Se aislaron 10 especies de la familia Saprolegniaceae del Centro Acuícola “El Zarco”, Estado de México, obteniéndose de muestras de agua de afluentes y efluentes del Centro y de huevos y peces infectados de trucha arcoiris. De estas 10 especies, 2 pertenecen al género Achlya y 8 a Saprolegnia. Se registra Saprolegnia ferax por primera vez para el Estado de México. Achlya ambisexualis, A. heterosexualis, S. australis, S. diclina, S. glomerata, S. parasitica, S. terrestris, S. uliginosa y S. unispora son citados por primera vez para México.
Ten species of the family Saprolegniaceae were isolated from the fish farm “El Zarco”, State of México, obtained from samples of influent and effluent water of the farm and from infected eggs and individual fish of rainbow trout. Two species belong to the genus Achlya and 8 to Saprolegnia. Saprolegnia ferax is recorded for the first time for the State of México. Achlya ambisexualis, A. heterosexualis, S. australis, S. diclinous, S. glomerata, S. parasitica, S. terrestris, S. uliginosa and S. unispora are cited for the first time from Mexico.
The family Saprolegniaceae includes widely distributed water molds which usually behave as saprophytes on plants and animal debris, or are parasitic. The Saprolegniaceae are eucarpic, monoecious or dioecious organisms (Lecler et al., 2000; Johnson et al., 2002). The main morphological characteristics are the coenocytic mycelium, 1 or 2 motile spore (planont) types, with or without gemmae; its asexual reproduction by means of monomorphic or dimorphic, biflagellate planonts, or by aplanetic spores, in terminal, subterminal, or intercalary sporangia, and sexual reproduction consisting of morphologically distinct gametangia, the feminine called oogonia, which can be terminal, lateral, or intercalary, and the masculine called antheridia which can be androgynous, monoclinous, diclinous, or exigynous, or limited to a hypogynous or hemihypogynous cell. The oospores, without periplasm, are formed from the entire content of the oogonium; 1 to many being produced; when mature, containing an oil reserve distributed within the ooplast; germinating to form mycelium directly, or to produce a hyphal segment bearing a terminal sporangium (Johnson et al., 2002). The family Saprolegniaceae, which is the most important family of the order Saprolegniales, contains 19 genera and about 150 species. The first classification of the taxon was established by de Bary and Woronin in 1881, and de Bary in 1888, and this was further developed by Coker and Mathews in 1937 (fideLecler et al., 2000).
One of the most relevant and widely accepted taxonomical works was done by Coker (1923), who described the Saprolegniaceae and established the genus classification based on the differentiation of asexual reproduction.
In Mexico, Céspedes and Castillo (1982) reported 18 species of the Chytridiomycetes and Oomycetes fungi. Aphanomyces, Dictyuchus, Geolegnia, Leptolegnia, and Saprolegnia species were isolated from soil and water from 10 different locations of 4 states in Mexico; these species were included in the class Oomycetes. The aim of this study was to detect the presence of species of Saprolegniaceae in the fish farm “El Zarco” in different substrates (eggs, fish and water) and contribute to the knowledge of fungal biodiversity in Mexico.
Materials and methodsSampling at the fish farm. The fish farm “El Zarco” is located at 32.5km from Mexico City, in proximity to the National Park “La Marquesa”. Its grid reference is 19°17'58'' N, 99°22'55'' W. Two samples of 250ml each were taken from water bodies in 25 different zones of the fish farm (Fig. 1). All samples were collected during the period of August 2006 to March 2008. Fishes of O. mykiss were sampled for the isolation of species of Saprolegniaceae. Mycelium of injured epidermis and the skin mucus were inoculated onto agar Sabouraud and sterile water. Samples of colonized eggs were inoculated in sterile water.
Isolation of Saprolegniaceae from water, trout's eggs and lesions. The method described by Willoughby (1984) was adopted with some modifications (Vega-Ramírez, 2008). Briefly, 5ml of water samples were mixed with 5ml of sterile water, and 0.32ml of GP culture medium (D-glucose 16.7mM; casein digest peptone 1.3g l-1; MgSO4•7H2O 0.5mM; KH2PO4 10mM; CaCl2 0.5mM and Na2HPO4 5mM) containing 500mg l-1 ampicillin, in 2 Petri dishes. One of them was incubated at 22° C and another at 4° C. We looked for fungal growth after 48h of incubation. The Saprolegnia colonies were washed several times with sterile water, and transferred to GP culture medium containing ampicillin. The process was repeated at least 3 times, until no bacteria were detected in the culture medium. The trout's eggs and lesions were washed several times using sterile distilled water; the egg membranes with fungal mycelia were separated, washed and placed into 4 plastic Petri dishes containing 7ml of sterile distilled water, mixed with 3ml of GP culture medium containing 500mg l-1 ampicillin. Two of the Petri dishes were incubated at 22° C and 2 at 4° C. We observed Saprolegnia growth after 48h of incubation. Water molds were washed in sterile distilled water and the Petri dishes were incubated at 22° C and 2 more at 4° C. We observed growth after 48h of incubation.
