Due to differential resource allocation by male and female plants, herbivores tend to feed more on male plants than on female ones. It is believed that the preferred food gives the organism a better performance. Buddleja cordatais an abundant dioicous plant in the “Pedregal de San Ángel”, México City. This plant is the food of the monophagous geometrid Acronyctodes mexicanaria. The objective of this study was to determine if the dioecy of B. cordataaffects nutritional and performance parameters of A. mexicanaria(consumption, metabolic use of the food, growth, and density of caterpillars on male and female trees). Feeding preference, development time of caterpillars, and 5 variables concerning consumption and growth efficiencies were analyzed for caterpillars collected from male and female trees, and which were fed in the laboratory with leaves from trees of each sex. There was no significant difference between caterpillar density on female and male trees; however, we found that caterpillars preferred feeding on female leaves, and they obtained better nutritional efficiencies and grew more quickly when feeding on female leaves than on male leaves.
La distribución de recursos diferenciada entre plantas femeninas y masculinas ocasiona que los herbívoros tiendan a alimentarse más de plantas masculinas. Se cree que el alimento preferido permite un mejor desempeño del herbívoro.Buddleja cordataes una planta dioica abundante en la reserva del Pedregal de San Ángel, en la ciudad de México. Esta especie es el alimento del geométrido monófago Acronyctodes mexicanaria.El objetivo de este estudio fue determinar si el dioicismo de B. cordataafecta algunos parámetros nutricionales y del desempeño de A. mexicanaria(consumo, uso metabólico del alimento, crecimiento y densidad de orugas en árboles de distinto sexo). Se analizaron las preferencias alimentarias, el tiempo de desarrollo y 5 variables de consumo y eficiencias alimentarias en orugas recolectadas de árboles femeninos y masculinos, y se alimentaron en el laboratorio con hojas de estos árboles. No se encontró diferencia significativa en la densidad de orugas entre árboles de diferente sexo, pero las orugas prefirieron alimentarse de hojas de árboles femeninos y presentaron mejores eficiencias alimentarias y un crecimiento más rápido que al alimentarse de árboles masculinos.
The interest shown in dioecious plant species and their effects on herbivores has grown over the past 20 years, although many issues are still poorly explored. The sex-biased herbivory hypothesis assumes that plant resource allocation between growth, reproduction and defense determines plant-herbivore interactions. Herbivore preferences and performance are thus mediated by nutritional traits, physical characteristics, and chemical defenses of male and female host plants (Fritz, Crabb, & Hochwender, 2003). In turn, sex-differential herbivory is believed to be one of the selective pressures that drive the evolution of plant dioecy (Avila-Sakar & Romanow, 2012). Most studies have found that herbivores damage male plants more than female plants (reviewed by Ågren, Danell, Elmqvist, Ericson, & Hjältén, 1999; Cepeda & Dirzo, 2002, 2010; Cornelissen & Stiling, 2005). This is thought to be because females usually allocate more resources toward reproduction and self-defense, and fewer resources toward growth, than male plants (Ågren et al., 1999; Cepeda & Dirzo, 2010; Cornelissen & Stiling, 2005); male plants are therefore more edible. Nevertheless, as of yet there is only limited direct evidence for these proximate explanations – of the 14,620 known dioecious angiosperm species (Renner & Ricklefs, 1995), only about 28 species have been studied (Fritz et al., 2003), and intersexual differences have not been widely tested (Avila-Sakar & Romanow, 2012). Furthermore, the effect that foraging on dioecious plants has on insect performance is poorly explored (Fritz et al., 2003). Feeding on plants of a specific sex affects insect performance and fitness.Krischik and Denno (1990)found that females of Trihabda bacharidis(Coleoptera: Chrysomelidae) that fed on male plant leaves of Baccharis halimifoliahad a higher daily fecundity than females that were fed with female plant leaves.Fritz et al.(2003)found that the gall-inducing sawfly Phyllocolpa leavitti(Hymenoptera: Tenthredinidae) had a higher survival probability when fed with female plants than when fed with male plants of Salix discolor. Hendricks and Collier (2003) studied the Mexican harlequin bug, Murgantia varicolor, on a dioecious tree, Forchhammeria pallida, and found that adult harlequin bugs occurred more on female than on male plants, although no feeding preferences were observed between plants of different sexes.
