Tumour necrosis factor alpha (TNFα) is a key molecule involved in the inflammatory response and thus related to the pathogenesis of several autoimmune and autoinflammatory diseases.
The blockade of TNF receptor (etanercept main effect) has been successfully used in psoriasis and psoriatic arthritis, among other rheumatologic diseases. The only approved indication for etanercept in dermatological disease is plaque psoriasis; however, the literature is full of case reports and case series where etanercept was used off-label, sometimes successfully. We review some of these indications.
El factor de necrosis tumoral alfa (FNTα) es una molécula clave en la respuesta inflamatoria y se relaciona con la patogenia de varias enfermedades autoinmunes y autoinflamatorias.
El bloqueo del receptor del FNTα (efecto principal del etanercept), ha sido utilizado con éxito en psoriasis y artritis psoriásica, así como en otras enfermedades reumatológicas. La única indicación aprobada para el uso de etanercept en padecimientos dermatológicos es en psoriasis en placa; sin embargo, la literatura está plena de reportes de casos y series de casos donde se utiliza etanercept con indicaciones no aprobadas en algunas ocasiones con éxito. Realizamos una revisión de algunas de tales indicaciones.
Tumour necrosis factor alpha (TNFα) is a proinflammatory mediator involved in several cellular functions, primarily proliferation, differentiation and apoptosis.1 Elevated levels of this cytokine are associated with excessive inflammation and severe organ injury,2 and it is also involved in the pathogenesis of several autoinflammatory and autoimmune diseases. This has led to the development of TNFα agonists.
TNF is produced by various different cells, including mast cells, macrophages, fibroblasts and keratinocytes.2
Etanercept is a fusion protein that acts as a TNFα inhibitor. Structurally, it is a dimer of the extracellular portion of human TNFR2 fused to the Fc-portion of human IgG1, and inhibits both soluble and transmembrane TNFα.1,3 Etanercept acts as a competitive inhibitor of TNFα4 by binding TNFα and preventing its interaction with the cell surface receptor.5
Etanercept also binds to soluble TNF by interacting with a single soluble TNF trimer to produce 1:1 complexes. It is administered subcutaneously, and has an estimated bioavailability of between 58% and 63% and a half-life of 70h.2
No drug interactions have been reported with etanercept because it is metabolised by proteolytic processes and eliminated in bile or urine.2
So far, it has been approved by the FDA (Food and Drug Administration) for inflammatory diseases such as moderate to severe rheumatoid arthritis, ankylosing spondylitis, Crohn's disease, moderate to severe plaque psoriasis, psoriatic arthritis and juvenile idiopathic arthritis.1,6,7 In dermatological diseases, it is only approved for moderate to severe recalcitrant or recurrent plaque psoriasis. Randomised, comparative, multicentre studies have shown etanercept to be safe and effective in various therapeutic regimens.8 It is usually administered subcutaneously at a dose of 50mg twice weekly, which can later been tapered to 25mg twice weekly.3,8
Other TNFα inhibitors include infliximab, adalimumab, and more recently, certolizumab and golimumab.
These drugs have so far proved to be safe. However, the main concern is the development of opportunistic infections and reactivation of latent tuberculosis infection.1 In a recent study, Guarneri and Polimeni9 describe a case of Nicolau syndrome following administration of etanercept, a complication that should be borne in mind and treated promptly at the first sign of symptoms.
Etanercept is usually well tolerated, with the most common adverse effect being injection site pain, reported in 40% of patients.3 Others frequently mentioned include a predisposition to respiratory infections (35%) and headache (20%).5 Reactivation of latent tuberculosis has also been reported with long-term use of etanercept, together with lymphomas. Evidence of the latter, however, is inconclusive.3 Other reactions reported are demyelinating disease of the central nervous system, aplastic anaemia, pancytopenia, onset or exacerbation of erythematous lupus, and sepsis.5 Sepsis in this context has been studied extensively by Díaz-Lagares et al.,10 in 344 patients with autoimmune disease receiving biological treatment. Of all the biological agents studied, the authors found that rituximab was associated with the highest risk for developing severe infection, and etanercept with the lowest.
