In landscapes where tropical dry forest was once the dominant vegetation type, traditional silvopastoral systems generate a range of natural and semi-natural habitats, namely fragments of secondary forest and fallow land in various stages of succession; essentially Acacia woodlots. This level of heterogeneity seems to favor the arrival and persistence of a large number of Scarabaeinae species. Changes in dung and carrion beetle assemblages were assessed in a silvopastoral landscape in Chiapas, Mexico by intensive sampling using baited pitfall traps. Total species richness decreased from late successional habitats to early successional habitats and average abundance per site was higher in the intermediate successional stages. The silvopastoral system studied is very dynamic; its components may remain constant or change over time, depending on management. These changing conditions lead to a variable local species richness and allow the permeability of certain species within the landscape. There was high species turnover among successional habitats, generating a gamma diversity similar to that of tropical dry forest landscapes and 2-fold higher than the highest recorded alpha diversity value. Silvopastoral systems can buffer the adverse effects of rapid expansion of open areas and the consequent reduction of tropical dry forest area generated by technified conventional systems.
En paisajes donde el bosque tropical seco fue la vegetación dominante, los sistemas silvopastoriles tradicionales generan una gama de hábitats naturales y seminaturales (fragmentos de bosques secundarios y acahuales en diversas etapas de sucesión), principalmente matorrales con Acacia. Este nivel de heterogeneidad parece favorecer la llegada y la persistencia de un gran número de especies de Scarabaeinae. Estudiamos los cambios en los ensambles de escarabajos copro-necrófagos en un paisaje silvopastoril en Chiapas, México. El muestreo fue intensivo y se llevó a cabo con trampas de caída cebadas. De acuerdo con nuestros resultados, la riqueza total de especies fue decreciendo de los hábitats sucesionales más complejos a los más simples y la abundancia promedio por sitio fue mayor en las etapas sucesionales intermedias. El sistema silvopastoril estudiado es muy dinámico y sus componentes pueden permanecer o cambiar con el tiempo, dependiendo de las decisiones de los ganaderos. Estas condiciones cambiantes provocaron que la riqueza local de escarabajos fuera muy variable y permitieron que el paisaje fuera permeable a la entrada y salida de algunas especies. Hubo un alto recambio de especies entre estados sucesionales, lo que dio como resultado una diversidad gamma similar a la de paisajes con bosque tropical seco y con valores 2 veces mayores a la mayor diversidad alfa encontrada. Los sistemas silvopastoriles pueden regular los efectos adversos de la rápida expansión de los espacios abiertos y la consiguiente reducción de la superficie ocupada por el bosque tropical seco, resultante de los sistemas ganaderos convencionales tecnificados.
To meet their needs, humans have used and transformed tropical landscapes through different forms of management (agricultural, livestock and forestry). To clearly understand the current status of conservation in anthropic landscapes, and to predict future trends, a deeper understanding of the biodiversity in actively managed rural landscapes is required (Ballam-Ballote and León-Cortés, 2010). In tropical dry landscapes, the conservation of species diversity depends not only on protecting forested areas, but also on managing the matrix in which they are embedded. Balanced management of the agrosilvopastoral matrix should influence the effects of productive activities on biodiversity and the ecological functions in the landscape (Harvey et al., 2006) and should sustain productivity while maintaining as much original ecosystem integrity as possible (Harvey et al., 2006; Harvey et al., 2008).