Pure cultures. These were obtained and maintained following the method described by Weston (1917). Briefly, spore suspension was seeded onto Sabouraud agar and a single germinated spore was transferred to fresh Sabouraud media.
Taxonomic classification. Observations were carried out in an inverted microscope and identifications of sexual or asexual structures, cyst ornamentation and pattern of germination (Willoughby et al, 1984). The sporulation and development of reproductive structures (asexual and sexual) were induced by melon seeds (Cucumis melo L.) in water. Petri dishes were incubated at 22° C and 4° C, examined every 24 hours to assess the growth of colonies. Cultures were observed under the inverted microscope (Motic AE31, Richmond, Canada). The images were scanned in order to document the growth of the fungus. All strains were characterized and identified according to Coker (1923), Seymour (1970) and Johnson et al. (2002).
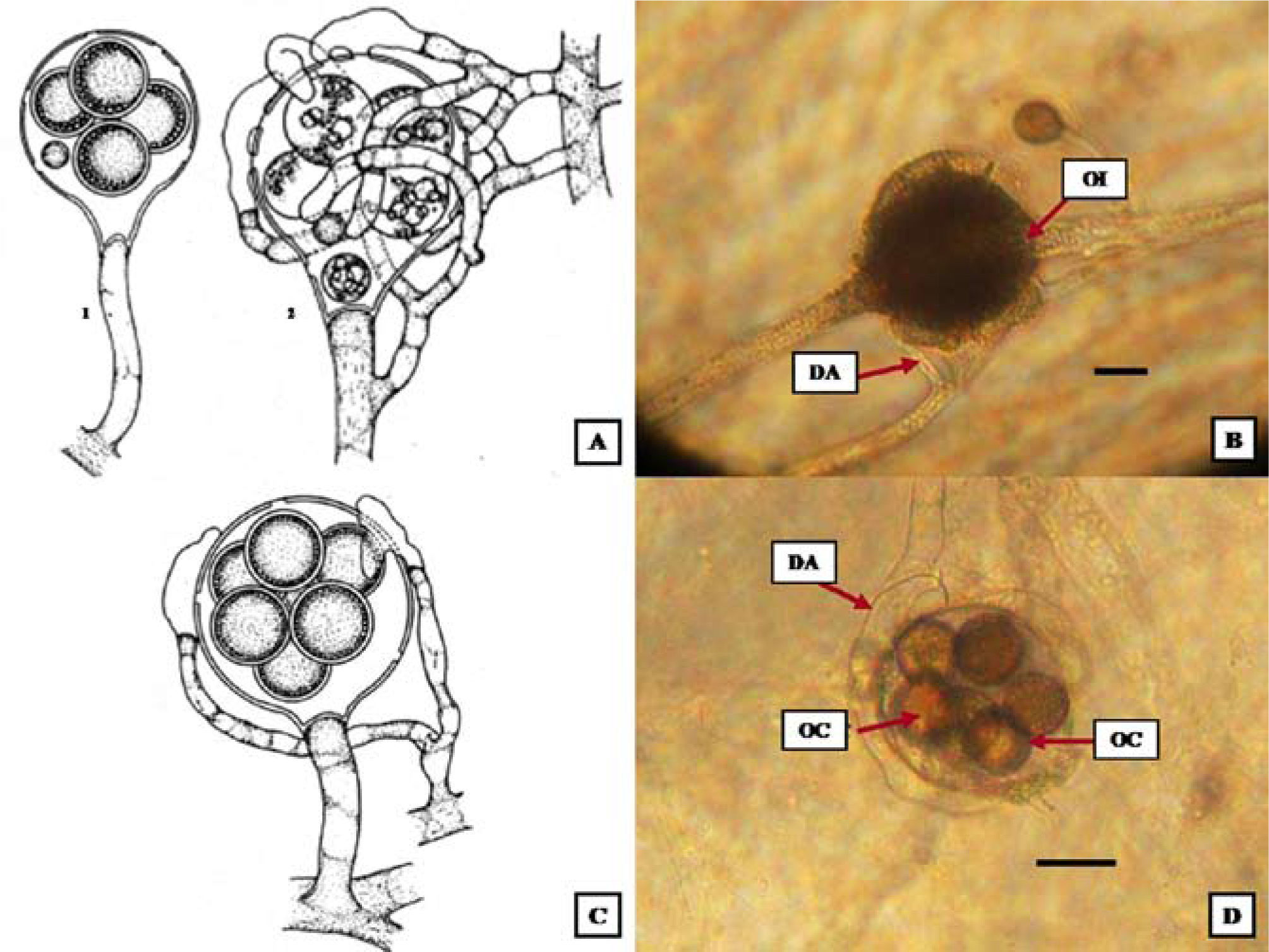
DescriptionsAchlya ambisexualis Raper 1939; Figs. 2A, B
Culture with melon seeds and indistilled water at 4° C. A) Achlya ambisexualis. Mature oogonium; wall pitting pattern; digitate antheridial cell attachment; oospores eccentric (Johnson et al., 2002). B), A. ambisexualis. Oogonium with antheridials cells with 6 oospores. Barr= 20μm. C), A. heterosexualis. Short-stalked oogonia; antheridial hyphae androgynous, monoclinous, or diclinous; antheridial cells attached laterally; oospores eccentric (Johnson et al., 2002). D), A. heterosexualis. Spherical oogonium, antheridial cells attached laterally, wall unpitted, oospores centric. Barr= 20μm. DA: diclinous antheridium, OE: oospores eccentric; OS: oospores subcentric; AC: antheridial cells; PI: pitted.