Etanercept is contraindicated in patients with a history of hypersensitivity to any of its ingredients and with any level of active tuberculosis.3
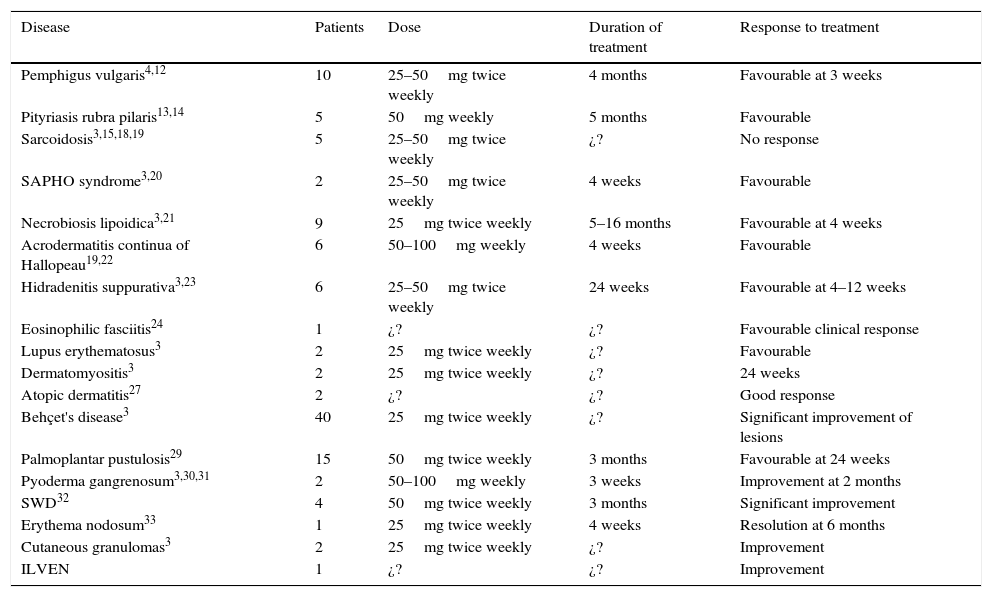
An increasing number of studies report good results for the off-label use of etanercept to treat dermatological diseases, and this has prompted us to undertake this review, Table 1 lists the off-label indications in dermatology7 and the regimens most commonly used in the studies reviewed.
Off-label uses of etanercept in skin diseases, dosage, and therapeutic response.
| Disease | Patients | Dose | Duration of treatment | Response to treatment |
|---|---|---|---|---|
| Pemphigus vulgaris4,12 | 10 | 25–50mg twice weekly | 4 months | Favourable at 3 weeks |
| Pityriasis rubra pilaris13,14 | 5 | 50mg weekly | 5 months | Favourable |
| Sarcoidosis3,15,18,19 | 5 | 25–50mg twice weekly | ¿? | No response |
| SAPHO syndrome3,20 | 2 | 25–50mg twice weekly | 4 weeks | Favourable |
| Necrobiosis lipoidica3,21 | 9 | 25mg twice weekly | 5–16 months | Favourable at 4 weeks |
| Acrodermatitis continua of Hallopeau19,22 | 6 | 50–100mg weekly | 4 weeks | Favourable |
| Hidradenitis suppurativa3,23 | 6 | 25–50mg twice weekly | 24 weeks | Favourable at 4–12 weeks |
| Eosinophilic fasciitis24 | 1 | ¿? | ¿? | Favourable clinical response |
| Lupus erythematosus3 | 2 | 25mg twice weekly | ¿? | Favourable |
| Dermatomyositis3 | 2 | 25mg twice weekly | ¿? | 24 weeks |
| Atopic dermatitis27 | 2 | ¿? | ¿? | Good response |
| Behçet's disease3 | 40 | 25mg twice weekly | ¿? | Significant improvement of lesions |
| Palmoplantar pustulosis29 | 15 | 50mg twice weekly | 3 months | Favourable at 24 weeks |
| Pyoderma gangrenosum3,30,31 | 2 | 50–100mg weekly | 3 weeks | Improvement at 2 months |
| SWD32 | 4 | 50mg twice weekly | 3 months | Significant improvement |
| Erythema nodosum33 | 1 | 25mg twice weekly | 4 weeks | Resolution at 6 months |
| Cutaneous granulomas3 | 2 | 25mg twice weekly | ¿? | Improvement |
| ILVEN | 1 | ¿? | ¿? | Improvement |
Pemphigus vulgaris is a bullous autoimmune disease characterised by autoantibodies that target different antigens, primarily desmogleins 1 and 3, which are adhesion molecules in the desmosomes of keratinocytes.5 TNFα is strongly associated with the pathogenesis of this condition,4 and evidence has shown levels of this cytokine in serum and blister fluid to be associated with the clinical activity of the disease.5
Etanercept has been successful in treating pemphigus vulgaris, and its use is justified by the significant involvement of TNF in the development of acantholysis.11
A number of studies and case series have reported the use of etanercept in the management of recurrent and/or recalcitrant pemphigus vulgaris. In these cases, the drug was used either alone or in combination with systemic immunosuppressants such as systemic steroids, azathioprine, methotrexate, dapsone and IV immunoglobulin. The disease was successfully controlled within 3–6 weeks of treatment, with a reduction in the number of bullae and fewer recurrences.3–5
In a recent report published by Fiorentino et al.,4 6 patients with severe pemphigus vulgaris were included in a double-blind, randomised, placebo controlled study and treated with etanercept 50mg twice weekly.