Tropical cattle production areas, where farmers use different proportions of fodder trees, shrubs and grasses, produce heterogeneous landscapes that may contribute to the conservation and maintenance of species diversity and ecological processes (Harvey et al., 2006; Giraldo et al., 2011; Rös et al., 2012). In contrast, conventional technified systems are those that feature a monoculture of grass with low or minimal tropical plant diversity: they are treeless and include irrigation systems to maintain the grazing. This type of management has resulted in a rapid expansion of open areas and the consequent reduction of original forest areas. Simplification in the structure and composition of vegetation, along with deforestation and species loss, causes changes in the dynamics of several ecological processes that are crucial to ecosystem functioning (Giraldo et al., 2011). In the anthropic landscapes of southern Mexico, where tropical dry forest was once the dominant vegetation, almost all cattle production activities involve extensive grazing (Gómez et al., 2002) conducted under a traditional agrosilvopastoral management scheme (Jiménez-Ferrer et al., 2007; Ramírez-Marcial et al., 2012). As a result, farms in tropical dry landscapes may include different proportions of grasses and trees: pastures with living fences and/or isolated trees or even forest fallows, where the land is put to a different use during the annual cycle (Nahed et al., 2009). However, some farms remain treeless as a result of management decisions taken by the landowners. The tree cover that is typically retained in silvopastoral landscapes may provide resources and habitats for a variety of organisms (Neita and Escobar, 2012) and may also increase the heterogeneity and functional connectivity of the landscape for certain animal species (Arellano et al. 2008a; Pineda-Diez de Bonilla et al., 2012). Vegetation dynamics and land use modifications can generate both predictable and unpredictable changes in the availability of certain habitats in a successional gradient (pastures, pastures with trees, living fences, secondary forest, managed Acacia habitats, tree and shrubby fallows). The species associated with these habitats could potentially undergo high rates of local extinction as a result of successional processes or disturbances, or may even disappear locally over the course of a few generations (Ballam-Ballote and León-Cortés, 2010); however, these changes could also enable the creation of new successional habitats for other species that depend on livestock activities (León Cortés et al., 2004), such as dung beetles (Scarabaeinae).
Dung and carrion beetles (Coleoptera, Scarabaeinae) are useful indicators of biodiversity in the tropics because their community structure responds rapidly to environmental changes, their biology is relatively well known and they are easy to sample (Favila and Halffter, 1997; McGeoch et al., 2002Nichols et al., 2007). Our aim was to assess changes in richness, abundance, composition and community structure of dung beetles across a representative range of successional habitats within a silvopastoral landscape in southern Mexico. Our working hypothesis was that species richness and abundance in this landscape would be higher in sites of intermediate successional stage, because these types of heterogeneous habitats would offer conditions suitable for a variety of species with different habitat preferences. We would also expect that this successional stage would support higher proportions of habitat generalists than would be case in early and late successional stages.
Materials and methodsStudy area. The landscape is located in the municipality of San Fernando, in Chiapas, Mexico (16°53'01”−16°47'57” N, 93°09'23−”93°13'58” W) (see description in Arellano et al., 2008b). The transformation and fragmentation of this tropical dry forest landscape occurred at the turn of the nineteenth century, so the area has been fragmented for at least 150 years.
The silvopastoral landscape. The intensification of livestock production in San Fernando has led to the promotion of silvopastoral landscapes. Depending on the management decisions made by the landowner, farms can include secondary forest fragments in various successional stages, embedded in a silvopastoral matrix integrated by different proportions of arboreal vegetation and pastures. The arboreal vegetation is mainly tree fallows and managed Acacia forests. Tree fallow areas correspond to different successional stages of tropical dry forest, characterized by low tree diversity with an open canopy, compared to that of secondary forest fragments, and also by small tree diameters. Managed Acacia woodlots are a typical successional habitat in tropical dry forest, normally used by livestock producers as part of the overall intensive management of pastures (Chazaro, 1977). Farmers move cattle into managed Acacia bush and forests during the period of fruit fall and use the pods as a dry-season feed. Mature Acacia plants deposit large numbers of seed pods below the canopy; these are eaten directly by livestock or harvested by the owners, and the seeds eventually dispersed by the livestock (Gutiérrez and Armesto, 1981). Seed pod dispersal largely depends on livestock movement and new Acacia forests can thus be readily established throughout the countryside. Pastures regenerate into a mixed scrub with a high proportion of spiny Acacia trees (León-Cortés et al., 2004). Cattle avoid eating the young Acacia plants with their conspicuous red spines, and concentrate on other, less noxious plants, thus encouraging the dominance of spiny Acacia trees in many parts of southern tropical Mexico (Greenberg et al., 1997). After approximately 5 years, high-density communities of Acacia plants are established (Cházaro, 1977). Competition for light and space results in the gradual disappearance of the weakest and sick trees, and after approximately 15–20 years, the Acacia woodlot becomes colonized by shrubs and secondary tree species. A community of Acacia forest would become a more diverse community over 15 to 20 years. On the other hand, when landowners remove Acacia plants, or when they burn the vegetation to favor grasses, managed Acacia woodlot becomes pasture (Cházaro, 1977). Landscapes with Acacia woodlots have a moderate level of transformation and are considered a management alternative for land use because they can mitigate the impact of humans on natural elements and processes (Ferguson et al., 2007).
