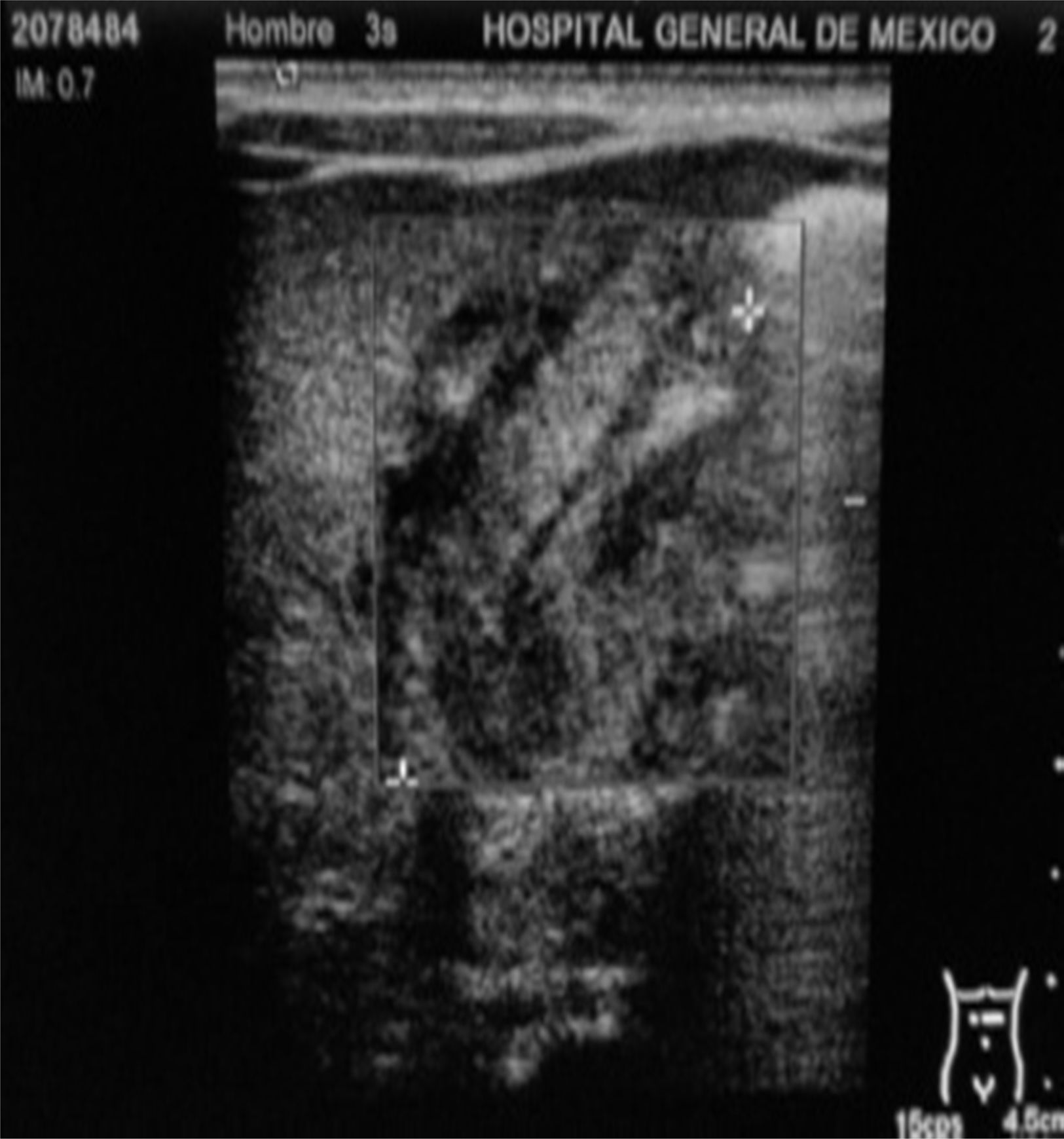
Management starts with fasting, oxygen therapy, ampicillin and amikacin for seven days due to premature membrane rupture. Insidious respiratory and infectious evolution. Sudden onset of postprandial non-biliary vomiting and increase of indirect bilirubin. Given the possibility of nosocomial infection, he is started on cefepime plus dicloxacillin. X-ray of chest and abdomen shows gastromegaly (Fig. 1). Oral ingestion is resumed, with enteral stimulation, but vomiting persists so he continues to fast, a central line is installed and parenteral nutrition is started. Gastric hyperperistalsis and palpation of pyloric mass. Blood gas shows normochloraemic metabolic alkalosis. Ultrasound shows pylorus 18.8mm long, with slices showing muscle wall thickness of up to 4.3mm (Fig. 2), confirming the diagnosis of HPS.
Surgical finding: 2cm pylorus with pearly appearance. Oral intake resumed 24h after surgery, well tolerated and accepting milk increments. Antibiotics suspended as there is no evidence of infection. Discharged home with follow-up as outpatient.