In another study, Tirado-Sánchez et al.,12 describe 4 cases in which recalcitrant pemphigus vulgaris was treated successfully with etanercept and methotrexate.
Pityriasis rubra pilarisPityriasis rubra pilaris (PPR) is an inflammatory disease of unknown aetiology, characterised by follicular hyperkeratosis and palmoplantar keratoderma that can progress to erythroderma in some cases. Opinions vary on the best treatment for PPR; only a few case studies and series have been reported, and the therapeutic approach is at the discretion of the attending specialist, based on severity. Some of the most effective treatments include oral retinoids (acitretin), methotrexate and PUVA therapy. A new approach with promising results involves the use of TNFα antagonists. In a recent study, Garcovich et al.,13 investigated 7 patients with PPR (6 with type 1 and 1 with type 2) that had responded poorly to different systemic therapy regimens. Three patients were started on IV infliximab 5mg/kg administered at week 2, week 6, and then weekly; 4 patients were started on etanercept 50mg weekly. The primary outcome measure, 75% improvement, was achieved with the 6 type 1 patients. The type 2 patient presented partial recurrence.
Guedes et al.,14 reported good results with etanercept in a 30-year-old patient diagnosed with PPR, with remission of lesions after 5 months of therapy.
SarcoidosisSarcoidosis is a chronic condition characterised by the formation of small, non-caseating granulomas. TNFα plays a pivotal role in the development of this disease.15–17
Several cases reporting beneficial results with etanercept have been published, above all in cutaneous sarcoidosis. Results with pulmonary and ocular sarcoidosis, however, have been disappointing. One of the most striking cases involves a man diagnosed with cutaneous sarcoidosis refractory to prednisone, hydroxychloroquine and methotrexate. The condition was resolved following the addition of etanercept 25mg twice weekly to the foregoing therapy.3 Another study mentions the case of a 43-year-old patient in whom skin lesions resolved after administration of etanercept as monotherapy.18 In other reports, however, etanercept was detrimental to the existing condition. One such case was that of a 71-year-old patient with ankylosing spondylitis treated with etanercept who developed cutaneous sarcoidosis.3 In another patient, a 43-year-old women with centrofacial sarcoidosis who presented a recurrence after treatment with prednisone, hydroxychloroquine and methotrexate, administration of etanercept 50mg twice weekly improved induration but greatly increased the extent of the plaque The patient was switched to adalimumab (another TNF inhibitor), with significant improvement.15 Yet another report involves a 37-year-old woman with a 5-year history of pulmonary and cutaneous sarcoidosis who did not respond to conventional immunosuppressant drugs. After switching to etanercept twice weekly (50mg), with poor results, she was eventually successfully treatment with adalimumab 80mg weekly.19
SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome)SAPHO syndrome is characterised by acne associated with synovitis, pustulosis, hyperostosis, and osteitis, and sometimes also with pyoderma gangrenosum. Although the aetiology is unclear, it is thought to be a reactive infectious osteitis due to Propionibacterium acnes.20
Some case reports have been published, such as that presented by Wegner et al. describing 2 cases of patients with SAPHO syndrome in whom etanercept 25mg twice weekly achieved rapid, sustained remission.3 Another case involved a 27-year-old man with a 7-year history of pyoderma vegetans and follicular occlusion triad with concurrent chronic hepatitis C and seronegative arthritis treated unsuccessfully with antibiotics and isotretinoin. He was started on etanercept 50mg twice weekly, and showed significant improvement in both skin manifestations and arthritis after 4 weeks of therapy.20
Necrobiosis lipoidicaTNFα is involved in the pathogenesis of necrobiosis lipoidica, a granulomatous disease.21 Although no treatment guidelines have been drawn up for this condition, TNFα inhibitors are usually considered to be effective.