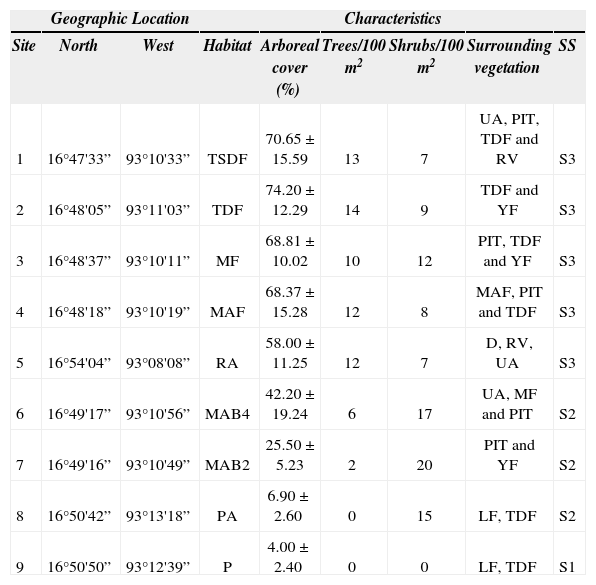
Site selection. The landscape elements were plotted on a 1:75 000 spatial orthophoto (Inegi, 2001), and digitized into a Geographical Information System (Arc View version 3.2 ©). To characterize the number of trees and shrubs, we randomly established 3 plots of 10×10m at each site. Shrubby tree cover or high (> 2m) was measured with a spherical densitometer. At each site, a fixed transect of 120m was established in which we measured tree cover in cardinal point directions (i.e. N, S, E, W). We then selected 9 sites, an average distance of 4km apart (minimum distance 610m), within a silvopastoral landscape. The sites were selected to represent different successional stages and management and were classified to 3 successional stages with a cluster analysis, using average linkage as the joining method and Euclidian distance as the distance measure (Fig. 1): i) early (S1), which includes a pasture with no trees or shrubs. ii) Intermediate (S2), including 2 areas of young managed Acacia shrubs (2 and 4 years old, respectively) and a pasture with Acacia pennatula, and iii) late (S3), with a tropical subdeciduous forest, a tropical deciduous forest, a mature tropical deciduous forest fallow, a ravine with tropical dry forest and a mature Acacia forest (Table 1).
Features of the study sites in a silvopastoral system in Chiapas, Mexico. TSDF= tropical subdeciduous forest, TDF= tropical deciduous forest, RA= Ravine in a tropical deciduous forest, MF= mature fallow, MAF= mature Acacia forest, MAB4= managed 4-year-old Acacia shrub, MAB2= managed 2-year-old Acacia shrub, PA= pasture with Acacia shrubs, P= pasture with no trees or shrubs. Successional stages (SS) are: early= S1, intermediate= S2, and late= S3. UA= urban area, PIT= pasture with isolated trees, RV= riparian vegetation, YF= young fallow, D= dam, LF= living fences
| Geographic Location | Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Site | North | West | Habitat | Arboreal cover (%) | Trees/100m2 | Shrubs/100m2 | Surrounding vegetation | SS |
| 1 | 16°47'33” | 93°10'33” | TSDF | 70.65±15.59 | 13 | 7 | UA, PIT, TDF and RV | S3 |
| 2 | 16°48'05” | 93°11'03” | TDF | 74.20±12.29 | 14 | 9 | TDF and YF | S3 |
| 3 | 16°48'37” | 93°10'11” | MF | 68.81±10.02 | 10 | 12 | PIT, TDF and YF | S3 |
| 4 | 16°48'18” | 93°10'19” | MAF | 68.37±15.28 | 12 | 8 | MAF, PIT and TDF | S3 |
| 5 | 16°54'04” | 93°08'08” | RA | 58.00±11.25 | 12 | 7 | D, RV, UA | S3 |
| 6 | 16°49'17” | 93°10'56” | MAB4 | 42.20±19.24 | 6 | 17 | UA, MF and PIT | S2 |
| 7 | 16°49'16” | 93°10'49” | MAB2 | 25.50±5.23 | 2 | 20 | PIT and YF | S2 |
| 8 | 16°50'42” | 93°13'18” | PA | 6.90±2.60 | 0 | 15 | LF, TDF | S2 |
| 9 | 16°50'50” | 93°12'39” | P | 4.00±2.40 | 0 | 0 | LF, TDF | S1 |
Dung beetle sampling. During the period July-August of 2003 and 2004, beetles were sampled along 18 transects (150m in length), with 6 baited pitfall traps/site (9 transects/year/site). Bait type (150g of human excrement, cow dung and squid) was alternated so the distance between traps with the same type of bait in each transect was 75m. Traps remained functional for 48h and total sampling effort was 2 268 hours.
