The abundance, species richness, similarity and dominance of braconid parasitoid wasps were estimated for 4 types of land use (secondary forest, rubber plantations, living fences and pastures), and remnants of preserved tropical rain forest in southern Mexico. We also analyzed whether specialist (koinobionts) taxa are more negatively affected by forest disturbance than generalists (idiobionts), and whether braconid abundance is correlated with adult host abundance. Braconids were sampled using 3 Malaise traps for each type of land use during March 2010 and May 2011. We collected 143 individuals belonging to 65 species and 15 subfamilies. Species richness and abundance were higher in preserved and secondary forests, than in other types of land use. Although abundance and richness were low in pastures, these sites potentially contain hosts for braconids. We detected no variation in abundance or species richness by land use, even when comparing idio- and koinobionts. The most dominant species belonged to the genera Apanteles (Microgastrinae) and Hetersopilus (Doryctinae) in all land use types, except pasture, where Bracon (Braconinae) dominated. We detected a positive relationship between braconids and adult host abundance. Altogether, the 4 types of land use and the preserved forest are able to host a diverse braconid community.
Se evaluó la abundancia, riqueza, similitud y dominancia de bracónidos, en 4 usos de suelo (vegetación secundaria, plantaciones de hule, cercas vivas y pastizales) y remanentes de selva conservada en el sureste de México. Se analizaron los cambios en la abundancia y riqueza de bracónidos especialistas (koinobiontes) y generalistas (idiobiontes), y la relación entre la abundancia de bracónidos y sus hospederos (adultos). Se utilizaron 3 trampas Malaise en cada uso de suelo, en marzo 2010 y mayo 2011. Se recolectaron 143 individuos pertenecientes a 65 especies y 15 subfamilias. La riqueza y la abundancia de especies fue mayor en la selva conservada y en la vegetación secundaria. Aunque la abundancia y la riqueza de bracónidos fueron bajas en los pastizales, éstos pueden albergar hospederos potenciales. No hubo diferencias en la abundancia y en la riqueza de especies de koino e idiobiontes entre usos de suelo. Los géneros con mayor número de especies en los 4 usos de suelo y en la selva conservada fueron: Apanteles (Micrograstrinae) y Heterospilus (Doryctinae), excepto en los pastizales que estuvieron dominados por Bracon (Braconinae). Se registró una relación positiva entre la abundancia de bracónidos y la abundancia de sus hospederos adultos. En conjunto, los 4 usos de suelo y la selva conservada albergan una comunidad diversa de bracónidos.
The Braconidae family represents the second largest family in Hymenoptera, for which 19,434 species have been described, though it is estimated to have more than 100,000 species (Jones, Purvis, Baumgart, & Quike, 2009; Yu, van Achterberg, & Horstmann, 2012). Braconids play an important role as parasitoids, helping regulate populations of herbivore insects and are thus essential for the maintenance of ecological processes and for contributing to the diversity of other organisms (e. g., plants) (Hanson & Gauld, 2006; LaSalle & Gauld, 1993; Veijalainen, Sääksjärvi, Erwin, Gómez, & Longino, 2012).The most common hosts for braconid wasps are species of Lepidoptera, Coleoptera and Diptera (van Achterberg, 1993). Braconids are often host specific, making them an attractive group for biological control programs and also as indicators of ecosystem health (Ávila, Berndt, & Holwell, 2013; Campos, 2001; Chay-Hernández, Delfín-González, & Parra-Tabla, 2006; Delfín & Burgos, 2000; LaSalle, 1993).
In the Uxpanapa region, located in Veracruz, Mexico, human activities have deforested vast areas of one of the most extensive zones of tropical rain forest in Mexico. This has resulted in a landscape composed of several land use systems, where natural forest is often patchily distributed among dominating agroecosystems. Among the main land use types in the area are pastures, rubber plantations, citrus orchards, living fences (i. e., lines of living trees connected with wire), riparian vegetation and secondary forest, which cover 63% of the original area of tropical rain forest (Rodríguez-Luna et al., 2011). These forest disturbances may significantly change the original composition of insect species, and therefore their ecological role in ecosystems (Cardoso, Erwin, Borges, & New, 2011).
Despite the ecological and economic importance of braconid wasps, relatively little is known about their richness, distribution or ecology, limiting our understanding of the impact of land use on their populations (Cardoso et al., 2011; Coronado-Blanco & Zaldívar-Riverón, 2013; Chay-Hernández et al., 2006; Chay-Hernández, Delfín-González, Meléndez-Ramírez, & González-Hernández, 2012; Lewis & Whitfield, 1999; Maeto, Neordjito, Sergey, & Fukuyama, 2009). For instance, in the last decade there have been an increasing number of publications describing new taxa, as well as biodiversity and systematics studies in Mexico, but there are no studies on the changes in braconid communities in relation to tropical rain forest destruction, which poses the main threat to these ecosystems. The Uxpanapa tropical rain forest has been considered one of the most important centers of plant diversity in Mexico (Wendt, 1989), even though it has been severely affected by human activity. Changes in plant structure, richness and diversity can negatively affect herbivore populations, as well as parasitoid communities (Idris & Hasmawati, 2002; Sääksjärvi et al., 2004). It has been shown that braconid wasps tend to be more abundant in habitats with diverse vegetation that have a high degree of architectural complexity, such as secondary forest, as these habitats provide the parasitoids with the resources they require (hosts, food, favorable microclimates) (Barbieri & Dias, 2012; Idris & Hasmawati, 2002; Menalled, Costamanga, Marino, & Landis, 2003; but see Auad, Resende, da Silva & Fonseca, 2012; Chay-Hernández et al., 2006; Klein, Steffan-Dewenter & Tscharntke, 2006; Steinbauer et al., 2006Steinbauer, Shot & Schmidt, 2006). In this context, we expect the richness and abundance of braconids, specifically koinobionts, to be higher in preserved rain forest and lower in secondary forest, rubber plantations, living fences, and pastures.