Mycellium of oogonial tallus dense, extensive; hyphae strout, branched. Sporangia clavate, renewed sympodially; 210–500×21–48μm. Gemmae abundant cylindrical, occasionally irregular; terminal or intercalary, single or catenulate. Mycelium of antheridial thallus diffuse; hyphae moderately stout, branched. Sporangia sparse, cylindrical, fusiform; renewed sympodially; 160–590×16–41μm. Gemmae cylindrical. Spores monomorphic in both thalli; discharge and behavior achlyoid; primary spore cysts 9–14μm in diameter; spore cluster persisting at exit orifice or disintegrating in part. Oogonia lateral or terminal; spherical or obpyriform,(33-) 50–85 (-110)μm in diameter. Oogonial wall pitted under the region of antheridial cell attachment; smooth. Oospores almost always maturing; eccentric; spherical; (1-) l0-l8 (-36) per oogonium, and generally filling it; (14-) 18–24 (-38)μm in diameter; germination not observed. Antheridial branches arising from one hypha (“male”); long, slender, irregular, and abundantly branched; often wrapping about the oogonium and its attendant hypha; persisting. Antheridial cells compound; tubular, branched or unbranched; persisting; attached in a digitate fashion or laterally; fertilization tubes not observed.
Taxonomic summary.Mexico. State of México, fish farm “El Zarco”, fishpond no. 9 (z-15). 05/FEB/2007. Vega-Ramírez Aa-15 (ENCB).
Remarks. A. ambisexualis is a dioecious species and can be distinguished by its oospheres predominantly maturing, oospores 18–24μm in diameter, generally 1–18 per oogonium and gemmae cylindrical in both antheridial and oogonial mycelia. A. bisexualisis is also a dioecious species, and is differentiated by its oospheres infrequently to rarely maturing, oospores 22–26μm in diameter, generally 5–10 per oogonium, and gemmae in oogonial mycelia spherical to short-cylindrical and spherical to cylindrical in antheridial mycelia. A. heterosexualis can be a dioecious or monoecious species and it is separated from both A. ambisexualis and A. bisexualis by producing monoclinous and androgynous as well as, diclinous antheridial branches. A. ambisexualisis is distributed in Africa, China, India, British Isles, South America, Canada and USA (Johnson et al., 2002); therefore this species is cited for the first time in Mexico.
Achlya heterosexualis Whiffen 1965; Figs. 2C, D
Mycelium diffuse; hyphae moderately stout, moderately branched; capable of self- or interspecific conjugation. Sporangia fusiform; renewed sympodially; sometimes with a lateral exit orifice; 142–309×23–42μm. Spores monomorphic; discharge and behavior achlyoid; primary spore cysts 9–11μm in diameter. Gemmae abundant; fusiform, cylindrical; often disarticulating; terminal or intercalary, single or catenulate. Oogonia lateral or terminal, obpyriform, or subglobose; (66-) 80–130 (-152)μm in diameter. Oogonial wall pitted; smooth. Oogonial stalks unbranched. Oospores not always maturing, but eccentric when mature; spherical; 3–18 per oogonium, and filling it or not; (18-) 20–26 (-39)μm in diameter; at germination forming a germ hypha. Antheridial branches in self-conjugating thallus diclinous or androgynous; persisting. Antheridial cells simple; fertilization tubes unknown.
Taxonomic summary.Mexico. State of México, fish farm: “El Zarco”, “El Zarco” limit (z-25). 05/FEB/2007. Vega-Ramírez Ah-25 (ENCB).
Remarks. A. heterosexualis is a dioecious or monoecius species and can be distinguished by its oospheres not always maturing, but eccentric when mature, oospores 20–26μm in diameter, generally 3–18 per oogonium and gemmae abundant; cylindrical; often disarticulating; terminal or intercalary, single or catenulate and produced only monoclinous and androgynous antheridial branches. In A. heterosexualis only the antheridial branches are cross-induced. The oogonia are self-induced, but function either with cross- or self- induced antheridial filaments. A. ambisexualis and A. bisexualisare are close to A. heterosexualis and the differences are presented in the remarks of the first species. A. heterosexualisis is distributed in USA (Johnson et al., 2002), and is recorded for the first time in Mexico.
Saprolegnia australis R. F. Elliott 1968; Figs. 3A, B
Culture with melon seeds indistilled water at 4° C. A), Saprolegnia australis. 1. Obpyriform oogonium; oospores subcentric; antheridia lacking. 2. Obpyriform oogonium and clasping/wrapping diclinous antheridial branch (Johnson et al., 2002). B), S. australis. Lateral spherical oogonium with more than 6 oospores subcentric, diclinous antheridial branch. Barr= 20μm. C), S. diclina. Spherical, lateral oogonium; oospores centric (Johnson et al., 2002). D), S. diclina spherical oogonium, wall unpitted, oospores centric. Barr= 25μm. DA: diclinous antheridium, OC: oospores centric, IO: immature oogonium.