Necrobiosis lipoidica is characterised by patches that can become ulcerated, and that resolve leaving behind atrophic, yellow plaques with raised borders and abundant telangiectasias. It is most prevalent in patients aged between 30 and 40 years, and usually presents on the lower limbs. It is associated with diabetes in up to 60% of cases.21 Ulceration occurs in approximately 35% of cases, and will determine response to treatment.21 First line therapies include potent topical or even intralesional steroids, diabetes management, and life-style changes, such a smoking cessation. Other treatment options with varying results include systemic steroids, topical tacrolimus, cyclosporine, mycophenolate mofetil, pentoxifylline, antimalarials, and autologous skin grafts.21
Only 7 studies on ulcerated necrobiosis lipoidica treated with etanercept have been published, all reporting successful outcomes.21
In one case of necrobiosis lipoidica that had previously been treated with systemic steroids and dapsone followed by autologous grafts, etanercept 25mg was given twice weekly for 16 weeks as maintenance treatment to prevent recurrence.3
In necrobiosis lipoidica, etanercept is primarily indicated for IV administration, although intralesional administration at the same dose and with good results has also been reported.3 In one such case, a 50-year-old patient with a 7-year history of ulcerated necrobiosis lipoidica on the anterior surface of the lower limbs had been treated unsuccessfully with topical and intralesional steroids, pentoxifylline and cyclosporine. Administration of etanercept 25mg twice weekly as monotherapy brought the dermatosis under control in 4 weeks, after which the patient continued to receive a maintenance dose for 5 months to prevent relapse.21
Acrodermatitis continua of HallopeauAcrodermatitis continua of Hallopeau is a pustular dermatosis considered to be a variant of pustular psoriasis. It is characterised by the formation of pustules on the distal phalanges of hands and feet, onychodystrophy, paronychia, and occasionally osteolysis of the adjacent phalange.22 There have been reports of successful outcomes with etanercept; however, the disease relapse when the therapy was withdrawn or the dose tapered. Better outcomes have been reported with adalimumab, although these were limited to single case reports, and no studies comparing etanercept and adalimumab have yet been undertaken.
In the foregoing case reports, etanercept did not consistently control the disease, and more studies are needed before reliable conclusions can be drawn. In acrodermatitis continua of Hallopeau, etanercept 50–100mg is usually administered weekly, divided into 2 separate doses.19 The most recent report involved a 72-year-old patient who responded favourably after 4 weeks of etanercept 50mg twice weekly combined with methotrexate 10mg once weekly. However, when both drugs were tapered the patient relapsed, with even more severe paronychia and pain, incapacitating her for activities of daily living. When etanercept was replaced by adalimumab, the lesions resolved and methotrexate could be withdrawn with no short-term relapse.22
Hidradenitis suppurativaHidradenitis suppurativa (HS) is a chronic form of dermatosis with inflammation of the apocrine sweat glands. It is characterised by deep, painful nodules and abscesses that progress to form fistulas, fibrosis and retractile scars.1 It presents in the axillae, perianal, submammary and/or inguinal region. The disease is recurrent and recalcitrant, causing considerable morbidity and incapacitating patients for activities of daily living, thus undermining their quality of life.
It usually presents during the third decade of life and its aetiology is unclear. However, it is thought to have an immune origin due to its association with conditions such as Crohn's disease, spondyloarthropathy and SAPHO syndrome.1 It has also been associated with obesity, high androgen levels, bacterial infections and smoking.23
Cusack et al.,3 used etanercept in 6 patients with HS, observing improvement in 64% after 24 weeks of treatment. A review of cases of HS treated with TNFα inhibitors retrieved 34 studies with a total of 105 patients, some treated with infliximab (52/105) and others with etanercept (37/105). Etanercept was given at a dose of 25–50mg, once or twice weekly, with acceptable improvement in 35 of the 37 patients treated: 23 responded during treatment but later relapsed, 12 showed continued improvement at 4–12 weeks after stopping treatment, and 2 patients did not respond to treatment.23
Eosinophilic fasciitis/Morphea profundaEosinophilic fasciitis (EF) is a type of rapid-onset localised morphea or scleroderma characterised by a painful induration of the extremities accompanied by peripheral eosinophilia. It is triggered by strenuous exercise. In the only study published to date, Heidary et al.,24 reported the case of a 50-year-old patient with a 3-month history of EF successfully treated with a combination of systemic steroids, methotrexate and etanercept.