We therefore set out to examine the effects of land conversion in Uxpanapa on the abundance and species richness of the Braconidae parasitoid wasp family. In particular, we asked: i) Does the abundance and species richness of braconid wasps vary with land use type? ii) Does response to land use differ between generalist parasitoids (idiobionts) and specialists (koinobionts)? and iii) Are these changes related to adult host abundance?
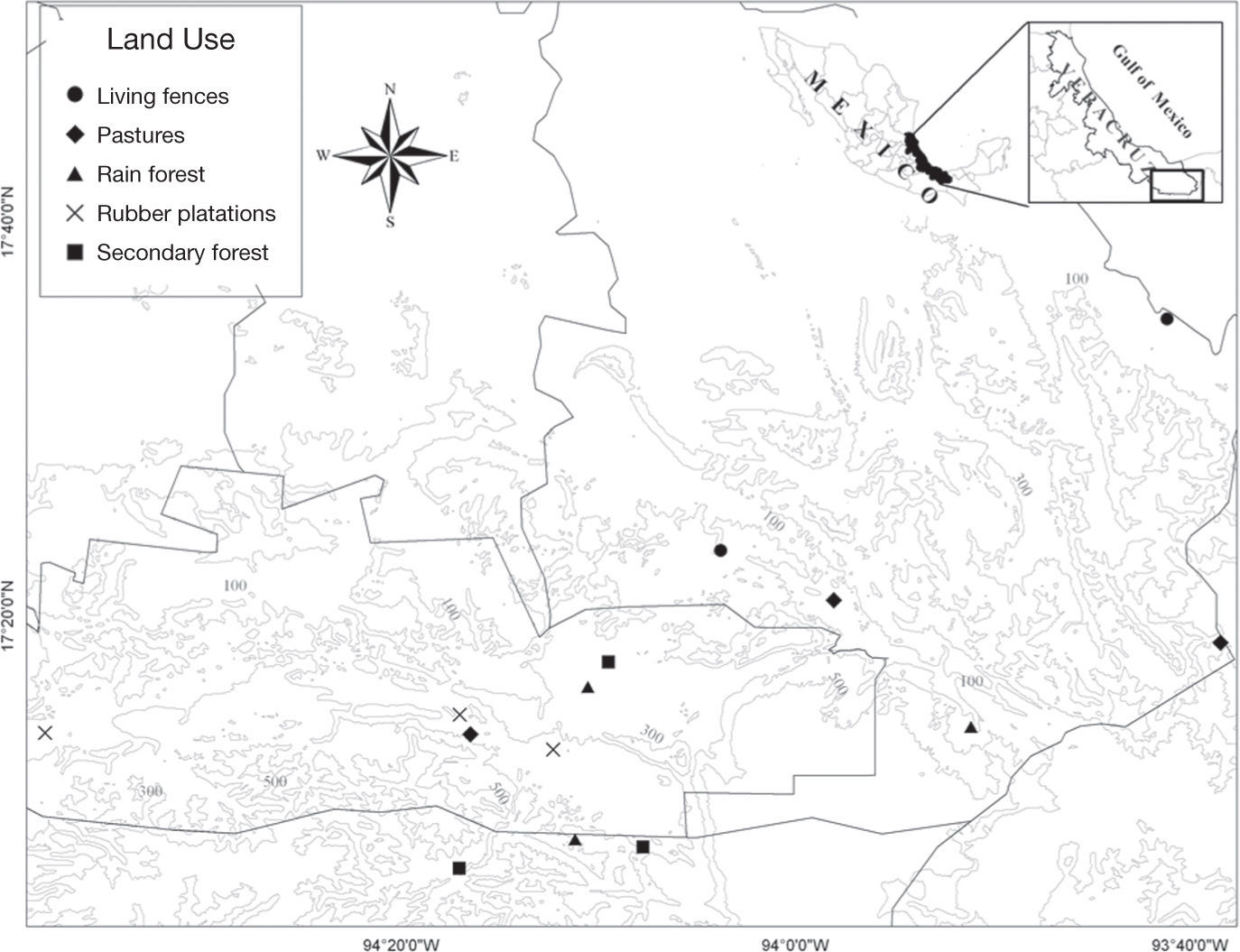
Materials and methodsStudy siteThe Uxpanapa region is located in the southwest of the state of Veracruz and neighbouring areas in the state Oaxaca, in Mexico (17°47’24”-16°59’24” N, 94°19’12”-93°43’ 12” W). The topography is relatively flat to gently sloping; elevation is 100- 300 m asl. Soils are mainly derived from sedimentary limestone rock, shale and sandstone (Williams-Linera, 1983). The climate is classified as hot and humid, with rains in the summer and a dry season from March to June. Average annual temperature is 24.4 °C and precipitation is 3,640 mm (Rodríguez-Luna et al., 2011). The predominant type of vegetation is tropical rain forest that usually grows on lateritic soils that are well drained and rich in organic matter, with a canopy height of 35 m and some individuals reaching 45 m. The most representative species in the understory are Rinorea guatemalensis, Astrocaryum mexicanum, Chamaedorea tepejilote and Brosimum alicastrum. The Uxpanapa region has been severely affected by human activity over the last 50 years, with an estimated 80% of the original vegetation being transformed. The current landscape is dominated by cattle pastures, rubber plantations and subsistence agriculture (Rodríguez-Luna et al., 2011). The most predominant understory plant species in the secondary forest are Psychotria grandis, Rinorea guatemalensis, Astrocaryum mexicanum, Guarea glabra and Chamaedorea tepejilote. Rubber plantations are characterized by Hevea brasilensis, Piper hispidum, Casearia commersoniana, Piper nitidum and Cecropia obtusifolia. Living fences are formed mainly by adult trees of Gliricidia sepium, Miconia argentea, Stemmadenia donnell-smithii, and saplings of Schizolobium parahyba. Finally, pastures have species such as Stemmadenia donnell-smithii, Zanthoxylum sp., Cordia alliodora, Miconia argentea and Psidium guajava (López-Acosta, pers.com.).
