The adult and pupa of a new species of Forcipomyia (Phytohelea) are described, illustrated and photographed. Pupae were collected from water held by Eryngium L. sp. (Apiaceae) in Corrientes province, Argentina and reared to adults in the laboratory. Adult and pupa are compared with the closely related congener F. (P.) jocosa Saunders, and the pupa is also compared with that of F. (P.) bromelicola (Lutz).
Se describen, ilustran y fotografían el adulto y la pupa de una nueva especie de Forcipomyia (Phytohelea). Las pupas fueron capturadas del agua acumulada por Eryngium L. sp. (Apiaceae) en la provincia de Corrientes, Argentina y criadas en el laboratorio hasta la emergencia del adulto. Se compara esta especie con su congénere más cercano, F. (P.) jocosa Saunders, y además, la pupa es comparada con la de F. (P.) bromelicola (Lutz).
The genus Forcipomyia Meigen, which includes 1 125 extant species placed in 36 subgenera (Borkent, 2013), is not only diverse in number of species but is also rich in morphological variation in each life stage. The species of the subgenus Phytohelea are one of the most common inhabitants of phytotelmata, such as those found in modified leaves, leaf axils, flowers, stem holes, open fruits and fallen leaves of terrestrial plants (Frank, 1983). Currently, there are 10 species in this subgenus in the Neotropical region (Borkent and Spinelli, 2007), and only F. jocosa Saunders was recently recorded from Argentina (Spinelli et al., 2010). Immature stages of species of F. (Phytohelea) have been found in the water held by such plants as Dracaena Vand. ex L., Musa L., Colocasia Schott, pineapple, and lily (de Meillon and Wirth, 1991). Spinelli et al. (2007) recorded and fully described the preimaginal stages of F. (P.) musae Clastrier and Delécolle from leaf axils of banana stems in Brazilian Amazonia. Recently, Marino et al. (2010) redescribed the immatures of F. (P.) bromelicola (Lutz) from bromeliads in Florida, USA. The purpose of this paper is to describe and illustrate the adult and pupa of a new species of F. (Phytohelea) found in Eryngium L, and to compare it with a closely related species.
Materials and methodsPupae of the new species were collected using a pipette to extract water from Eryngium L. sp., located in the province of Corrientes (Fig. 23). They were carried to the laboratory and placed individually in vials with a drop of water. Observations were made daily until adult emergence. Measurements and ratios of the pupa were taken using a binocular microscope (BCM). For observation with the BCM, specimens were slide-mounted in Canada balsam following the technique described by Borkent and Spinelli (2007). Ink illustrations were made with a camera lucida. Photographs were taken with a digital camera, Micrometrics SE Premium, using a Nikon Eclipse E200 microscope. Terms for the pupae follow Borkent (2012) with addition of the following abbreviations of measurements: DAL, dorsal apotome length and DAW, dorsal apotome width, and those for adults follow Spinelli et al. (2012). The map was traced from Google Earth and the track was kept in KLM format. Afterwards, the format was turned into figures through the GPS Visualizer site (http://www.gpsvisualizer.com/gpsbabel/gpsbabel_convert) and drawn with OziExplorer version 3.95.4. Types are deposited in the collection of the División Entomología, Museo de La Plata, Argentina (MLP).