Lupus erythematosusLupus erythematosus is an autoimmune disease that affects the skin, the joints and various organs, and can present in an acute, subacute or chronic form. The benefit of etanercept in the management of lupus erythematosus is controversial. Some studies have reported that it can trigger an outbreak of lupus erythematosus or exacerbate an existing condition, although others report cases in which lupus erythematosus lesion have responded well to treatment with etanercept. Norman et al. describe the case of a 42-year-old patient with subacute lupus erythematosus unresponsive to antimalarials, methotrexate and prednisone. Therapeutic success was achieve by the addition of etanercept 25mg twice weekly to the regimen.3 Fautrel reported the case of the 65-year-old man with rheumatoid arthritis and lupus erythematosus in whom arthritis and skin lesions improved after administration of etanercept.3
DermatomyositisDermatomyositis is an autoimmune connective tissue disease that mainly affects the skin and striated muscle tissue. It is characterised by inflammation of the proximal striated muscles with characteristic cutaneous findings. As in the case of lupus erythematosus, the benefits of etanercept in the treatment of this condition are unclear. One case report concerned a 42-year-old patient with dermatomyositis refractory to methotrexate, hydroxychloroquine, mycophenolate mofetil and systemic steroids whose cutaneous and muscle symptoms improved 24 weeks after treatment was switched to etanercept 25mg twice weekly plus methotrexate.3 In contrast, Lannone et al. reported therapeutic failure in 5 dermatomyositis patients treated with etanercept alone.3
Atopic dermatitisAtopic dermatitis is a common inflammatory disease. It is associated with an epidermal barrier dysfunction derived from mutations in the gene coding for filaggrin, a protein that aggregates keratin filaments into keratin fibrils, and contributes to skin hydration. Atopic dermatitis is characterised by periods of skin inflammation associated with elevated IgE levels.25 Treatment is mainly focussed on application of emollients to restore the skin barrier and life-style changes. However, when these measures fail, various topical and/or systemic therapies are used. Systemic drugs are used as second-line therapy, and include cyclosporin, azathioprine, methotrexate, systemic steroids, mycophenolate mofetil, leflunomide, and more recently omalizumab (a biological anti-IgE).26 Rullan et al obtained good results with etanercept in two pediatric patients with severe, recalcitrant atopic dermatitis, with no significant adverse events.27
Behçet's diseaseBehçet's disease (BD) is a chronic inflammatory disease of unknown aetiology. It is characterised by recurrent oral and genital ulcers, polymorphous skin lesions and uveitis, with involvement of the gastrointestinal, respiratory, vascular and central nervous systems.1 It is more common in the third and fourth decades of life, and both sexes are equally affected, although more cases of BD refractory to conventional therapy have been reported in men.28
Some recent reports have described the use of etanercept as monotherapy to treat BD. In a randomised study of 40 BD patients, etanercept 25mg twice weekly was compared to placebo. Patients in the treatment arm presented significant improvement in the number of oral ulcers and skin lesions.3 Estrach et al., however, reported the case of a BD patient who showed no response to etanercept administered for 3 months at the foregoing dose.3
Palmoplantar pustulosisPalmoplantar pustulosis (PPP) is a recurrent, chronic disease characterised by pustules, erythema and keratoderma on the palms and soles of the feet. Treatment is rarely effective in controlling the disease, and relapse is inevitable in the long- or short-term. For this reason, new treatment options are needed. Bissonnette et al.,29 studied 15 cases of PPP. Patients were randomised to receive either etanercept 50mg twice weekly or placebo for 3 months, after which both groups received etanercept 50mg twice weekly for a further 3 months. The authors observed a significant improvement in skin lesions in the treatment group, and concluded that etanercept is a good treatment option for this disease.