Specimen samplingThree Malaise traps (BioQuip product no. 2875A) were set up on each land use type (cattle pasture, living fences, rubber plantations, secondary forest) and in the preserved forest during March 2010 and May 2011. The collecting head of each trap was modified in order to use ethanol as preserving solution. Initially we intended to sample during both the dry and rainy seasons, however it was only possible to do so during the dry season as rainy season flooding was so intense in the region as to prevent sampling.
In total, 15 Malaise traps were used (3 per land use type). In each land use type, the traps were left open for 1 month and were separated by more than 1km to ensure that we were sampling relatively homogeneous internal conditions, but far enough apart to ensure their independence (Gotelli & Ellison, 2004) (Fig. 1). Every 8 days, insects were collected from the traps to avoid losing specimens. We lost 1 Malaise trap at a living fence due to intense wind and rain, so we only include the results from 2 traps for this land use type. We used Malaise traps because they are generally considered the best method for obtaining large, representative samples of Ichneumonidae from most habitats (Noyes, 1989; Sääksjärvi et al., 2004), providing high quality material for further taxonomic analysis (Missa et al., 2009). Braconid specimens from the samples were sorted and subsequently identified to the subfamily and genus level using the specialized literature (Hanson & Gauld, 2006; Wharton, Marsh & Sharkey, 1997). The morphospecies identified were designated as either koinobionts or idiobionts, following Hanson and Gauld (2006). Koinobiont species generally allow the host to continue its development after ovoposition, and they have become specialized to tolerate the host's immunological and chemical defences, whereas the more generalist idiobionts paralyse permanently or even kill the host (Hanson & Gauld, 2006).
In addition to the braconids, the other insects collected in each Malaise trap were separated and assigned to 1 of the 3 main potential host orders (Lepidoptera, Coleoptera and Diptera). All of the insects from these orders were counted and used as surrogates for host abundance. Specimens were deposited in the Colección Nacional de Insectos (CNIN) at the Instituto de Biología, Universidad Nacional Autónoma de México.
Data analysesSpecies richness and abundanceDifferences in species richness among land use types were compared using sampled-based extrapolation rarefaction curves, with 1,000 randomizations and extrapolating to 6 samples for each type of land use (Colwell et al., 2012). The analysis was run in EstimateS (ver. 9.1.0). Changes in the abundance and species number of all braconid wasps with land use were analysed using a generalized linear model (GLM) with a Poisson distribution and a log link function. Differences in the abundance and number of koinobiont and idiobiont species were analysed using a GLM with a binomial distribution and a logit link function. Response variables included both the number of individuals and the number of species of braconid wasps, and the explanatory variable was land use type. Statistical analyses were run in R 2.5.1 (R Development Core Team, 2011) using the glm function. Contrasts were performed with the estimable function (in the g models library; Warnes, 2007). Descriptive statistics are presented as mean ± standard error.
Species similarity and dominanceTaxonomic similarity between land use types was assessed using Sørensen's quantitative index of similarity (CZ) as =2∑1nW/Na∑Nb, where W is the lowest abundance of each of the nth taxa found at both sites; Na and Nb are the total number of individuals at site a, and site b, respectively (Magurran, 2004). The analysis was run in EstimateS (ver. 9.1.0) (Colwell, 2013). Changes in braconid community structure with land use were assessed, using Whittaker plots (Magurran, 2004). For each type of land use, we plotted the relative abundance of each species against species rank, from the most to the least abundant species (Magurran, 2004).
Braconid wasps and their relationship to adult host abundanceTo determine whether the abundance of braconid species is related to the abundance of their potential hosts (Lepidoptera, Coleoptera and Diptera), a Pearson's correlation analysis was run. Data were square root transformed and analysed using the statistical package R ver. 2.5.1 (R Development Core Team, 2011).
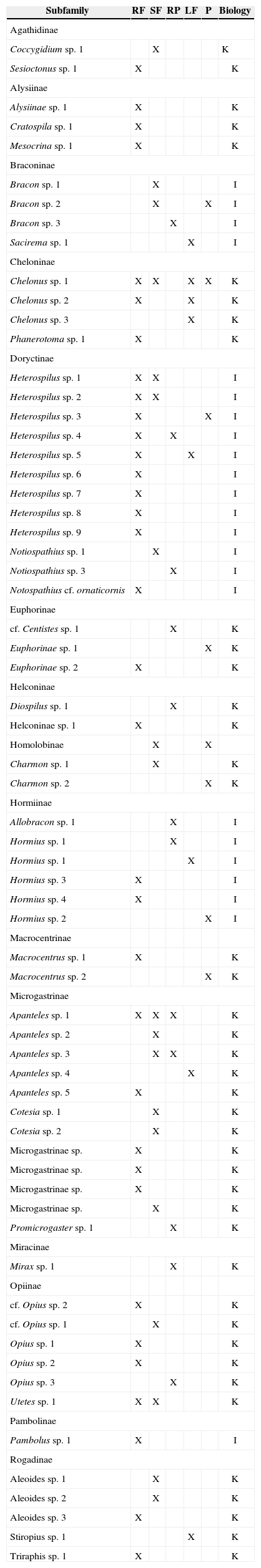
ResultsSpecies richness and abundanceA total of 143 individuals were collected, corresponding to 15 subfamilies and 65 species (Appendix). Micrograstrinae and Doryctinae were the subfamilies with the most species, accounting for 33 and 21% of the total number of individuals, respectively.