Buddleja cordata(Scrophulariaceae, before in Loganiaceae family) is a dioecious tree and it is the dominant tree species in Ciudad Universitaria, in México City (González-Rebeles, 2012).González-Cortés and Cano-Santana (1998a, b) found different epiphyte insect community compositions on male and female B. cordataover the course of 1 year, as well as seasonal variations in the rate of herbivory in female trees. In addition, González-Cortés and Cano-Santana (1998a, b) found that the fourth instar of Acronyctodes mexicanaria(Lepidoptera: Geometridae) preferred feeding on female plants during a feeding trial. These results suggested the possibility of differential performance in A. mexicanaria, depending on the sex of B. cordataused as food source. The objective of this research was to determine whether the sex of this dioecious plant affected the performance of a monophagous caterpillar.
Materials and methodsField site and study speciesOur study site was located in preserved and gardened areas of the main campus of Universidad Nacional Autónoma de México, in México City (19°19’ N, 99°11’ W, 2,250 m). The natural reserve inside the campus, the “Pedregal de San Ángel” (176,244 ha), was also sampled. The climate is temperate subhumid with a summer rainy season. Mean temperature is 15.5 °C with an annual rainfall of 870mm (Valiente-Banuet & De Luna, 1990). The original vegetation of this field site is xerophytic scrub (Rzedowski, 1978) with a rocky substrate of volcanic origin (Carrillo, 1995).
Buddleja cordataKunth is a dioecious tree measuring 1.5- 6.0 m high. This species presents perfect flowers whose sex appears to be functionally determined by the atrophy of either the gynoceum or androceum (personal observation). One of its arthropod herbivores is Acronyctodes mexicanariaWalker (Vázquez, 1936). The caterpillar of this species is reported to be monophagous (González-Cortés & Cano-Santana, 1998a, b), and can be observed in the studied area from the last week of June, at the beginning of the rainy season when B. cordatais flowering (Elizalde, 1995), to January or February (García- García pers.obs., González-Cortés & Cano-Santana, 1998a, b.), when the plant is producing fruits (Meave, Carabias, Arrigaga, & Valiente-Banuet, 1994). The total larval period of A. mexicanarialasts about 2 months with 5 instars (García-García, 2004).
Feeding preferencesWe collected 6 fourth and fifth instar caterpillars from female trees, and 6 from male trees on October 12, 2002. We recorded the sex of the plant from which the caterpillars were collected because it has been reported that previous feeding experiences can modify food choice (Jolivet, 1998). Ten caterpillars were placed inside individual transparent plastic cages measuring 21.5cm diameter ×10cm deep, and then were deprived of food for 48 h. We collected 12 leaves from 6 different female trees and male trees each. Since it has been reported that young leaves tend to be more nutritious (Slansky Jr., 1993), we controlled leaf age preference by collecting 6 young leaves and 6 older ones from both tree sexes. The leaves were cut into discs measuring 2cm in diameter. Four discs were pinned equidistantly in a circular arrangement inside each cage. One disc from each of the following categories was placed in every cage, in an alternate fashion: Fy(young leaf from a female tree), Fo(old leaf from a female tree), My(young leaf from a male tree), and Mo(old leaf from a male tree). A caterpillar was then placed in the middle of the cage, on a humid piece of cotton. The foliar area remaining after 3, 7, 20 and 24 h was recorded. We calculated the percentage of consumed accumulated foliar area at each time point using a sheet of transparent plastic with dots separated equidistantly by 1mm (Cano-Santana, 1987; Cano- Santana & Oyama, 1992). These data were transformed into percentage of consumed accumulated foliar area (% CF) using the formula % CF= (total foliar area-remaining area) ×100/total foliar area. We used repeated measures Anova to compare the consumed foliar area of male and female tree leaves, transforming the data with arcsenx (Zar, 1999).