Mycelium dense, diffuse; hyphae slender or stout. Sporangia cylindrical; renewed internally, primary ones 250×32μm; secondary ones usually shorter, but up to 600μm long. Spores dimorphic; discharge and behavior generally saprolegnoid; primary spore cysts 11.1m in diameter. Gemmae abundant; clavate; terminal or intercalary, usually single. Oogonia generally terminal, lateral or intercalary, when intercalary 59–80μm in diameter. Oogonial wall pitted, smooth. Oogonial stalks in length; straight, curved, twisted, or irregular; unbranched. Oospores may or may not mature, or may abort; when mature, subcentric; spherical to subspherical; 4–12 per oogonium, but usually not filling it; 22–27μm in diameter; germination not observed. Antheridial branches, predominantly diclinous, monoclinous or androgynous. Antheridial cells simple or branched, persisting; tubular or attached in a digitated fashion; fertilization tubes present or absent, not persisting.
Taxonomic summary.Mexico. State of México, fish farm: “El Zarco”, spring (z-10), spawning room (z-14), fishpond no.6 (z-21). 05/FEB/2007. Vega-Ramírez Sa-10, Sa-14, Sa-21 (ENCB).
Remarks. S. australis is a monoecious species and can be distinguished by its subcentric oospores (types I and III) with the refractive droplets surrounding the plasma or only partially so. The oospheres may or may not mature, or the oosphere may develop and then abort. S. australis and S. diclina have pitted and obpyriform oogonial wall, but in S. australis are primarily terminal and in S. diclina are these are predominantly lateral. S. australisis is distributed in Canada, Japan, New Zealand and USA (Johnson et al., 2002); this species is cited for the first time in Mexico.
Saprolegnia diclina Humphrey 1892; Figs. 3C, D
Mycelium sparingly to moderately branched. Sporangia cylindrical, clavate, fusiform; straight or slightly curved; renewed internally or basipetalous succession; 75-1050×20–80μm. Spores dimorphic; discharge and behavior saprolegnoid; encysted spores 9–12μm in diameter.
Gemmae, when present, pyriform, cylindrical, clavate, or irregular; terminal or intercalary, single or catenulate. Oogonia sparse to moderately abundant, and often appearing in culture only after prolonged incubation; terminal, lateral, or intercalary, single or catenulate; spherical, or subspherical when intercalary; spherical ones (30-) 50–70 (-130)μm in diameter, subspherical or obpyriform ones 54–146×18–72μm. Oogonial wall unpitted, pitted, or with pits only under the region of attachment of antheridial cells; pits sometimes inconspicuous; smooth. Oospores centric or subcentric, both types occurring in some oogonia; spherical (1-) 8–16 (>100) per oogonium, and may or may not fill it; (12-)18–26 (-44)μm in diameter; at germination forming a slender, irregular germ tube terminating in a small, cylindrical or clavate sporangium. Antheridial branches diclinous. Antheridial cells simple, very rarely compound; fertilization tubes, when present, persisting or deliquescing.
Taxonomic summary.Mexico. State of México, fish farm: “El Zarco”, spawning room (fishpond 2) (z-11), fishpond no.5 (z-20), channel (z-22), “El Zarco” limit (z-25), eggs (CH-11), trout (T-1, T-6) fish farm: “El Zarco”, water-05/ FEB/2007, eggs-06/FEB/2008, trout's lesions-09/ENE/09. Vega-Ramírez Sd-11, Sd-20, Sd-25, CH-11, T-1, T-6 (ENCB).
Remarks. S. diclina is a monoecious species and can be distinguished by the predominance of diclinous antheridial branches, these are often very abundant and may indeed enclose the oogonia partially or fully. Like S. ferax, S. diclina produces both centric and subcentric oospores, and the 2 types may occur in the same oogonia. Milanez (cited by Johnson et al., 2002) observed subcentric oospores in some oogonia of his specimens of Humphrey's species. Willoughby et al. (1984) recognized 3 types of Saprolegnia diclina (parasitic forms from salmonids and perch, and strictly saprophytic ones) based upon the ratio of oogonium length to diameter. S. diclina is distributed in Belgium, British Isles, Canada, Czechoslovakia, Denmark, France, Finland, Germany, Iceland, India, Iraq, Japan, Latvia, Middle Europe, Nepal, Poland, Portugal, Republic of China, Rumania, South America, Switzerland, USA and West Indies (Johnson et al., 2002); this species is cited for the first time in Mexico.
Saprolegnia ferax (Gruith.) Kütz 1843; Figs. 4A, B
Culture with melon seeds and indistilled water at 4° C. A), Saprolegnia ferax. Obpyriform, pitted oogonium, centric or slightly oospores subcentric (Johnson et al., 2002). B), S. ferax. Obpyriform oogonium, oospores centric. Barr= 20μm. C), S. glomerata. Mature oogonia; short, contorted, branched, or peg-like hyphal elements; oospores centric (Johnson et al., 2002). D), S. glomerata. Terminal oogonium, antheridial diclinous branch. Barr= 20μm. OC: oospores centric; OS: oospores subcentric; AC: antheridial cells; PI: pitted.