DescriptionForcipomyia (Phytohelea) bilobata Marino, n. sp. (Figs. 1–23)
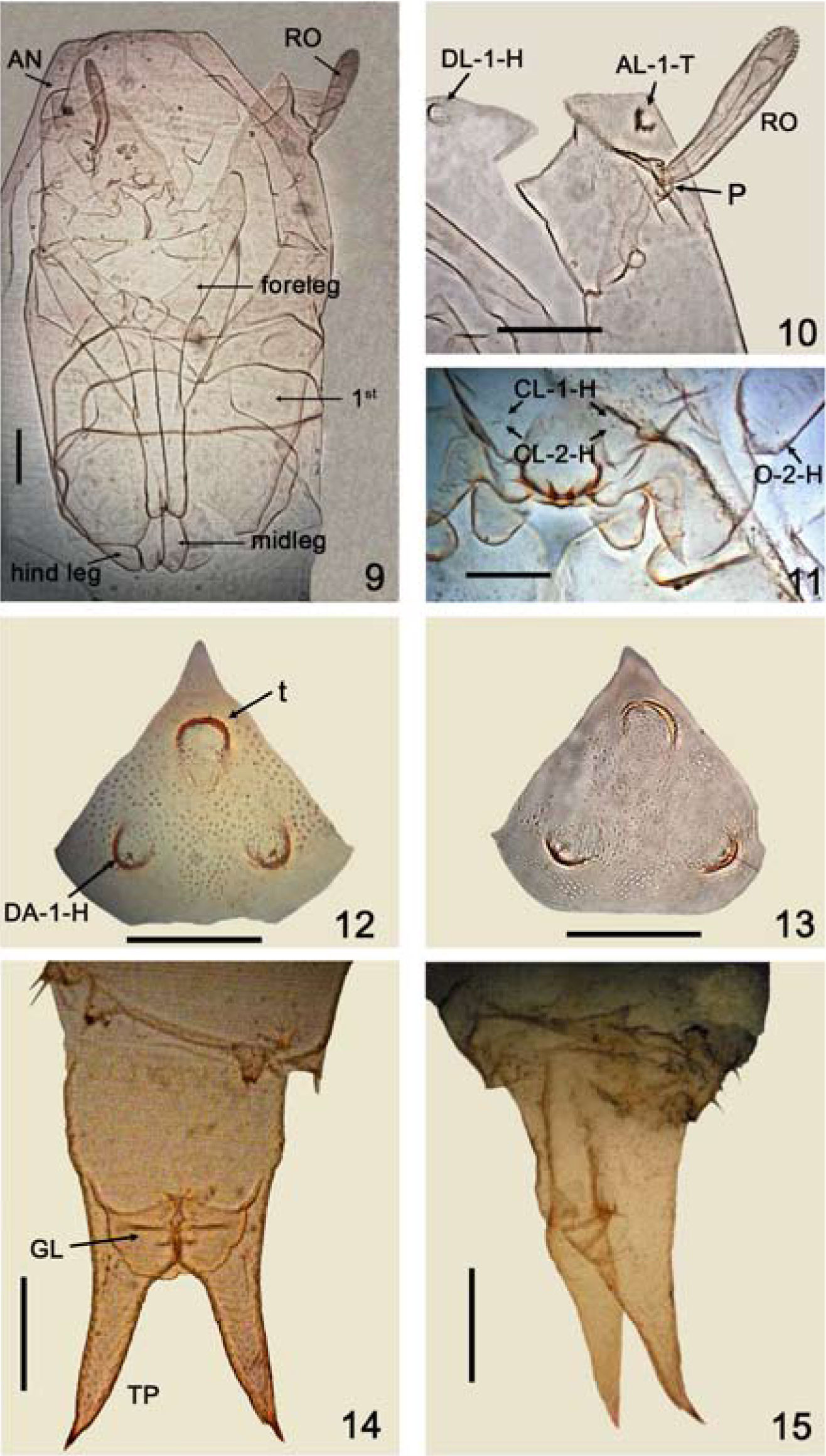
Forcipomyia (Phytohelea) bilobata, pupal structures. 9-12, 14, male; 13, 15, female. 9, cephalothorax (dorsal view); 10, detail of cephalothorax (anterodorsal view); 11, clypeal/labral sensilla (CL) and ocular sensillum (O) (ventral view); 12-13, dorsal apotome; 14-15, segment 9 (male, ventral view and female, lateral view). Antenna (AN); anterolateral sensillum (AL); clypeal/labrals (CL), dorsal apotome sensilla (DA); dorsolateral cephalic sclerite sensillum (DL); genital lobe (GL); ocular (O); pedicel (P); respiratory organ (RO); terminal processes (TP). Scale bars 0.05mm.