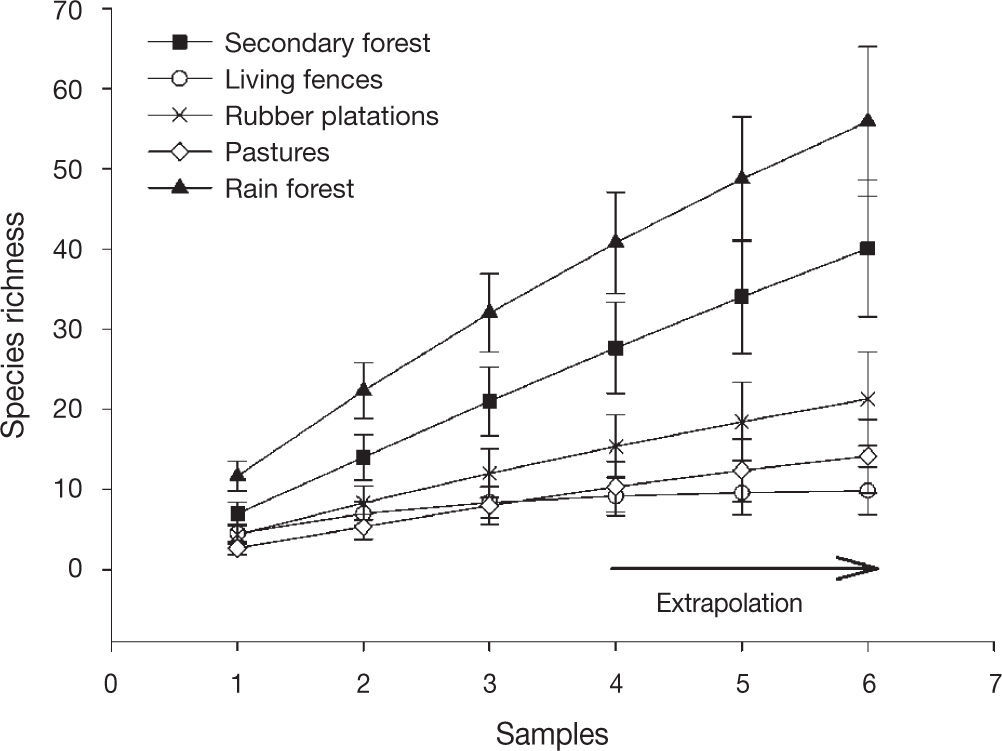
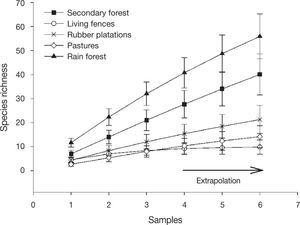
The sampled-based-extrapolation curves revealed that species richness was greatest in preserved rain forest, followed by secondary forest. Braconid richness was lower in rubber plantations, living fences and pastures (with no difference among them) than in secondary forest or rain forest. With our sampling effort (3 Malaise traps) species richness is underestimated for the rain forest and secondary forest, but not for the rubber plantations, living fences or pastures. When sampling effort is increased, the trends in braconid richness between land use types remain the same (Fig. 2).
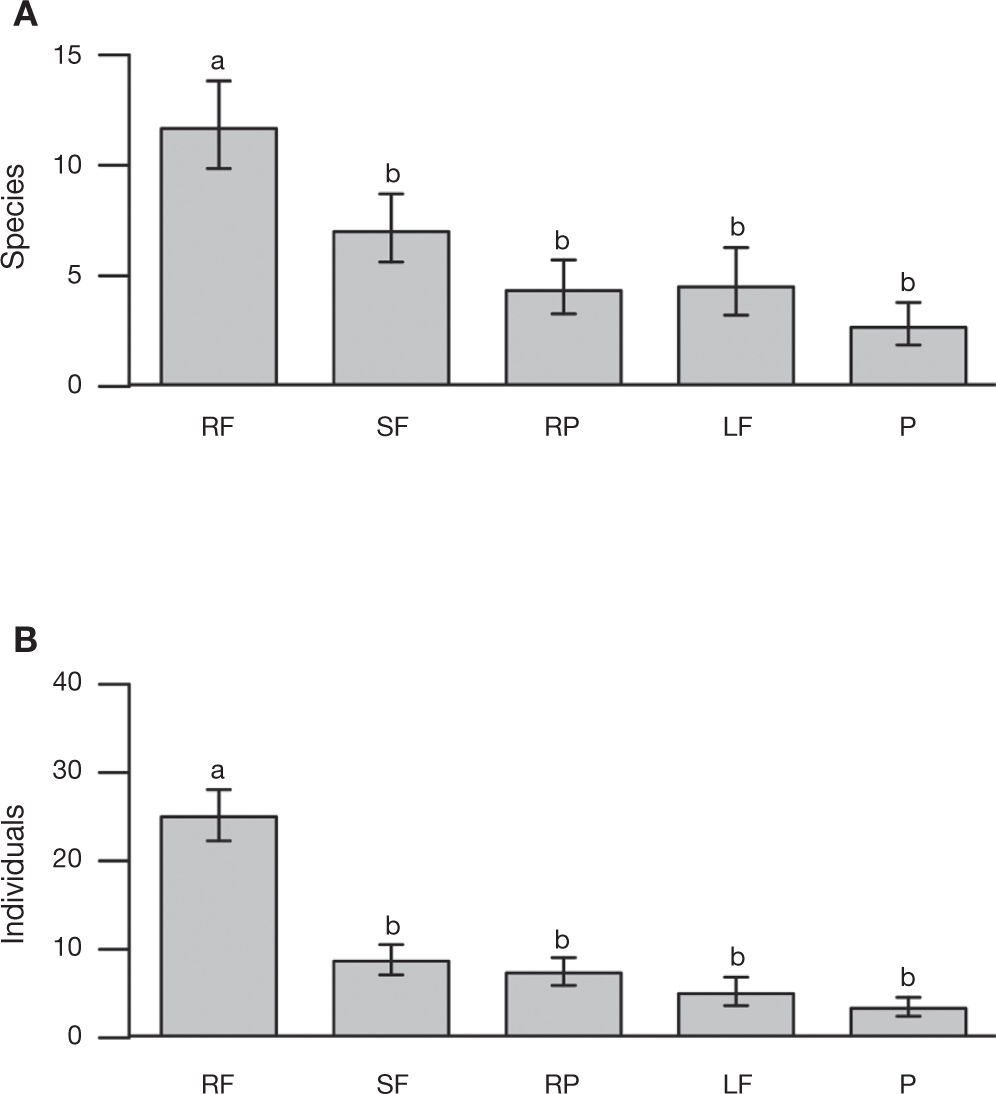
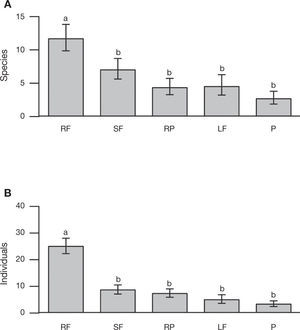
We recorded significant differences in both the total number of individuals and the species of braconid wasps as a function of land use type (χ2= 75.45, df= 4, p< 0.001; χ2= 22.36, df= 4, p< 0.001; respectively). There were more individuals and species in the rain forest than in the other land use types (χ2= 64.38, df= 1, p< 0.001; χ2= 20.50, df= 1, p< 0.001, respectively) (Fig. 3A and B). The rain forest had 52% of the individuals and 41% of species collected. However in terms of host specialization (koinobionts, idiobionts), neither abundance (rain forest 14 idiobionts and 23 koinobionts, secondary forest 7 idiobionts and 15 koinobionts, rubber plantation 5 idiobionts and 8 koinobionts, living fences 3 idiobionts and 6 koinobionts and pastures 3 idiobionts and 5 