Data analysis. The final slope of the species accumulation curves was used to determine survey completeness (Hortal and Lobo, 2005). This choice had the advantage of identifying a cut-off value to determine the rate of finding new species in additional samples, which suggests a comparative measure of the survey completeness. As the inventory is being completed, new records of species become rare, so that the slope of the curve decreases. In addition to a decrease in this slope, the sampling effort required to add a significant number of species to the inventory increases, and therefore the balance between extra effort and number of new species becomes less favorable. Therefore, species that are missing from the inventory may be locally rare species that can nonetheless still be found (see Moreno and Halffter, 2000).
The Michaelis-Menten (MM) equation was used as a reliable estimator for dung beetles, since MM commonly used in diversity inventory assessment and is an adequate function for a relatively small-intermediate number of samples (Colwell and Coddington, 1994; Colwell, 2009). For this equation, and using number of individuals as a unit of sampling effort, the survey can be considered sufficiently reliable despite being incomplete, even when the slope becomes approximately <0.1. Using individuals, one could establish the slope cut-off at 0.05 or 0.01, which would imply a new species every 20 or 100 individuals, respectively. However, if the final slope is much higher than the 0.1 cut-off point, it may be that we are using such a low number of (i) sampling places, (ii) sampling methodologies, or (iii) sampling dates, that we are unable to capture reliable inventories.
Kruskal Wallis tests were used to examine changes in the median and range of richness and abundance per site, while Mann Whitney tests were used to examine changes in the median and range of richness and abundance between early-intermediate successional stage and late successional stage. Early and intermediate stages were grouped together because the early stage included only 1 site. The influence of vegetation variables on species richness and abundance in every site (alpha diversity) was explored using Spearman correlations.
Rank-abundance curves, based on the number of individuals per species, were used to compare the changes in community structure for each habitat on the successional gradient. The equitability values (J') per site were obtained. Ancova analysis was used to compare abundances among sites from rank abundance curves.
To identify indicator species for every successional stage in the silvopastoral landscape, we used INDVAL (Dufrene and Legendre, 1997). Changes in the composition of the dung and carrion beetle communities in different habitats were analyzed according to: a) food relocation method: the proportion of tunnelers, endocoprids and rollers, b) food preference: the proportion of generalists (species for which less than 80% of total individuals were caught in copro- or necro-baited traps) to specialists (species for which more than 80% of the individuals were collected in either copro- or necro-baited traps), c) daily activity: the proportion of nocturnal to diurnal species, d) beetle size: the proportions of large (18–28mm), medium-sized (9–17.99mm) and small (< 9mm) species (Halffter and Arellano, 2002), and e) habitat preference: the proportion of habitat generalists (species for which the individuals were collected in more than one specific habitat) to habitat specialists (species for which more than 80% of total individuals were caught in a specific habitat). The R × C test of independence, using the G-test, was performed to compare the proportion of individuals in each functional trait per habitat (Sokal and Rohlf, 1995).
A Venn diagram was used to show the total number of species, shared species and exclusive species per successional stage. The spatial arrangement of sites based on species composition was analyzed with a non-metric multidimensional scaling ordination procedure (NMDS). Bray-Curtis dissimilarity indices were used for NMDS ordination, since they robustly identify relationships between site dissimilarity and ecological distance among species (Faith et al., 1987). P values were based on 1 000 permutations.