Nutritional ecologyWe collected 12 fifth instar caterpillars on October 28 and 29, 2002. We deprived them of food for 24 h to prevent food from accumulating in their intestines. Six caterpillars were fed every other day with 1 g of leaf from male or female trees over a period of 6 days. We recorded the fresh weight of caterpillars, feces, and leaves using an analytic balance (B300 D, Ohaus). Water loss was controlled for leaf weight by also recording the weight of 3 uneaten leaves over the same time period. We also recorded the dry weight of the feces and leaves after drying in a 40 °C oven. The dry weight of caterpillars was estimated using a correlation of fresh vs.dry weight, obtained from 46 caterpillars of different instars dried at 40 °C until reaching constant weight.
Nutritional indices were obtained according to Waldbauer's formulas (1968)with the following modifications:
(a) Weight gain: (G;mg):
during the feeding period.
(b) Mean weight (B; mg):
where wis the caterpillar weight at time “t” and T= number of times the caterpillar is weighed.
(c) Relative growth rate (RGR; mg g-1day-1):
where t= number of days.
(d) Relative consumption rate (RCR; mg g-1day-1):
(e) Ingested food in fresh weight (Cfw;mg):
where fwiis the initial fresh weight of the leaves and fwris the remaining fresh weight of the leaves once they have been partially consumed by the caterpillar;
where dwreis the remaining dry weight of the leaves that the caterpillar fed, and dwrcis the dry weight of the control leaves at the end of the feeding period.
(f) Ingested food in dry weight (Cdw;mg):
(g) Efficiency of conversion of ingested food to biomass (ECI; %):
where Gdwis the weight gain in dry weight.
(h) Efficiency of conversion of digested food to biomass (ECD; %):
(i) Approximate digestibility (AD; %):
The variables in fresh and dry weight were compared between treatments (caterpillars fed with leaves from female and male trees) with one-way Anova, transforming the data with arcsenx (Zar, 1999).
GrowthWeight gainWe collected 20 third and fourth instar caterpillars on August 20 and 24, 2002, from both male and female trees. Half of the caterpillars were fed with female tree leaves and the other half with male tree leaves every 2 to 3 days. We therefore had 4 feeding schemes: Ff(caterpillars from female trees fed with leaves of the same tree sex), Fm(caterpillars from female trees fed with leaves from male trees), Mm(caterpillars from male trees fed with leaves of the same tree sex),and Mf(caterpillars from male trees fed with leaves from female trees). Caterpillars were weighed using an analytic balance until reaching pupation.
Development timeWe collected 16 and 12 fourth or fifth instar caterpillars on October 28 from female and male trees, respectively. Half of the caterpillars from each tree sex were then fed with female tree leaves, and the other half with male tree leaves. Categories were thus the same as in the previous section: Ff(N= 7), Fm(N= 9), Mf(N= 6) and Mm(N= 6). Caterpillar weight gain and development time were compared according to the sex of the tree from which they were collected and the sex of the tree leaves on which they were fed, using repeated measures Anova.
DensityWe recorded the number of caterpillars found on 20 pairs of male and female trees (2 to 5.5 m high) close to each other (distance from 2 to 8 m) every 3 weeks, 4 times (starting on August 27, 2002). The density of caterpillars per individual tree, per crown cover, and per crown volume, was calculated using these measurements. Crown cover was estimated using the formula
and crown volume estimated with
h, height; d1,maximum diameter of the crown; d2,perpendicular diameter to d1; h, height; hr, height of the first branch. Densities were compared using a paired ttest on each date, correcting data with x+0.5 (Zar, 1999).
All statistical analyses were conducted using Statistica software (StatSoft, 1998 ver.6.0). Tukey's posthoc test was performed after significant effects were found with Anova.