koinobionts) nor species richness (rain forest 22 idiobionts and 53 koinobionts, secondary forest 7 idiobionts and 19 koinobionts, rubber plantations 8 idiobionts and 14 koinobionts, living fences 3 idiobionts and 7 koinobionts and pastures 4 idiobionts and 6 koinobionts) varied significantly with land use type (χ2= 0.96, df= 4, p= 0.92; χ2= 0.56, df= 4, p= 0.97; respectively).
Mean (±SE) species richness (A) and abundance (B) of braconid wasps, collected from 4 types of land use and in preserved forest in the Uxpanapa region: Rain forest (RF), Secondary forest (SF), Rubber plantations, Living fences (LF), and Pastures (P).Different letters indicate statistical significant differences (p< 0.05).
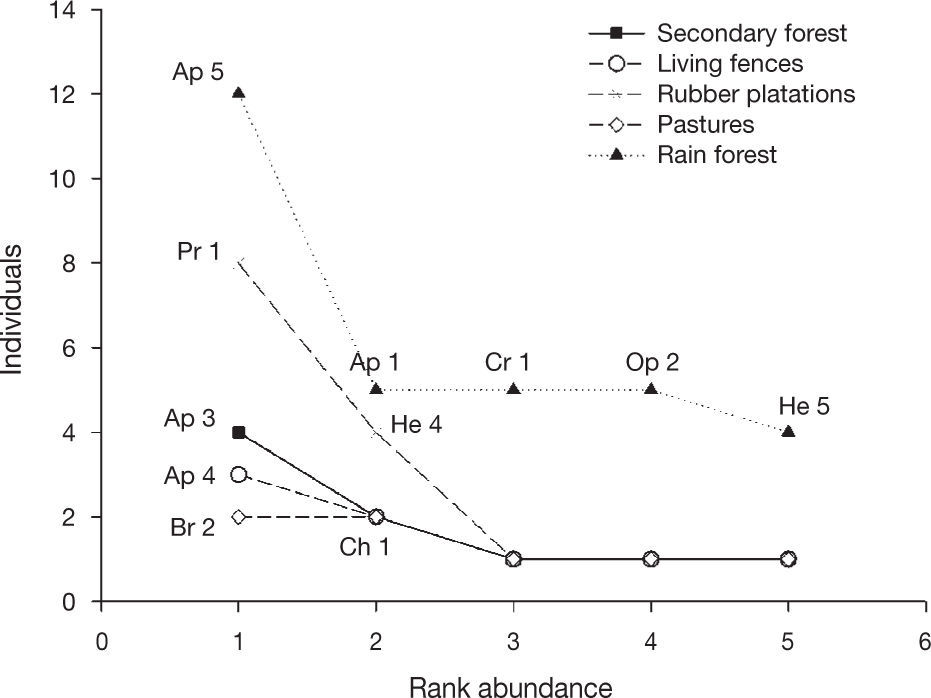
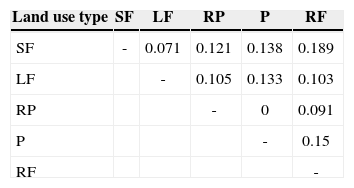
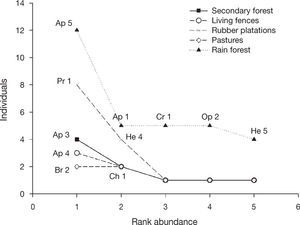
The similarity of the braconid community was low between land use types (0 and 18%; Table 1), with secondary forest and rain forest the most similar communities. Generally, most of the species collected in the rain forest were absent from other land use types. While Apanteles and Heterospilus were the most dominant genera for all land use types except pastures, the other dominant species differed among land use types: the rain forest was heavily dominated by Apanteles sp. 5, secondary forest by Apanteles sp. 3 and living fences by Apanteles sp. 4. In contrast, the most dominant species in the rubber plantations was Promicrogaster sp. 1, and pastures were dominated by Bracon sp. 2 (Fig. 4).