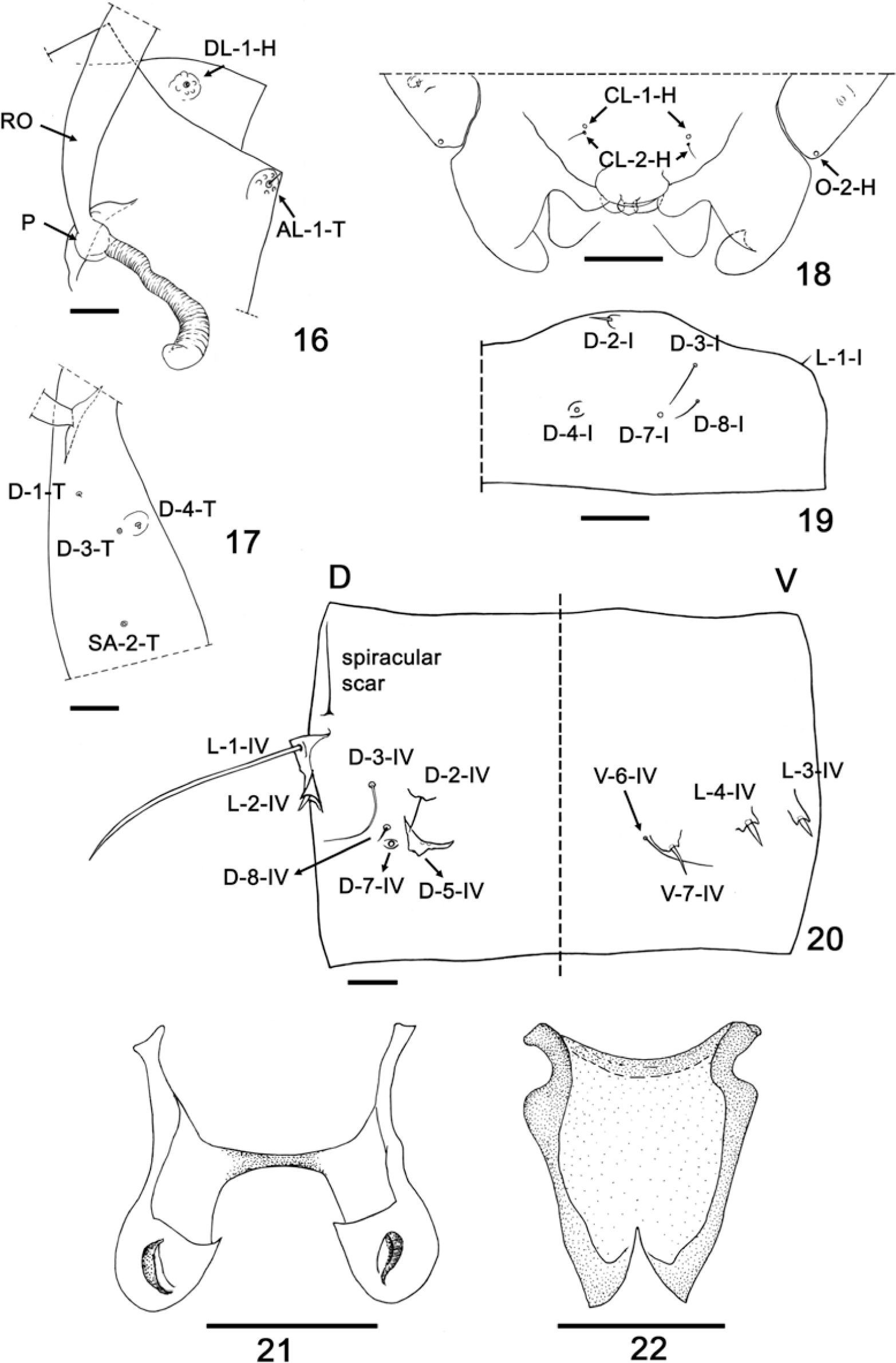
Forcipomyia (Phytohelea) bilobata, pupa and male adult. 16–20, pupa; 21–22, male adult. 16, dorsolateral cephalic sclerite sensillum (DL); anterolateral sensillum (AL); pedicel (P), respiratory organ (RO); 17, dorsal setae and supralar sensillum (SA); 18, clypeal/labral sensilla (CL) and ocular sensillum (O) (ventral view); 19, first abdominal segment (dorsal view); 20, fourth abdominal segment (dorsal and ventral view); 21, parameres; 22, aedeagus. Scale bars 0.05mm.