Mycelium stout, hyphae moderately to sparingly branched. Sporangia cylindrical, or slightly irregular, sometimes nearly spherical; renewed internally with secondary ones nesting inside discharged primary ones, or partially emerged through orifices of empty sporangia and forming bead-like chains or cylindrical segments, or emerging fully through orifices of previously emptied sporangia; rarely renewed in a basipetalous or cymose manner; 31–624×18–67μm. Spores dimorphic; discharge and behavior saprolegnoid; primary spore cysts 9–12μm in diameter. Gemmae variable in shape and position. Oogonia lateral, terminal, or intercalary, infrequently occurring in emptied sporangia or sessile; (28-) 60–80 (-194)μm in diameter. Oogonial wall generally conspicuously and abundantly pitted, rarely unpitted; smooth or rarely with 1 or 2 short, papilliform evaginations, or apiculate. Oospores centric or subcentric, spherical or ellipsoidal; (1-) 10–18 (-54) per oogonium and nearly filling it, (12-) 22–28 (-44)μm in diameter; germinating by a slender germ hypha that may or may not bear a small, apical, clavate sporangium. Antheridial branches sometimes absent; when present, monoclinous or androgynous, rarely diclinous; slender, may vary slightly to prominently irregular, unbranched or very sparingly branched; persisting. Antheridial cells simple; generally tubular or clavate, occasionally irregular, infrequently once-branched; usually persisting; laterally oppressed, very rarely attached apically; fertilization tubes present, not persisting.
Taxonomic summary.Mexico. State of México, fish farm: “El Zarco”, spring (z-10), spawning room (z-14), fishpond no.39 (z-17), fishpond no.6 (z-21). Fish farm: “El Zarco”, 05/FEB/2007. Vega-Ramírez Sf-10, Sf-14, Sf-17, Sf-21 (ENCB).
Remarks. S. ferax is a monoecious species and can be distinguished by its oospores centric or subcentric, spherical or ellipsoidal; 10–18 per oogonium and nearly filling it, 22–28μm in diameter and gemmae variable in shape and position. Generally, it is most easily recognized by reliance on a combination of predominating characters: large, conspicuously or sparsely pitted oogonia, centric and subcentric oospores (sometimes in the same oogonium), occasional development of oogonia in discharged sporangia, and a preponderance of androgynous or monoclinous antheridial branches (when these filaments are present at all). Saprolegnia ferax also is associated with ulcerative dermal necrosis. S. ferax is distributed in Asia, Australia, Austria, Belgium, British Isles, Canada, Czechoslovakia, Denmark, France, Germany, India, Iraq, Japan, Lapland, Latvia, Middle Europe, Nepal, Netherlands, Poland, Republic of China, Romania, South America, Switzerland, USA and USRR (Johnson et al., 2002). This species is cited for the first time in the State of México.
Saprolegnia glomerata (Tiesenh.) A. Lund 1934; Figs. 4C, D
Mycelium delicate; some principal hyphae stout and provided with numerous short, scattered or clustered lateral, often twig-like branches. Sporangia abundant or sparse; cylindrical, fusiform, clavate, or irregular; renewed internally; 40–220x18–28μm. Spores dimorphic; discharge and behavior saprolegnoid, rarely aplanoid; primary spore cysts 10–14μm in diameter. Gemmae very sparse; clavate or obpyriform; terminal, single. Oogonia spherical, obpyriform, napiform, or subspherical; lateral, occasionally terminal, rarely intercalary; (32-) 46–60 (107)μm in diameter. Oogonial wall pitted or unpitted; smooth; stout; straight, curved, or bent; unbranched or with 1 or more short, lateral branches. Oospores centric; spherical, often nearly filling the oogonium; (1-) 6–16 (28) per oogonium; (18-) 23–26 (-30)μm in diameter; at germination producing a germ hypha. Antheridial branches androgynous or monoclinous; slender, usually contorted, twisted, or irregular and sparingly branched; persisting. Antheridial cells simple, generally tubular, occasionally clavate; fertilization tubes present, not persisting. S. glomerata is readily recognizable by the short, branched or unbranched, contorted, lateral (and often clustered) hyphal extensions.
Taxonomic summary.Mexico. State of México, fish farm: “El Zarco”, fishpond no.6 (z-21). Fish farm: “El Zarco”, 05/FEB/2007. Vega-Ramírez Sg-21 (ENCB).
Remarks. S. glomerata is a monoecious species and can be distinguished by its centric oospores; spherical, often nearly filling the oogonium, 6–16 per oogonium; 23–26μm in diameter; at germination producing a germ hypha and gemmae variable very sparse; clavate or obpyriform; terminal, single. Saprolegnia glomerata is readily recognizable by the short, branched or unbranched, contorted, lateral (and often clustered) hyphal extensions. Secondary characters of recognition are the contorted, branched antheridial filamentsand the short, lateral evaginations on many of the oogonial stalks. In S. litoralis, the hypha immediately below a terminal oogonium, may bear short, lateral branches as does S. glomerata, but in the former, the hypha is usually swollen at its juncture with the oogonial septum. In any case, the oospores in S. litoralis are occasionally subcentric, a condition not known to occur in S. glomerata. S. glomeratais distributed in British Isles, Czechoslovakia, Denmark, Germany, Iceland, India, Japan, Latvia, Poland, Switzerland and USA (Johnson et al., 2002); this species is cited for the first time in Mexico.