Sorensen coefficient index (similarity index) of braconid wasps collected in 4 types of land use and preserved rain forest in the Uxpanapa region.
| Land use type | SF | LF | RP | P | RF |
|---|---|---|---|---|---|
| SF | - | 0.071 | 0.121 | 0.138 | 0.189 |
| LF | - | 0.105 | 0.133 | 0.103 | |
| RP | - | 0 | 0.091 | ||
| P | - | 0.15 | |||
| RF | - |
Mexico. Secondary forest (SF), living fences (LF), rubber plantations (RP), pastures (P), and rain forest (RF).
Rank abundance plots for braconid wasps from 4 types of land use and preserved forest in Uxpanapa, Mexico, from the most abundant to the least abundant species.Ap 5 (Apanteles sp. 5), Ap 1 (Apanteles sp. 1), Cr 1 (Cratospila sp. 1), Op 2 (Opius sp. 2), He 1 (Heterospilus sp. 1), Pr 1 (Promicrogaster sp.1), He 4 (Heterospilus sp. 4), Ap 3 (Apanteles sp. 3), Ch 1 (Chelonus sp. 1), Ap 4 (Apanteles sp. 4), Br 2 (Bracon sp. 2).
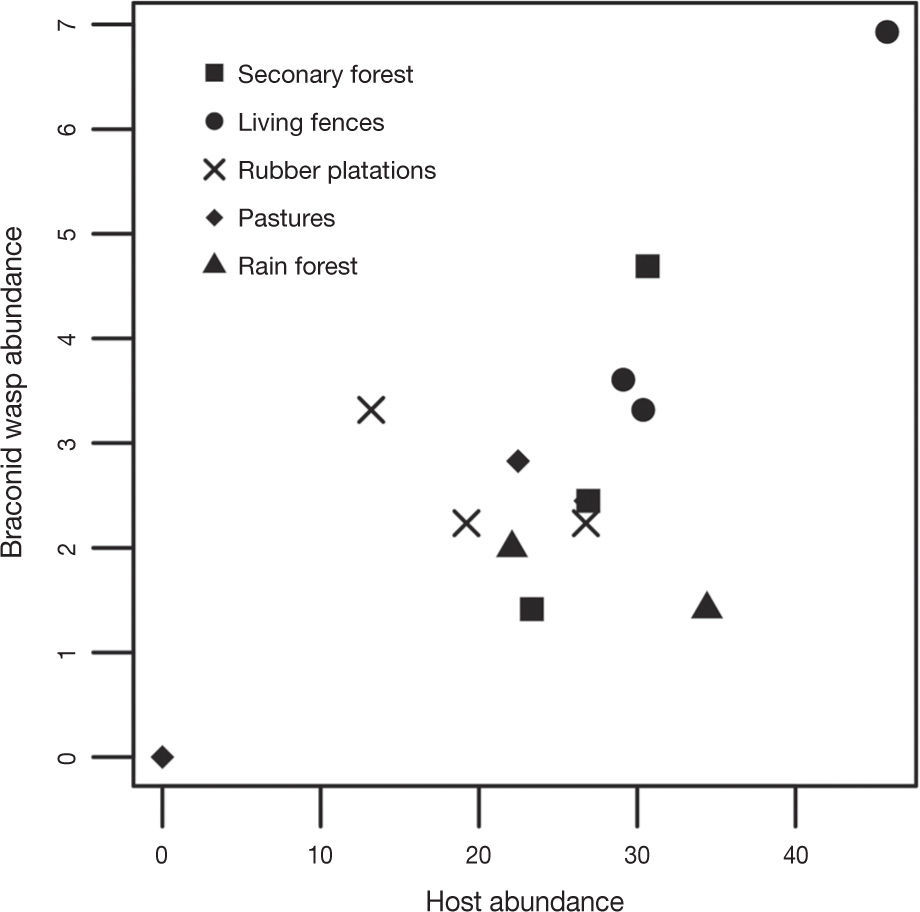
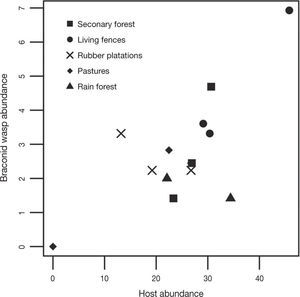
There was a positive relationship between braconid and adult potential host abundance (r= 0.71; t=4.21, df= 11, p= 0.001; Fig. 5).