Diagnosis. Male adult: the only Neotropical species of the F. (P.) bromelicola species group with the apex of the aedeagus bilobed. Female adult: not diagnosable at this time. Pupa: only species of Neotropical Forcipomyia (Phytohelea) with the dorsal apotome triangular, dorsal apotome sensilla long and a terminal process of segment 9 with a medium-sized, outer seta that extends to 1/3 of its total length.
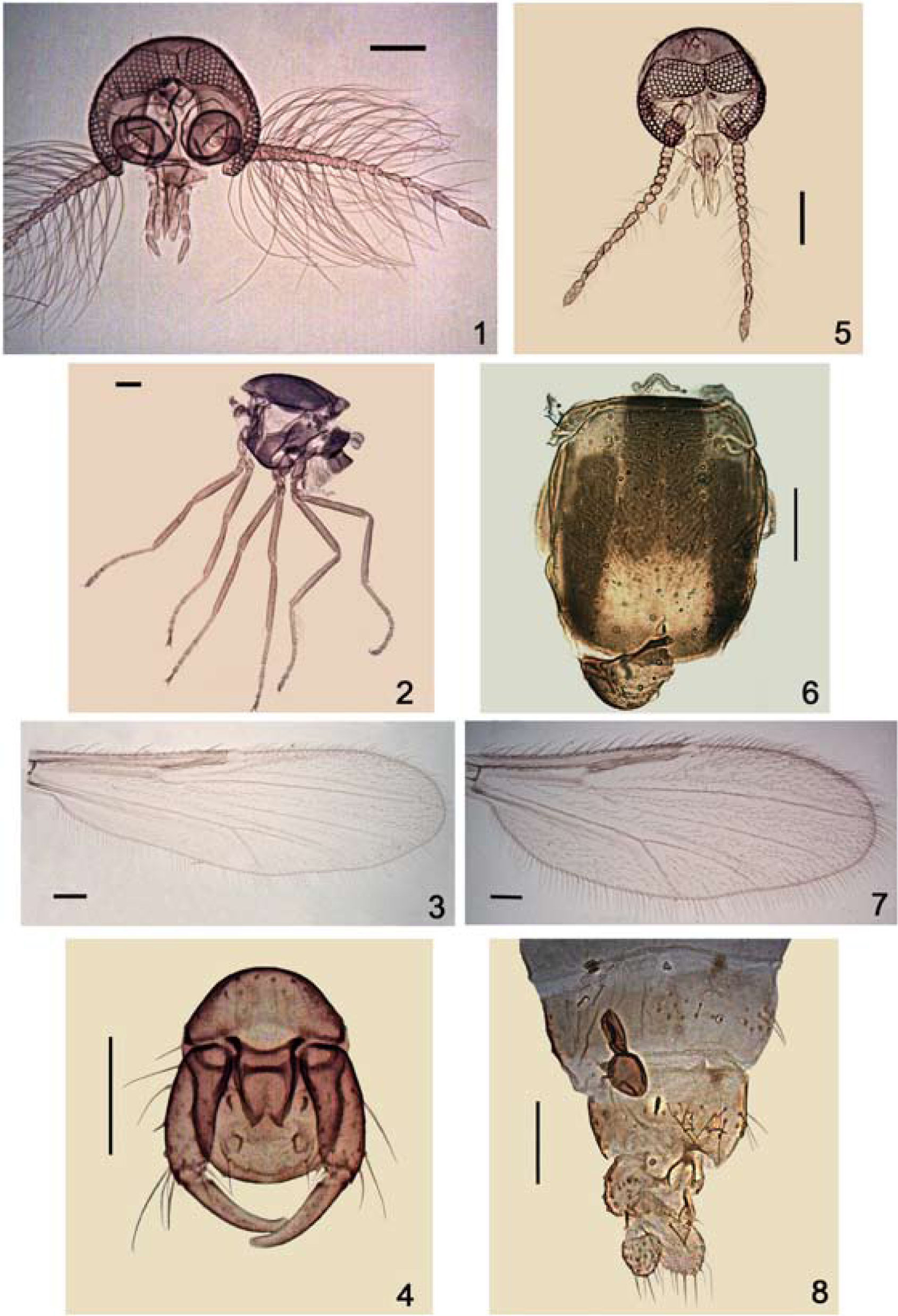
Male. Head (Fig. 1). Brown. Eyes abutting medially for length of 3 ommatidia. Antenna (Fig. 1) pale brown with plume setae poorly developed, flagellomeres 2-4 partially fused, flagellomeres 5-8 separate, flagellomere 10 0.5 X shorter than flagellomere 11, flagellomeres 1013 elongate; flagellomere 13 with apical nipple slightly constricted basally; AR 0.50. Palpus (Fig. 1) pale brown; third segment slightly swollen at midlength, with shallow, small sensory pit opening on swollen portion; segments 4, 5 completely fused; PR 1.83. Thorax (Fig. 2). Dark brown except humeral areas, scutellum pale brown; scutellum with 6 strong, 4 more slender setae. Legs (Fig. 2) pale brown; apex of hind tibia with 4 spines, tarsi paler except fifth tarsomere slightly darker; tarsomere 1 of foreleg with row of thick setae; foreleg TR 2.28, midleg TR 2.42, hind leg TR 2.37; claws curved, moderately stout, slightly bifid at tip. Wing (Fig. 3) plain; both radial cells obliterated; fork of cubitus situated at same level as apex of costa; wing length 0.96mm; breadth 0.30mm; CR 0.47. Halter brownish. Abdomen. Uniformly brown. Genitalia (Fig. 4): Brown. Tergite 9 moderately elongate, extending to level of apex of gonocoxite; posterior margin rounded; cercus lobe-like, slightly