ResultsFeeding preferencesDue to a differential resource allocation of female and male plants, herbivores tend to feed more on plants of a given sex. In order to determine whether caterpillars of A. mexicanariahad sex-related feeding preferences, we compared the percentage of foliar area consumed of male and female tree leaves. Caterpillars marginally consumed a greater foliar area of female tree leaves than male ones (Fig.1) (F1,40= 4.141, p= 0.049). However, the leaf age did not significantly influence the caterpillars’ feeding preferences (F1,40= 1.525, p= 0.224), nor did the plant sex × leaf age interaction (F1,40= 1.887, p= 0.177).
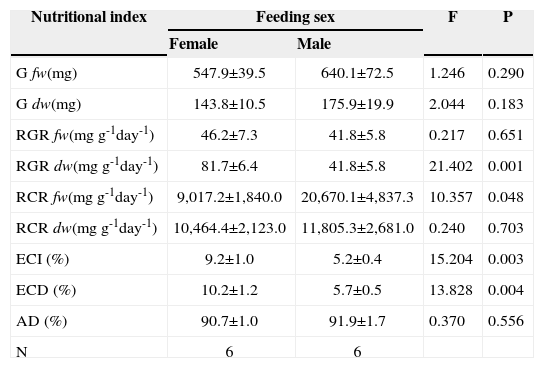
It is believed that by displaying sex-biased feeding preferences, herbivorous organisms may have a better performance related to the nutritional quality of dioecious plants. To determine the possible effect of the sex of B. cordataon nutritional efficiencies and growth of A. mexicanaria,we compared several nutritional indices between caterpillars fed with either male or female tree leaves. We observed that compared to caterpillars fed with male tree leaves, the relative growth rate (in dry weight; RGRdw), the efficiency of conversion of ingested food to biomass (ECI) and the efficiency of conversion of digested food to biomass (ECD) were significantly higher in caterpillars fed with leaves from female trees (Table 1). In contrast, the relative consumption rate in fresh weight (RCRfw) of caterpillars fed with male tree leaves was significantly higher than for caterpillars fed with female ones. The other indices (weight gain in dry and fresh weight, relative growth rate in fresh weight, relative consumption rate in dry weight, and approximate digestibility) did not show significant differences between caterpillars fed with leaves of either sex (Table 1).
Repeated measures Anova evaluating, in fresh weight (fw) and in dry weight (dw), weight gain (G), relative growth rate (RGR), relative consumption rate (RCR), efficiency of conversion of ingested food to biomass (ECI), efficiency of conversion of digested food to biomass (ECD), and approximate digestibility (AD) of fifth instars of A. mexicanariafed with male and female tree leaves during 6 days
| Nutritional index | Feeding sex | F | P | |
|---|---|---|---|---|
| Female | Male | |||
| G fw(mg) | 547.9±39.5 | 640.1±72.5 | 1.246 | 0.290 |
| G dw(mg) | 143.8±10.5 | 175.9±19.9 | 2.044 | 0.183 |
| RGR fw(mg g-1day-1) | 46.2±7.3 | 41.8±5.8 | 0.217 | 0.651 |
| RGR dw(mg g-1day-1) | 81.7±6.4 | 41.8±5.8 | 21.402 | 0.001 |
| RCR fw(mg g-1day-1) | 9,017.2±1,840.0 | 20,670.1±4,837.3 | 10.357 | 0.048 |
| RCR dw(mg g-1day-1) | 10,464.4±2,123.0 | 11,805.3±2,681.0 | 0.240 | 0.703 |
| ECI (%) | 9.2±1.0 | 5.2±0.4 | 15.204 | 0.003 |
| ECD (%) | 10.2±1.2 | 5.7±0.5 | 13.828 | 0.004 |
| AD (%) | 90.7±1.0 | 91.9±1.7 | 0.370 | 0.556 |
| N | 6 | 6 | ||
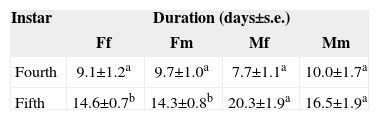
The performance of an organism may be influenced by the adjustment of an organism's growth. To identify the possible effects of the sex of tree leaves on the growth of A. mexicanaria, we compared the weight gain and development time of caterpillars collected from and fed with leaves from different tree sexes. Caterpillars did not present differences in weight gain based on the sex of their tree of origin (F1,37= 2.117, p= 0.154) (Table 1). Neither was weight gain influenced by the sex of the tree leaves used for feeding (F1,37= 1.078, p= 0.306) (Table 1). The interaction of origin × sex of the tree leaves used for feeding did not influence weight gain either (F1, 37= 0.677, p= 0.416). In contrast, we found that fifth, but not fourth, instar caterpillars collected from female trees developed faster than caterpillars found on male plants. This was observed in the marginally meaningful effect of origin on instar duration (p= 0.070) and on the meaningful interaction of caterpillar origin × instar (p= 0.017). In addition, the sex of the tree leaves used for feeding marginally influenced the development time of fifth instar caterpillars, as observed in the interaction of leaves used for feeding according to tree sex × instar (p= 0.065) (Table 2).