DiscussionThe accelerated rate of land transformation has been identified as the main cause of species extinction on Earth; the vast majority of these species pertaining to insect taxa (Cardoso et al., 2011). Unfortunately, in tropical regions we are still far from comprehending the magnitude of species richness loss and the consequences in terms of their role in ecological processes. In this study, we report that the abundance and species richness of braconid wasps decreased as a result of the most destructive types of land use. Species similarity among types of land use was low and the most similar land use types were rain forest and secondary vegetation. The most dominant species belong to Apanteles and Hetersopilus except in pastures, which were dominated by Bracon sp. 2. We also found a positive relationship between adult host and braconid abundance.
Although the insects were collected over a 1 month period and in the dry season, the species richness and braconid subfamilies recorded in Uxpanapa highlight the relevance of this region in terms of the diversity of this group. Together with the Chimalapas in Oaxaca and the El Ocote Natural Reserve, this region is one of the last areas of continuous tropical rain forest in North America (Rodríguez-Luna et al., 2011). Likewise, it has been identified as a conservation priority owing to its extraordinary diversity of flora and fauna and because of the accelerated rate that land is being converted to accommodate human activities (Wendt, 1989). Unfortunately, the biological diversity of Uxpanapa has been poorly studied; to our knowledge this is the first report of its braconid diversity. Our results indicate that this region is able to host a notable diversity of braconids, and with them a variety of ecosystem functions. According to the most recent inventory of Mexican Braconidae (Coronado-Blanco & Zaldívar-Riverón, 2013), the number of species (65) and subfamilies (15) we collected represent 9 and 43%, respectively, of the total reported for Mexico, and account for 57% of the species described for the state of Veracruz, which is the state with the second highest number of braconid species in the country. While samples are usually only collected from a limited number of localities (González-Hernández, Lomelí-Flores, & Ruiz-Cancino, 2011), ours is the first study that has assessed changes in braconid communities for a range of land use types and their importance as reservoirs for braconid diversity in the Uxpanapa region. While Peris-Felipo, and Jiménez-Peydrón (2011) reported a positive relationship between rise in temperature and an increased number of captures of Alysiinae in Spain, several studies have reported an increase in abundance and richness during the rainy season (Cirelli & Penteado-Dias, 2003). It is therefore likely that species richness we recorded for the region would be greater if had we also sampled during the rainy season.
The species accumulation curves did not reach the asymptote for preserved rain forest or secondary forest, indicating a lack of sampling. Cirelli, and Penteado-Dias (2003) suggested sampling over 12 months would be sufficient to reach the asymptote when collecting braconids in Cerrado, Brazil. In our study, traps were only open for a month. Even so, the extrapolation model suggests that with an increase in the sampling effort, the differences in richness observed between sites would be maintained, i. e. braconid richness would be highest in rain forest followed by secondary forest, rubber plantations, pastures, and living fences. However, more extensive sampling is necessary to corroborate whether these differences still occur by land use. This information may help confirm that these species may be used as indicators of habitat disturbance (Delfín & Burgos, 2000).
As we predicted, the site with the greatest richness was the rain forest, followed by the secondary forest. The secondary forest may be working as a transitional system that can sustain individuals from conserved and disturbed sites and therefore act as a home for more species. Similarly, secondary forest has a notable proportion of light demanding or pioneer species that are more palatable to insect herbivores, resulting in an increase in their abundance (Coley & Barone, 1996). Changes in the relative abundance of braconid species as a function of the conservation status of a site is a response that has been observed in different vegetation types (Barbieri & Dias, 2012; Chay-Hernández et al., 2006; Idris & Hasmawati, 2002; Querino, Couceiro, Queiroz & Penteado-Dias, 2011). The higher number of individuals and species found in rain forest and secondary forest may be related to the fact that these sites provide more microhabitats, and better protection against predators, as well as an abundance of diverse resources and nesting substrates (Barbieri & Dias, 2012; Idris & Hasmawati, 2002; Maeto et al., 2009; Santos, Bichara-Filho, Resende, Cruz & Marques, 2007). We have evidence that the sites sampled in the rain forest and secondary forest have more species of woody plants than other land use types, and greater structural complexity (López-Acosta., pers.com.). These results concur with those of other studies (Auad et al., 2012; Chay-Hernández et al., 2006; Idris & Hasmawati, 2002).
Pastures host more braconid species than do living fences. These results may be explained by the presence of light-demanding plant species at these sites. Other studies have reported an increase in diversity and abundance in modified systems (Chay-Hernández et al., 2006; Lewis & Whitfield, 1999). For example, some species of the subfamily Microgastrinae are parasites on lepidopteran larvae and are associated with open or disturbed sites (Wahl & Sharkey, 1993). Cirelli, and Penteado- Dias (2003) report that members of the Aphidiinae were only captured at the most disturbed site because these parasitoids used homoptera, which are grass pests, as hosts. In our pastures, the most abundant species were Bracon sp. 2 and Macrocentrus sp. 2.
The composition of braconid wasps was generally very different among land use types; with less than 18% of the total number of species shared. The most similar sites were the rain forest and secondary forest. This reflects that braconid communities found in rubber plantations, living fences and pastures do not represent a subsample of the rain forest community, but rather these areas host a group of species different to those described for the rain forest. The most abundant species in the rain forest, secondary forest, and living fences belong to Apanteles (Microgastrinae). In contrast, rubber plantations and pastures were dominated by Promicrogaster and Bracon, respectively.
We also found that braconid abundance in Uxpanapa was positively correlated with an increase in potential host abundance. We cannot generalize based on these results because the analysis only included a sample of potential hosts, i. e., we only considered adults and some braconid species that are parasitoids during their larval stages. This first approximation indicates that the change in land use type not only affects braconid species but also has a negative effect on herbivorous insects.