To analyze differences in species composition (using abundances) for successional stages, we applied for Adonis function using R, a multivariate ANOVA based on dissimilarities, and the betadisper function for the homogeneity of groups and beta diversity (Oksanen, 2012) based on a dissimilarity matrix (Bray Curtis index), for successional stages (grouping early and intermediate stages). A test was used to measure differences in the multivariate homoscedasticity among the sites of every successional stage; this test is analogous to a Levene test, for homoscedasticity and is used to measure the dispersal in species composition at every successional stage.
ResultsWe recorded 1 230 individuals, representing 33 species across the entire landscape (Table 2). In general, the total number of species decreased from the late successional to early successional habitats: there were 28 species in the late successional stages, 18 in the intermediate successional stages and 8 in the early successional stages. Similarly, the average number of species/transects was higher in S3 (with the exception of the ravine) than in the other successional stages (Fig. 2a). The highest abundances were obtained in TDF and in the pasture with Acacia (Fig. 2b). The average number of individuals per site was higher in S2 than in the other successional stages.
Dung beetle species and numbers, recorded in a silvopastoral landscape in Chiapas, Mexico. DA= daily activity, N= nocturnal, D=diumal. FP= food preference. C= coprophagous, G= generalist, NE= necrophagous. FR= Food relocation, T= tunneler, R= roller, E= endocoprid. BS= beetle size, S= small, M= medium, L= large. HP= habitat preference, Pasture with Acacia shrubs= PA. Pasture with no trees or shrubs= P. Ravine in a tropical dry forest= RA. Managed 4-year-old Acacia shrub= MAB4. Managed 2-year-old Acacia shrub= MAB2. Tropical subdeciduous forest= TSDF. Mature fallow=MF. Mature Acacia forest= MAF. Numbers in the first column are those used to identify species in Figure 2
| Late stage S3 | Intermediate stage S2 | Early stage S1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | DA | FP | FR | BS | HP | TDSF | TDF | MAF | MF | RA | MAB4 | MAB2 | PA | P | Total | |
| 10 | Agamopus lampros | N | C | T | S | P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 21 | Ateuchus rodriguezi | N | G | T | S | G | 1 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 5 |
| 22 | Canthidium laetum | D | C | T | S | G | 4 | 1 | 6 | 1 | 0 | 3 | 1 | 0 | 0 | 16 |
| 2 | Canthon (Glaphyrocanthon) delgadoi | D | G | R | S | G | 2 | 0 | 1 | 10 | 8 | 23 | 11 | 60 | 10 | 125 |
| 26 | Canthon lucreciae | D | C | R | S | G | 8 | 8 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 23 |
| 18 | Canthon femoralis | D | C | R | M | S3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 6 | Canthon cyanellus cyanellus | D | NE | R | M | S3 | 6 | 31 | 1 | 14 | 15 | 23 | 22 | 20 | 16 | 148 |
| 4 | Canthon humectus sayi | D | C | R | M | P | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 22 | 0 | 24 |
| 1 | Canthon indigaceus chiapas | D | C | R | M | P | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 152 | 28 | 182 |
| 9 | Copris lugubris | N | C | T | M | G | 0 | 0 | 0 | 1 | 0 | 2 | 3 | 2 | 0 | 8 |
| 31 | Copris laeviceps | N | C | T | M | S3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 16 | Copris incertus | N | C | T | M | G | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 3 |
| 7 | Coprophanaeus corythus | N | NE | T | L | P | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 8 | 2 | 12 |
| 30 | Deltochilum lobipes | N | NE | R | L | S3 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| 27 | Deltochilum gibbosum | N | NE | R | L | S3 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| 28 | Deltochilum scabriusculum | N | C | R | L | S3 | 5 | 31 | 9 | 27 | 0 | 0 | 0 | 0 | 0 | 72 |
| 15 | Dichotomius amplicollis | N | C | T | L | S3 | 10 | 29 | 1 | 20 | 8 | 15 | 2 | 0 | 0 | 85 |
| 11 | Dichotomius colonicus | N | C | T | L | P | 0 | 3 | 7 | 1 | 0 | 0 | 1 | 1 | 0 | 13 |
| 13 | Euoniticellus intermedius | D | C | T | M | P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 20 | Eurysternus mexicanus | D | C | E | M | G | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 7 |