produced beyond 1/2 length of gonocoxite; sternite 9 broad, posterior margin slightly concave. Gonocoxite slender, 3 X longer than greatest breadth; gonostylus 0.80 as long as gonocoxite, nearly straight, tip blunt. Gonocoxal apodemes reduced. Parameres (Fig. 21) H- shaped, basal arms slender, nearly straight, posterior process swollen distally, tip rounded. Aedeagus (Figs. 4, 22) stout, triangular, lateral margins sclerotized, gradually narrowed to bilobed, pointed tip; basal arch extending to 1/10 of total length; lateral arms extending laterally.
Female. As for male, with following differences: head (Fig. 5) with antenna brown, flagellomeres 1-2 partially fused, 3-5 subspherical, 6-8 vasiform; flagellomeres 9-13 elongate, flagellomere 13 with apical nipple not constricted basally, proportions as shown in Fig. 5; AR 0.95. Palpus (Fig. 5) pale brown; third segment slightly swollen at midlength with small, shallow sensory pit; segments 4, 5 completely fused, its combined length shorter than third segment; PR 2.50. Thorax. Scutum with pattern as in Fig. 6, with distinct submedial, lateral vittae. Legs pale brown except tibiae darker, apex of hind tibia with 4-5 spines; tarsomeres progressively darker, foreleg TR 2.40, midleg TR 2.29, hind leg TR 2.46, claws curved. Wing (Fig. 7) with abundant macrotrichiae covered membrane; first radial cell reduced; second radial cell narrow; fork of cubitus situated proximad to level of apex of costa; wing length 0.87mm; breadth 0.33mm; CR 0.48. Abdomen. Tergites uniformly brown. Genital sclerotization as in Fig. 8. Two pyriform, sclerotized spermathecae (Fig. 8) with short necks, measuring 0.036 by 0.034mm, and 0.032 by 0.030mm. Cercus brown.