DiscussionHypertrophic pyloric stenosis is a very rare condition in preterm infants. The literature describes an incidence rate of 1 to 2.9 cases per 1000 pre-term births.10,13,14 It is a very common cause of surgery in the first month of life. Despite the radical change of this condition's presentation in the last 10 years,23 the rarity of its presentation in preterm infants is still emphasised.12
In full-term newborns it appears from the second to the seventh week after birth, and its incidence rate is much higher than in pre-terms.10 This year, Stark et al. reported a series of 2466 newborns with HPS, 208 (8.43%) of whom were premature, compared with 2258 (91.57%) full-term babies with the problem, showing how rare this condition is in babies with less than 37 weeks of gestation (wog).10
The literature describes factors associated to preterm newborns with HPS: mothers with multiple pregnancies, exposure to macrolides and male gender10; however, the greater the prematurity, from 28 to 31 wog, a ratio of 1:1 F–M is reported. This ratio increases to 2:1 F–M from 32 to 36 wog, and for full-term NBs, the F–M ratio is 5:1.12 Our patient was of the gender primarily reported in the literature.
Predominance of the male gender has a genetic component, as the greater risk of gastrointestinal malformations in that gender is well documented, as is the risk of rotavirus infections in childhood. This suggests considerable differences in the development, maturity and function of the gastrointestinal tract between men and women.15–19
Mutations in chromosomes 2q, 3p, 5q, 7p, 11q, 16p and even in chromosome x play an important role in the development of HPS.14,17–21 However, despite a large variety of studies, it has not been possible to document a Mendelian inheritance pattern, not even when present in monozygotic twins, which supports the theory that environmental factors are fundamental in its presentation.22
Other risk factors associated to HPS are: caesarian birth and mothers smoking during pregnancy.12,13 However, caesarian birth as a risk factor is not fully clear, as in some series the birth method is consistent with maternal infection. Vaginal birth has also been described as beneficial, as it prevents hormonal cascades and stress factors in newborns. Our patient was born vaginally and therefore not affected by such a risk factor.
The association with smoking has also been attributed to tobacco substances that cause pyloric spasm and muscular hypertrophy.12,13 The mother of the NB in question denied any toxic habits.
Mothers under 20 years of age, diabetic and with a low education level have also been associated to a greater risk of HPS, as are women exposed to pesticides, as they act as endocrine disruptors.20,23 None of these factors were present in this case.
On the other hand, prostaglandin infusion at standard doses has also been reported as a cause of the condition, due to direct pharmacological effects on the pyloric muscle.24 This drug was not administered to our patient, as he did not present a ductus arteriosus-dependent cardiopathy, but it is important to consider this undesirable effect in patients who do.
The clinical presentation often consists of vomiting that could initially be mistaken for reflux, yet episodes often lead to metabolic alkalosis due to loss of hydrogen ions and chlorine. This is consistent with our patient's symptoms. This presentation in pre-term newborns is often diagnosed and treated as gastroesophageal reflux, as premature babies have multiple risk factors such as the use of methyl-xanthines and immaturity of the upper oesophageal sphincter. This makes it difficult to consider HPS as the primary origin of vomiting.25
Our patient was initially treated with anti-reflux measures, using widely recommended drugs at standard doses.25–27
The patient was examined, palpating a pyloric mass, a pathognomic sign of the condition.28
Abdominal ultrasound is considered to be a highly sensitive (91%) and specific (100%) test, which also makes it possible to distinguish duodenal membranes or other malformations as differential diagnoses.28,29 In our patient, the ultrasound showed the findings described in Table 1 and Fig. 2, which are consistent with the literature.28,29 Three factors have been suggested for the condition's diagnosis: the presence of delayed gastric output by ultrasound, pyloric sphincter muscle thickness of ≥2.5mm and muscle length ≥14mm.30
Other diagnostic tests mentioned in the literature are a gastroduodenal oesophageal series and endoscopy, which can also have therapeutic purposes.28,30–32
Laparoscopic, or minimally invasive, surgery is increasingly recommended in the literature, but requires appropriate medical instruments and equipment to tackle the physiology of pre-term infants and their reduced surgical fields,30–33 with small incisions through the umbilical scar and excellent results, starting food intake the following day. However, our hospital does not yet have the equipment required for this procedure in pre-term infants. Even so, the results were excellent in our patient, operated on with a conventional technique, enabling him to feed after 24h, with no complications and outpatient follow-up.
ConclusionThe objective of presenting this case report is to consider HPS as a possible diagnosis in pre-term NB patients with food intolerance symptoms,10–13,25,29 even when gestational age and days of onset are not commonly reported in the literature, as presentation has radically changed in the last 10 years and even male predominance is not common in such patients. This could prevent said diagnosis, delaying treatment, increasing the duration of hospitalisation and inherent complications.
It is therefore important to broaden our diagnostic approach and consider this condition in our patients, as the tendency towards preterm birth in intensive or intermediate care units, mothers aged 20 years or less, with low educational levels, smoking, and the use of macrolides and prostaglandins for commonly associated conditions are increasingly common in our hospital population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interests.