| 23 | Onthophagus acuminatus | N | C | T | S | G | 0 | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 7 |
| 24 | Onthophagus batesi | D | C | T | S | G | 0 | 0 | 1 | 1 | 0 | 3 | 0 | 0 | 0 | 5 |
| 8 | Onthophagus crinitus | D | C | T | S | G | 0 | 6 | 10 | 12 | 0 | 2 | 1 | 7 | 1 | 39 |
| 5 | Onthophagus igualensis | D | C | T | S | G | 3 | 4 | 17 | 71 | 6 | 6 | 1 | 22 | 0 | 123 |
| 25 | Onthophagus landolti | D | C | T | S | S3 | 0 | 1 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 7 |
| 19 | Onthophagus violetae | N | C | T | S | S3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 32 | Pedaridium maya | N | C | T | S | G | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 12 | Phanaeus endymion | D | G | T | M | S3 | 5 | 43 | 5 | 28 | 0 | 5 | 3 | 1 | 1 | 104 |
| 3 | Phanaeus tridens | D | C | T | M | G | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 31 | 0 | 40 |
| 33 | Phanaeus wagneri | D | C | T | L | G | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 29 | Phanaeus demon | D | C | T | L | S3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 14 | Scatimus ovatus | N | C | T | S | P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 17 | Uroxys deavilai | N | C | T | S | S3 | 2 | 152 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 158 |
| Total number of individuals | 55 | 320 | 86 | 198 | 49 | 98 | 50 | 320 | 60 | 1230 | ||||||
| Number of species | 12 | 15 | 15 | 17 | 9 | 12 | 11 | 12 | 8 | |||||||
The final slope of the species accumulation curve was 0.0058 for the entire landscape; i.e. a new species was added for every 171 individuals collected (Fig. 3). For early (S1), intermediate (S2) and late stage (S3) successional habitats, a new species was collected every 182, 85 and 88 individuals, respectively. According to the Michaelis-Menten estimator (MM), at least 94% of the potential species richness for the entire landscape was collected, representing 88% of species in the early stage, 71% in the intermediate stage, and 82% in the late stage.
No significant differences were detected in local richness (Mann Whitney (z=−1.49, p=0.14) or in abundance (Mann Whitney (z=0.245, p=0.80) between early-intermediate successional stages and S3. No significant differences were detected in local abundance (Kruskal-Wallis, H(8, n=9)=8, p=0.43) and in local richness (Kruskal-Wallis, H (8, n=9)=7.73, p=0.46), among sites. The minimum and maximum number of species per site was 8 and 17 species, respectively. We did not find significant correlations for the values of vegetation features among successional stages (Table 3).
Spearman-rank correlations among dung beetle species richness and abundance with descriptive vegetation variables. p-values are shown in parenthesis
| Variables | Species richness | Species abundance |
|---|---|---|
| Vegetation cover | 0.508 (0.15) | 0.167 (0.637) |
| Total number of trees | 0.321 (0.364) | −0.083 (0.814) |
| Total number of shrubs | 0.425 (0.229) | 0.338 (0.34) |
| Species richness | 0.675 (0.056) |
The equitability value was 0.67 for S1 while this value in S2 increased with the increasing structural complexity (Fig. 4). In S3, the increase of equitability was apparently related to the increased relative humidity in the wooded sites. These trends might reflect dominant-diversity curves that show the highest slopes in sites with low structural complexity and in wooded sites with low relative humidity (Fig. 5). Significant differences in abundances were detected among the species rank of successional stages (ANCOVA, F=35.05, p<0.0001). Canthon (Glaphyrocanthon) delgadoi was distributed in almost all sites and was highly abundant in all successional stages. In early successional stage and in intermediate successional stage, C. indigaceus chiapas was the dominant species, followed by C. cyanellus cyanellus and C. (G.) delgadoi. In late stages, the most abundant species in the forest fragments were Uroxys deavilai and C. (G.) delgadoi, while in the fallows Onthophagus igualensis and Phanaeus endymion were the most abundant (Fig. 5).
Rank-abundance curves (Whittaker's plots), comparing beetle abundance distribution in 3 successional stages within a silvopastoral landscape in Chiapas, Mexico. Species identification numbers are those used in Table 2.