Male pupa. (Figs. 9-12, 14, 16, 18-20). Total length 3.09mm. General coloration of exuviae pale brown. Dorsal apotome (Fig. 12) triangular, with disc 0.70 X longer than greatest width; posterior margin with pointed and smooth apex; surface covered with small, rounded spicules, anterior margin nearly straight, dorsal apotome sensilla (Fig. 12) long, thin, located on well developed tubercle, without pore at base; disc with subapical, strong, rounded tubercle (t); DAL 0.16mm; DAW 0.23mm; DAW/DAL 1.60. Cephalothorax (Fig. 9) rectangular, surface smooth, length 1.15mm, width 0.64mm with short medial crest. Cephalothoracic sensilla (Figs. 10, 16) as follows: one peg dorsolateral cephalic sclerite sensillum on well developed rounded tubercle; one medium-sized, strong anterolateral seta; 3 dorsals present: D-1-T, D- 4-T short setae, D-3-T near D-4-T, D-4-T on rounded tubercle, supraalar 2 present. Respiratory organ (Figs. 9–10, 16) nearly straight, pale brown, 4.25 X longer than broad, with 12-14 apical pores; RO length 0.20mm, RO width 0.05mm; pedicel (Figs. 10, 16) smooth, short, pedicel length 0.024mm, P/RO 0.12. Two clypeal/labrals (Figs. 11, 18), CL-1-H coeloconica sensillum, CL-2-H medium-sized, thin sensillum; one ocular campaniform sensillum (Figs. 11, 18). Abdominal segments covered with small pointed spicules. First abdominal segment (Fig. 19) with sensilla as follows: D-2-I short, stout seta on rounded small tubercle, D-3-I medium-sized seta, D-4-I, D-7-I pores on posteromesal portion, D-8-I short seta; one lateral sensillum: L-1-I short, stout seta. Second abdominal segment similar to first, except sensilla longer, and by the presence of 2 additional sensilla: one long, thin seta, one short, stout seta. Fourth abdominal segment with sensillar pattern (Fig. 20) as follows: D-2-IV, D-3-IV thin setae, D-2-IV medium-sized on flattened small tubercle, D-3-IV longer than D-2-IV; D-4-IV absent, D-5-IV stout tubercle without sensillum, D-7-IV pore, D-8-IV short, thin seta; L-1-IV very long, stout seta, L-2-IV short seta, both located on stout triangular tubercles, L-3-IV, L-4-IV short, stout setae, both located on apically blunt tubercles; 2 ventral sensilla, V-6-IV long, thin seta, V-7-IV medium-sized, stout seta located on rounded tubercle. Segment 9 (Fig. 14) length 0.42mm, width 0.20mm, approximately 2 X longer than width; ventral surface with posteriorly directed stout spicules located on anterolaterally portion; genital lobe ventral; terminal process long, slightly divergent, with dark pointed tip, length 0.26mm; base with medium-sized, thin outer seta on small tubercle.
Female pupa (Figs. 13, 15, 17). Similar to male with usual sexual differences. Total length 3.03mm. Exuviae pale brown. Dorsal apotome (Fig. 13) with DAL 0.14mm; DAW 0.19mm; DAW/DAL 1.33. Dorsals as in Fig. 17. Respiratory organ length 0.204mm; width 0.036mm; pedicel length 0.02mm; P/RO 0.11. Cephalothorax length 1.03mm, width 0.684mm. Segment 9 (Fig. 15) length 0.44mm, terminal process length 0.33mm.
Taxonomic summaryDistribution. Argentina, known only from the type locality (Fig. 23).
Types. Holotype male with pupal exuviae on microscope slide, labeled “HOLOTYPE Forcipomyia (Phytohelea) bilobata Marino. Argentina, Corrientes prov., arroyo Toropí, 28°35'40.4” S, 59°02'00.9” W, 55m, 16-IX-2010, R. Campos, ex Eryngium L., (MLP). Paratype female with pupal exuviae: same data as holotype (MLP). The pupae were collected at the same locality and date from Eryngium L. sp., and laboratory reared pupae emerged in 2–3 days at 12°C.
Etymology. The name bilobata (divided in 2 lobes) refers to the bilobed apex of the aedeagus of this species.
Remarks. This new species belongs to the F. bromelicola species group defined by de Meillon and Wirth (1979) by the presence of 2 spermathecae and 13 antennal flagellomeres. The adult of F. (P.) bilobata is very similar to F. (P.) jocosa Saunders by the presence of well developed empodia; however, the latter species exhibits an elongate third palpal segment, the 8 basal flagellomeres are strongly compressed, and the thorax is entirely dark brown. The male has the apex of aedeagus with 2 lobes separate by a shallow gap. The pupa of F. jocosa was incompletely described by Saunders (1956) based on 2 pupal exuviae; however, the outer seta of the terminal process of F. jocosa is very reduced, and the D-5-IV of the fourth abdominal segment is clearly stouter. The sensillar pattern of the fourth abdominal segment of this new species is similar to that of F. (P.) bromelicola. The pupa of F. bromelicola differs from F. bilobata by the presence of a rounded dorsal apotome, with a minute sensilla and by the terminal processes of the segment 9 as long as their bases, each of them with a longer outer seta.