Saprolegnia parasiticaCoker 1923; Figs. 5A1-5A10.
Culture with melon seeds and indistilled water at 4° C. A), Saprolegnia parasitica. 1, mature zoosporangia; 2, release mobile zoospores; 3, formation of secondary zoosporangia; 4, primary zoospore; 5, primary cyst. 6, germinated cyst; 7, secondary zoospore; 8, secondary cyst with “boat hooks”; 9, germinated cyst; 10, hyphal growth. B), S. parasitica. B1, formation of secondary sporangia. B2, mature sporangia. B3, formation of sporangia. Barr= 75μm. B4, catenulate gemmae. C), S terrestris. 1–3, oogonia with attendant androgynous antheridial branches; oospores subcentric (Johnson et al., 2002). D), S. terrestris. Lateral spherical oogonium, wall pitted androgynous, monoclinous and diclinous antheridial branches. Barr= 25μm. OC: oospores centric; AC: antheridial cells; PI: pitted.
Gemmae abundant, size and shape very variable; often in chains, mostly terminating hyphae, but sometimes intercalary. Sporangia variable, but usually bent and irregular. At times up to 0.7mm, long, very oftenproliferating from the side below as in Achlya; when growing through others sometimes discharging spores through the side wall of the old sporangium; spores dimorphic; discharge and behavior saprolegnoid, 9-11.5μm. Sexual reproduction not observed so far in our strains and very rarely observed in others. Our isolation developed as a parasite on fish or in water, rarely on trout eggs.
Taxonomic summary.Mexico. State of México, fish farm: “El Zarco”, dam (z-16), fishpond no. 5 (z-20), channel (z-22), eggs (CH-11, CH-9, CH-13, CH-1), trout's lesions (T-1, T-3, T-9, T-11, T-15, T-22). Fish farm: “El Zarco”, water-05/FEB/2007, eggs-06/FEB/08, trout's lesions- 09/ENE/09. Vega-Ramírez Sp-16, Sp-20, Sp-22, CH-11, CH-9, CH-13, CH-1, T-1, T-3, T-9, T-11, T-15, T-22 (ENCB).
Remarks. S. parasitica can be distinguished by gemmae abundant, size and shape very variable; often in chains, mostly terminating hyphae; but sometimes intercalary. Sporangia usually bent and irregular, at times up to 700 μm long with rounded ends, containing the zoospores (9–11.5μm). The isolates obtained from lesions of live rainbow trout with characteristic bundles of hairs and retracted germination pattern. Sexual reproduction not observed. S. parasitica is distributed in Japan, United Kingdom, Brazil, Netherlands and Russian Federation (Global Biodiversity Information Facility, 2012); this species is cited for the first time in Mexico.
Saprolegnia terrestris Cookson ex R. L. Seym. 1970; Figs. 5C, D
Hyphae slender, sparingly branched. Sporangia fusiform, obpyriform, or clavate, frequently spherical, often irregular and contorted; renewed internally, by cymose branching, or in basipetalous succession; 60–400}16–48μm. Spores dimorphic; discharge and behavior saprolegnoid; primary spore cysts 6–11μm in diameter. Gemmae, when present, fusiform or obpyriform, infrequently conspicuously irregular or branched; terminal or intercalary. Oogonia lateral or terminal, infrequently intercalary; spherical or obpyriform, sometimes oval or apiculate, (35-) 60–65 (9l)μm in diameter, inclusive of papillae, if any. Oogonial wall pitted or unpitted; smooth or occasionally very sparsely papillate. Oospores subcentric; spherical or ellipsoidal; (1-) 2–11(-18) per oogonium, and nearly filling it; (20-) 24–32 (-41)μm in diameter; germination not observed. Antheridial branches androgynous and sometimes arising close to the oogonial septum, or infrequently monoclinous; stout or delicate, irregular, infrequently branched; persisting. Antheridial cells simple; generally clavate; not persisting; apically or laterally appressed; fertilization tubes sometimes present, not persisting.
Taxonomic summary.Mexico. State of México, fish farm: “El Zarco”, fishpond no.9 (z-15). Fish farm “El Zarco”, 05/FEB/2007. Vega-Ramírez St-15 (ENCB). Remarks. S. terrestris is a monoecious species and can be distinguished by oospores subcentric 2–11 per oogonium and 24–32m in diameter. Like Saprolegnia litoralis, S. terrestris commonly has androgynous antheridial branches, and the general configuration of the laterally produced sexual apparatus in both is very similar. The oospores of S. litoralis are generally slightly larger than those of S. terrestris, but the latter usually has subcentricones while those of the former are consistently centric. S. terrrestrisis distributed in Australia, British Isles, Canada, Iceland, Iraq, New Zealand and Republic of China (Johnson et al., 2002); this species is cited for the first time in Mexico.