According to the INDVAL analysis, the species with the highest indicator values for the early and intermediate successional stages (pasture and pasture with Acacia) was Canthon indigaceus chiapas (IV=99.4, p=0.036), a diurnal, coprophagous, median roller and Copris lugubris (IV=88.2, p=0.05) a nocturnal, coprophagous, median tunneler for the intermediate successional stage; while for the late successional stage Deltochilum scabriusculum (IV=100, p=0.006) a nocturnal, coprophagous, large roller and Phanaeus endymion (IV=85.3, p=0.007) a diurnal, generalist, median tunneler had the highest values. There were no significant values for the other species and their indicator values (IV) were lower than 75.
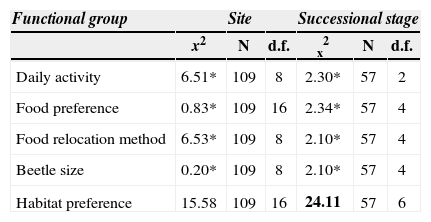
The proportions of the species categories for every functional trait were independent of the tree cover at each site, with the exception of habitat preferences that were associated with successional stage (x2=24.11, N=57, d.f.=6, p=0.05). In pastures, there were no forest species or mature Acacia forest species. In tropical deciduous forest, pasture species represented 7% of all the species captured, while young managed Acacia bush (2-year-old) contained 9%, and mature Acacia forests had 12%.
A large number of species were lost between successional habitats: 22 species were lost from S3 to S1, while 12 species were lost from S3 to S2 and 12 species were lost from S2 to S1. Figure 6 shows the total number of species and shared and exclusive species per successional stage. In S1, Euoniticellus intermedius and Scatimus ovatus were exclusive species, in S2 Eurysternus mexicanus and Agamopus lampros were exclusive and in S3, there were 12 exclusive species; e.g. Canthon (Glaphyrocanthon) lucreciae, Copris laeviceps, Phanaeus wagneri, Canthon femoralis, Pedaridium maya and Onthophagus violetae.
The Multivariate ANOVA based on dissimilarities detected significant differences between early-intermediate successional stages and late succesional stages (Pseudo- F=2.29, p (perm)= 0.038). In Axis 1 of the NMDS, sites included in S3 are separated from the S2 and S1 sites (Fig. 7). This distribution of species is explained by arboreal coverage (DC). Stress type 1 (weak ties) was 0.06.
DiscussionThe studied landscape has a variegated structure (see Ros et al., 2012) and dynamic structure, the latter caused by the action of cattle and frequent fires. It is formed by remnants of tropical dry forest, Acacia woodlots and grasslands. At landscape level, the diversity of species of Scarabaeinae (33 species) is similar to that of other landscapes where the original vegetation was tropical dry forest (Arellano and Halffter, 2003; Hernández et al., 2003; Harvey et al., 2006). The heterogeneous nature of the silvopastoral landscape and the dynamism of many of its elements allow the entry of foreign species that had abundant populations in other landscapes; e.g. Agamopus lampros, Canthon femoralis, Euoniticellus intermedius, Onthophagus violetae, Pedaridium maya, Phanaeus wagneri, P. demon, etc.
In the pasture land with Acacia shrubs, species abundance was higher than in other sites, and was similar to that recorded in TDF. In contrast, other studies report higher abundance in early successional stages than in late successional stages (Manrique, 2009; Ballam-Ballote and León-Cortés, 2010). In Tizimín, Yucatán, Mexico, Basto-Estrella et al. (2012) found the highest abundance on livestock farms (> 90 000 individuals), perhaps because heliophiles and open site species (Digitonthophagus gazella, Canthon indigaceus chevrolati, Onthophaguslandolti) were favored by the continuity and large expanses of open livestock areas in that region.