It has been suggested that braconids can be used as biological indicators of ecosystem disturbance (Barbieri & Dias, 2012; Delfín & Burgos, 2000). We report the greatest richness of braconid species occurred in the least altered types of land use (secondary vegetation and primary rain forest), a finding that concurs with those of Chay-Hernández et al. (2006). Host specificity, however, is not related to the degree of disturbance, given that koinobiont species were dominant in all land use types. These results are similar to those reported by other authors who found that koinobionts can occur in both the early, as well as the late successional stages of vegetation (Cirelli & Penteado-Dias, 2003; Chay-Hernández et al., 2006; Delfín & Burgos, 2000).
The low abundance and richness of Braconidae we report for living fences, pastures and rubber plantations reflects a negative effect of changes in land use on the braconid community. Our study revealed that the braconid wasp fauna of the preserved rain forest was different from that of other, more anthropic land use types, but even the latter offer some hosts for braconid wasps. This underscores the importance of all of the landscape elements in the conservation of braconid wasps. Together, the 4 land use types and preserved forest of Uxpanapa may host different assemblages of braconid species that can contribute to different ecological processes.
The authors are grateful to Mauricio Juárez and José Luis Libreros for their assistance with the field work and for their careful work separating the insects. We also thank C. MacSwiney for logistical support and S. Armenta for her assistance preparing the map of the study site. Bianca Delfosse revised the final version of the manuscript. The study was funded by the Conacyt and the Government of Veracruz (grant No 108990).
List of braconid wasps collected in 4 types of land use and preserved forest at Uxpanapa region
| Subfamily | RF | SF | RP | LF | P | Biology |
|---|---|---|---|---|---|---|
| Agathidinae | ||||||
| Coccygidium sp. 1 | X | K | ||||
| Sesioctonus sp. 1 | X | K | ||||
| Alysiinae | ||||||
| Alysiinae sp. 1 | X | K | ||||
| Cratospila sp. 1 | X | K | ||||
| Mesocrina sp. 1 | X | K | ||||
| Braconinae | ||||||
| Bracon sp. 1 | X | I | ||||
| Bracon sp. 2 | X | X | I | |||
| Bracon sp. 3 | X | I | ||||
| Sacirema sp. 1 | X | I | ||||
| Cheloninae | ||||||
| Chelonus sp. 1 | X | X | X | X | K | |
| Chelonus sp. 2 | X | X | K | |||
| Chelonus sp. 3 | X | K | ||||
| Phanerotoma sp. 1 | X | K | ||||
| Doryctinae | ||||||
| Heterospilus sp. 1 | X | X | I | |||
| Heterospilus sp. 2 | X | X | I | |||
| Heterospilus sp. 3 | X | X | I | |||
| Heterospilus sp. 4 | X | X | I | |||
| Heterospilus sp. 5 | X | X | I | |||
| Heterospilus sp. 6 | X | I | ||||
| Heterospilus sp. 7 | X | I | ||||
| Heterospilus sp. 8 | X | I | ||||
| Heterospilus sp. 9 | X | I | ||||
| Notiospathius sp. 1 | X | I | ||||
| Notiospathius sp. 3 | X | I | ||||
| Notospathius cf. ornaticornis | X | I | ||||
| Euphorinae | ||||||
| cf. Centistes sp. 1 | X | K | ||||
| Euphorinae sp. 1 | X | K | ||||
| Euphorinae sp. 2 | X | K | ||||
| Helconinae | ||||||
| Diospilus sp. 1 | X | K | ||||
| Helconinae sp. 1 | X | K | ||||
| Homolobinae | X | X | ||||
| Charmon sp. 1 | X | K | ||||
| Charmon sp. 2 | X | K | ||||
| Hormiinae | ||||||
| Allobracon sp. 1 | X | I | ||||
| Hormius sp. 1 | X | I | ||||
| Hormius sp. 1 | X | I | ||||
| Hormius sp. 3 | X | I | ||||
| Hormius sp. 4 | X | I | ||||
| Hormius sp. 2 | X | I | ||||
| Macrocentrinae | ||||||
| Macrocentrus sp. 1 | X | K | ||||
| Macrocentrus sp. 2 | X | K | ||||
| Microgastrinae | ||||||
| Apanteles sp. 1 | X | X | X | K | ||
| Apanteles sp. 2 | X | K | ||||
| Apanteles sp. 3 | X | X | K | |||
| Apanteles sp. 4 | X | K | ||||
| Apanteles sp. 5 | X | K | ||||
| Cotesia sp. 1 | X | K | ||||
| Cotesia sp. 2 | X | K | ||||
| Microgastrinae sp. | X | K | ||||
| Microgastrinae sp. | X | K | ||||
| Microgastrinae sp. | X | K | ||||
| Microgastrinae sp. | X | K | ||||
| Promicrogaster sp. 1 | X | K | ||||
| Miracinae | ||||||
| Mirax sp. 1 | X | K | ||||
| Opiinae | ||||||
| cf. Opius sp. 2 | X | K | ||||
| cf. Opius sp. 1 | X | K | ||||
| Opius sp. 1 | X | K | ||||
| Opius sp. 2 | X | K | ||||
| Opius sp. 3 | X | K | ||||
| Utetes sp. 1 | X | X | K | |||
| Pambolinae | ||||||
| Pambolus sp. 1 | X | I | ||||
| Rogadinae | ||||||
| Aleoides sp. 1 | X | K | ||||
| Aleoides sp. 2 | X | K | ||||
| Aleoides sp. 3 | X | K | ||||
| Stiropius sp. 1 | X | K | ||||
| Triraphis sp. 1 | X | K | ||||
RF, rain forest; SF, secondary forest; RP, rubber plantations; LF, living fences; P, pastures. Biology corresponds to host specialization: koinobiont (K) and idiobiont (I).