Saprolegnia uliginosa Johannes 1950; Figs. 6A, B
Culture with melon seeds and indistilled water at 4° C. A), S. uliginosa. Oogonia with oospores centric; monoclinous antheridial branches (Johnson et al., 2002). B), S. uliginosa. Spherical oogonium with 5 centric oospores. Barr= 20μm. C), S. unispora. Cluster of oogonia; antheridial components lacking; variations in oogonial stalk length (Johnson et al., 2002). D), S. unispora. Oogonium with 1 oospore, without antheridial branches. Barr= 20μmm. DA: diclinous antheridium, OC: oospores centric, AC: antheridial cells.
Mycelium moderately dense, extensive; hyphae slender, flaccid. Sporangia clavate, cylindrical or fusiform, sometimes irregular, renewed internally, rarely in basipetalous succession; 108–266×12–42μm. Spores dimorphic; discharge and behavior saprolegnoid; primary and secondary spore cysts 9–12μm in diameter. Gemmae sparse; spherical, obpyriform, cylindrical, fusiform, or irregular; terminal or intercalary, single or catenulate. Oogonia sparse or abundant; lateral, rarely terminal, occasionally intercalary; spherical, infrequently napiform or obpyriform; (32-) 60–68 (-91)μmm in diameter. Oogonial wall pitted under the region of attachment of antheridial cells; smooth. Oospores centric, rarely subcentric; spherical; (2-) 5–7 (-25) per oogonium, and nearly filling it; (21-) 25–33(-36) ^m in diameter; germination not observed. Antheridial branches predominantly monoclinous, and arising very near the oogonial stalk; infrequently androgynous; rarely diclinous; slender, slightly irregular, occasionally producing 1 or 2 short, lateral branches; persisting. Antheridial cells simple; generally tubular, straight or curved, occasionally faintly cylindro-clavate; laterally appressed, persisting; fertilization tubes present, not persisting.
Taxonomic summary.Mexico. State of México, fish farm: “El Zarco”, fishpond no.39 (z-17). Fish farm: “El Zarco”, 05/FEB/2007. Vega-Ramírez Su-17 (ENCB).
Remarks. S. uliginosa is a monoecious species and can be distinguished by oospores centric, 5–7 per oogonium, and nearly filling it; 25–33μmm in diameter; germination not observed. Like Saprolegnia turfosa, S. uliginosa usually has spherical oogonia borne laterally on short stalks and containing predominantly centric oospores. The similarity between these 2 species does not go beyond these features since the antheridial branches of S. uliginosa generally are monoclinous whereas androgynous ones are clearly predominate in S. turfosa. S. uliginosais is distributed in Germany, Iceland, India and USA (Johnson et al., 2002); this species is cited for the first time in Mexico.
Saprolegnia unispora (Coker and Couch) R. L. Seym., 1970; Figs. 6C, D
Mycelium extensive; hyphae stout, sparingly branched. Sporangia clavate, fusiform, pyriform, cylindrical, or subspherical basally and attenuated apically; often slightly irregular or curved; renewed internally or in a cymose fashion, rarely sympodially; 88–317×21–127μm. Spores dimorphic, or rarely monomorphic; discharge and behavior saprolegnoid, rarely dictyucoid; primary spore cysts 10–12μm in diameter. Gemmae abundant, fusiform, pyriform, obpyriform, cylindrical, spherical, subspherical, or irregular; terminal or intercalary; single or catenulate. Oogonia lateral, occasionally terminal or sessile, rarely intercalary; frequently clustered on the hyphae or arranged in a sympodially branched glomerulus; spherical or obpyriform, occasionally subspherical, oval, or broadly clavate, very rarely angular, sometimes cylindrical (in old sporangia); (18-) 45–55 (-91)μm in diameter, inclusive of wall ornamentations. Oogonial wall pitted or unpitted; smooth or very rarely with 1 to a few short, inconspicuous broad papillae, or a single apiculus. Oogonial stalks (1/8-) ½ -11/4 (-3) times the diameter of the oogonium, in length; stout, straight or curved, sometimes slightly irregular; unbranched, branched, or forming a glomerulus. Oospores centric or subcentric; spherical or compressed at one side, or ellipsoidal; 1–2 (-4) per oogonium, and generally not filling it; (l6-) 32–38 (-43)μm in diameter; germination not observed. Antheridial apparatus absent. Saprolegniaunispora is recognized chiefly by its short-stalked, large, generally spherical oogonia (often in glomeruli or on once- branched stalks) usually containing a single, large, centric or subcentric oospore, but having no attendant antheridial filaments.
Taxonomic summary.Mexico. State of México, fish farm: “El Zarco”, spring (z-10). Fish farm: “El Zarco”, 05/FEB/2007. Vega-Ramírez Sun-10.
Remarks. S. unispora is a monoecious species and can be distinguished by oospores centric or subcentric; 1–2 per oogonium; 32–38μm in diameter; germination not observed. Antheridial apparatus absent. Saprolegnia unispora is recognized chiefly by its short-stalked, large, generally spherical oogonia (often in glomeruli or on once- branched stalks) usually containing a single, large, centric or subcentric oospore, but having no attendant antheridial filaments. S. unisporais distributed in Australia, British Isles, Canada, Czechoslovakia, India, Japan, Republic of China, USA, and USSR (Johnson et al., 2002); this species is cited for the first time in Mexico.