Changes in species proportions of the functional groups, according to site and successional stage in a silvopastoral landscape in Chiapas, Mexico. *NS. The only significant value (p=0.001) is in bold
| Functional group | Site | Successional stage | ||||
|---|---|---|---|---|---|---|
| x2 | N | d.f. | x2 | N | d.f. | |
| Daily activity | 6.51* | 109 | 8 | 2.30* | 57 | 2 |
| Food preference | 0.83* | 109 | 16 | 2.34* | 57 | 4 |
| Food relocation method | 6.53* | 109 | 8 | 2.10* | 57 | 4 |
| Beetle size | 0.20* | 109 | 8 | 2.10* | 57 | 4 |
| Habitat preference | 15.58 | 109 | 16 | 24.11 | 57 | 6 |
Contrary to our hypothesis, and the results of other studies with insects (Manrique, 2009; Ballam-Ballote and León-Cortés, 2010), total species richness was not higher in the intermediate successional stages (the ravine and young managed Acacia bush), but instead decreased from late to early successional habitats. For instance, 22 species were lost from S3 to S1, while only 2 species were gained. Structural complexity seems to be an important factor for beetle diversity. Ravines, as in other tropical Mexican landscapes, are sites that allow species with different environmental requirements and biogeographic affinities to colonize silvopastoral landscapes.
Within the landscape, alpha diversity values vary between sites (especially among communities with Acacia) due to the presence of species represented by few individuals. Gamma diversity of the landscape increases in relation to the dry tropical forests, due to heterogeneity and its influence on beta diversity. Intriguingly, the presence of “tourist” species, their number and frequency is highly variable between different successional stages. Therefore, the high number of tourist species is associated with livestock management in the landscape.
When food availability is high, a habitat becomes suitable for larger dung beetle species (Hanski and Cambefort, 1991). In the landscape we studied, small species dominated due in all likelihood to the low availability of dung and the conditions in certain habitats such as the managed young Acacia bush (high densities of trees with many thorns, dry soil, etc.). This pattern is consistent with previous assessments that suggest that open-habitat communities are characterized by highly abundant small species (e.g. Nichols et al., 2007; Caballero et al., 2009Caballero and León-Cortés, 2012). Throughout this landscape, species that are characteristic of wooded habitats dominate, while those typical of pastures account for a low percentage of the beetle assemblages. This silvopastoral landscape has a high proportion of generalist (53%) and forest species (22%) that have apparently been favored by the landscape structure and for which the functional connectivity is relatively high.
We conclude that the traditional management of tropical dry landscapes has created habitat conditions suitable for the persistence of a variety of species. However, we are concerned that landowners may decide to switch from traditional practices to conventional cattle production techniques with low plant diversity and high dependence on chemical fertilizers and herbicides. Such a transformation would be likely to cause a dramatic change in the abundance of species within the landscape, the local extinction of those species favored by landscape heterogeneity and, in the long term, landscape-scale extinctions.
This silvopastoral landscape allows for a high diversity of beetles while maintaining a very profitable economic activity (livestock production). Acacia pennatula is a native species that increases the profitability of livestock due to its multiple uses, and might -in the long term- allow a potential reestablishment of tropical deciduous forest habitats. A well managed population of Acacia makes it possible to intensify land use while reducing pressure on the forest remnants. Specifically, the use of Acacia farnesiana has been recommended to control erosion and improve soil quality. These trees can reduce peak daytime temperatures near the soil surface, and modify soil physical and chemical conditions, increasing fertility and moisture retention (Cházaro, 1977).
Finally, the persistence of insect species with restricted distributions such as Baronia brevicornis (Lepidoptera: Papilionidae), which is associated with silvopastoral landscapes such as the one we studied (León-Cortés et al., 2004), underlines the importance of maintaining traditionally managed systems in tropical Mesoamerican landscapes. There have been perhaps 10 000 or more insect generations that have been able to persist and interact in these man-made landscape elements, and this alone provides good reason to reconcile landscape management with traditional practices.
Manuel Girón, Arcángel Molina, José G. Alonso and José Luis Díaz-Valdivieso helped in the field. Leonardo Delgado, David Edmonds, Bert Kohlmann, Fernando Vaz de Mello and Mario Zunino confirmed our species identifications. Special thanks to Vicente Jiménez and Reinalda Hernández for allowing us to conduct this study on their land. The first author is grateful to SEP-CONACYT for a doctoral grant (175987) and El Colegio de la Frontera Sur in San Cristóbal de las Casas for funding and the use of their instalations. We thank the Instituto de Ecología, A. C., for laboratory facilities. Bruce Ferguson, Jorge Macías and José Nahed made helpful comments and suggestions on the manuscript. Keith MacMillan corrected the English.