Pyoderma gangrenosumPyoderma gangrenosum (PG) is a neutrophilic dermatosis characterised by deep, painful ulcers often localised to the lower extremities. It can be associated with other autoimmune diseases, particularly inflammatory bowel disease.1 First-line treatment for PG is systemic steroids, although infliximab has also been shown to be effective. A few studies have reported the use of etanercept in the management of PG. McGowan et al.30 reported a case study of a 30-year-old patient with PG refractory to systemic steroids in which a good response was achieved with the addition of etanercept 100mg weekly to the steroid regimen. Goldenberg et al. reported the case of a 30-year-old patient with PG and autoimmune hepatitis in whom lesions improved after a regimen of etanercept 25mg twice weekly combined with prednisone.3 Another report concerned a 49-year-old patient with vegetative PG associated with psoriasis and pyoderma vegetans who improved after 3 weeks of etanercept therapy.31
Sneddon-Wilkinson disease (subcorneal pustular dermatosis)Sneddon-Wilkinson disease (SWD) is a chronic form of dermatosis characterised by superficial pustules in an annular pattern on intertriginous areas of the skin, mainly on the trunk and upper extremities. It is more common in women in the fourth and fifth decade of life. Although its aetiology is unknown, it can be associated with other autoimmune diseases such as inflammatory bowel disease, pyoderma gangrenosum and rheumatoid arthritis in which TNFα is a contributing factor. For this reason, although dapsone is considered the treatment of choice in SWD, favourable results can also be achieved with TNF inhibitors.32
Four cases of SWD successfully treated with etanercept have so far been reported. The most recent study is a report of 2 cases, the first concerning a 51-year-old patient with SWD refractory to topical steroids and acitretin. The patient was started on etanercept 50mg twice weekly, and after 3 months of therapy the extent of the disease had been reduced from 20% to <5% of the body surface area. After the extent of the disease had increased to 7%, acitretin 25mg every third day was added to etanercept and the dermatosis was resolved.32 The second case involved a 61-year-old patient diagnosed with recalcitrant SWD affecting 10% of the body surface area. The patient was given etanercept 50mg twice weekly in combination with topical steroids, and after 9 months the condition had improved considerably.32
Erythema nodosumErythema nodosum (EN) is the most common panniculitis and represents the prototype of a septal panniculitis. EN can be caused by a variety of factors, the most important being streptococcal or mycobacterial infections, including M. tuberculosis, inflammatory bowel disease, sarcoidosis and Behcet's disease. It can also occur with sensitivity to certain medications, and up to 55% of cases can be idiopathic. EN is a delayed hypersensitivity reaction involving Th1-derived cytokines such as interleukin 2 (IL2) and interferon γ.33
TNFα stimulates expression of several cytokines, including IL-6, IL-1, granulocyte-macrophage colony-stimulating factor, and IL-8, which modulate the activity of the disease. For this reason, TNFα is an option for the management of EN.
Boyd reported the case of a 22-year-old patient with a 5-year history of EN with frequent relapses requiring repeated regimens of prednisone, potassium iodide, indomethacin, methotrexate and dapsone, with no benefit. One month after starting etanercept 25mg twice weekly, the symptoms had improved, and the dermatosis resolved after 6 weeks of therapy.33
Cutaneous granulomasPasternack et al.,3 published a report of 2 patients with adjuvant-induced granulomas treated with etanercept 25mg twice weekly. One patient presented total remission of the lesions, and in the other, improvement was limited to erythema and pain.
Another report describes a patient treated with etanercept for rheumatoid arthritis associated with the appearance of multiple facial granulomas at the site of an earlier silicon injection.3
Inflammatory linear verrucous epidermal nevus (ILVEN)ILVEN presents as verrucous papules distributed in a linear pattern following Blaschko's lines which, when inflammatory, can be erythematous and intensely pruritic. ILVEN can be congenital or present during the first months of life. In 75% of cases, onset is before 5 years of age.
There is no effective treatment for all cases of ILVEN. Whenever possible, treatment consists of partial or total surgical excision. In the case of extensive tumours, a range of treatments including topical or intralesional steroids, keratolytics, calcipotriol, dermoabrasion, cryosurgery and CO2 laser have been used, with mixed results.3 The use of etanercept has been reported in a few couple case studies, also with conflicting results.
ConclusionsThe aim of this review of off-label uses of etanercept in dermatological diseases has been to explore the different regimens available and the variability of response to treatment, even with high doses and long-term administration.
Conflict of interestThe authors have no conflict of interest.