DiscussionThe largest numbers of isolated water molds were found during the coldest months: November to February from water samples (Table 1). S. diclina was isolated with a frequency of 60% from trout eggs. S. parasitica was isolated with a frequency of 90% from trout lesions. Female trout sampled here predominantly had lesions in hate region (41%), while majority of male trout presented lesions in the caudal fin region (73%). One trout presented lesions on the entire body including the dorsal and anal fins. None of the saprolegniasis positive fishes survived.
Saprolegniaceae isolated in fish farm “El Zarco”
| Species | Frequency | Source | |
|---|---|---|---|
| Achlya ambisexualis | 2/50 | 4% | Water |
| A.heterosexualis | 2/50 | 4% | Water |
| Saprolegnia australis | 6/50 | 12% | Water |
| S. diclina | 8/50 | 16% | Water |
| S. ferax | 6/10 | 60% | Eggs |
| S. glomerata | 8/50 | 16% | Water |
| 2/50 | 4% | Water | |
| S. parasitica | 6/50 | 12% | Water |
| 26/28 | 92.8% | Trout's lesions | |
| 02/10 | 20% | Eggs | |
| S. terrestris | 02/50 | 4% | Water |
| S. uliginosa | 02/50 | 4% | Water |
| S. unispora | 02/50 | 4% | Water |
| Another water molds | 16/50 | 32% | Water |
| 07/73 | 9.6% | Skin mucus | |
| No development | 16/50 | 32% | Water |
| 02/28 | 7% | Trout's lesions | |
| 66/73 | 90.4% | Skin mucus | |
| 02/10 | 20% | Eggs | |
Three isolates of S. parasitica were obtained from water samples taken from breeding fishponds. The presence of long threads bundles with a hook-shaped ending in secondary cysts is decisive to originate an infection in the fish. Long thread bundles are typically found in pathogenic species such as S. parasitica (Diéguez-Uribeondo et al., 2007; Fregeneda-Grandes et al., 2009; Woo and Bruno, 2010). Secondary cysts of S. parasitica showed indirect germination and abundant chlamydospore chains, and S. diclina showed individual short thread bundles (Fig. 7).
We observed that S. parasitica did not form oogonia, instead formed abundant chlamydospore chains, which are secondary cysts having hooked hairs in bundles, and they persisted for a long time. S. parasitica was isolated from trout lesions and water. S. diclina isolated from trout eggs produced abundant oogonia and the secondary cysts showed no long hairs.
With best of our knowledge the following species were isolated for the first time in Mexico: Achlya ambisexualis, A. heterosexualis, Saprolegnia australis, S. diclina, S. glomerata, S. parasitica, S. terrestris, S. uliginosa and S. unispora (Fig. 8).
Culture with melon seeds and indistilled water at 4° C. A), A. ambisexualis. Spherical oogonium with more than 6 oospores eccentric. B), A. heterosexualis. Oogonium antheridial cells with 6 oospores. C), S. australis. Lateral spherical oogonium with more than 6 oospores subcentric. D), S. diclina. Spherical oogonium, wall unpitted, oospores centric. E), S. glomerata. Terminal oogonium, antheridial diclinous branch. F), S. terrestris. Lateral spherical oogonium, wall pitted androgynous, monoclinous and diclinous antheridial branches. G), S. uliginosa. Spherical oogonium with 5 oospores centric. H), S. unispora. Oogonium with 1 oospore, without antheridial branches. I), S. parasitica. Typical zoosporangia with mature zoospores. A-C, E, G- I: Barr= 20μm. D, F: Barr= 25μm. I: Barr= 100μm. AA: androgynous antheridium, DA: diclinous antheridium, OC: oospores centric, OE: oospores eccentric, OS: oospores subcentric, AC: antheridial cells, PI: pitted, Z: zoospores, ZP: zoosporangia.
The fact that Saprolegnia and Achlya were found in most of the water samples obtained during the winter, but not from those obtained during the summer months, does not actually exclude the possibility of the presence of water molds all year long, but rather suggests the influence of hormonal and environmental factors on the susceptibility of rainbow trout influencing saprolegniasis infections. The great economic toll caused by high morbidity and mortality by saprolegniasis in rainbow trout warrants further research, and requires immediate attention on the sanitary issues in fish farms to prevent any further spread of S. parasitica.
The study of the genus Saprolegnia in Mexico is scarce. There is little background information to help clarify what is the impact of such bodies in trout production activity; most of the data we have collected over the years assumed releases from the same fish farmers we have studied. This work is one of the first contributions in the study of the taxonomy of the Saprolegniaceae family in Mexico.
The authors are greatly indebted to staff members of the fish farm “El Zarco” for their skillful and technical assistance. To Dr. Miguel Aguilar-Santelises for his critical review on this manuscript. R. Valenzuela thanks COFAA and IPN for the financial support for their research in the project SIP-20130034.