Duration of fourth and fifth instar of A. mexicanaria(days±s. e.) collected from female and male trees of B. cordata(Fand M) and fed with leaves from male and female trees (mand f)
| Instar | Duration (days±s.e.) | |||
|---|---|---|---|---|
| Ff | Fm | Mf | Mm | |
| Fourth | 9.1±1.2a | 9.7±1.0a | 7.7±1.1a | 10.0±1.7a |
| Fifth | 14.6±0.7b | 14.3±0.8b | 20.3±1.9a | 16.5±1.9a |
Different letters denote significant differences between male and female source trees for fifth instar caterpillars, with p< 0.05 (Tukey test).NFf= 7, NFm= 9, NMf= 6 and NMm= 6.
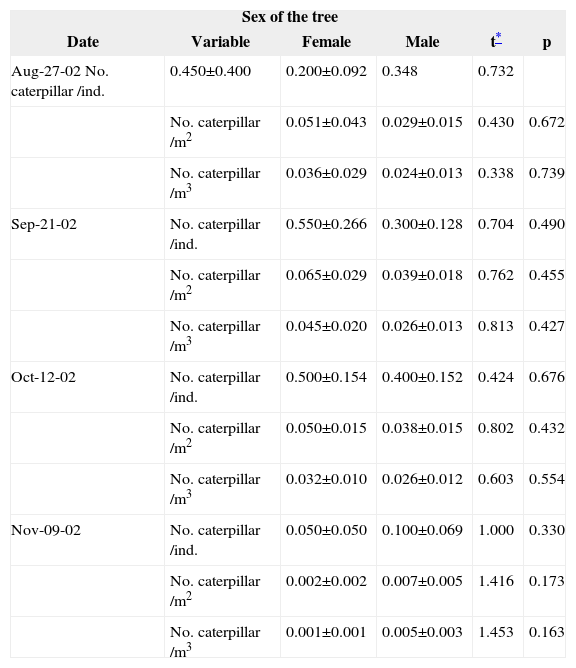
If the sex of the host is an important factor for oviposition selection, different caterpillar densities should be expected on trees of different sexes. We tested this notion by comparing the density of caterpillars on male-female tree pairs over a 3-week period (Table 3). No differences were found between the caterpillar densities on female or male trees on any date of observation.
Comparison of caterpillar density (mean±s.e.) on female and male trees of B. cordata(d.f.= 19).
| Sex of the tree | |||||
|---|---|---|---|---|---|
| Date | Variable | Female | Male | t* | p |
| Aug-27-02 No. caterpillar /ind. | 0.450±0.400 | 0.200±0.092 | 0.348 | 0.732 | |
| No. caterpillar /m2 | 0.051±0.043 | 0.029±0.015 | 0.430 | 0.672 | |
| No. caterpillar /m3 | 0.036±0.029 | 0.024±0.013 | 0.338 | 0.739 | |
| Sep-21-02 | No. caterpillar /ind. | 0.550±0.266 | 0.300±0.128 | 0.704 | 0.490 |
| No. caterpillar /m2 | 0.065±0.029 | 0.039±0.018 | 0.762 | 0.455 | |
| No. caterpillar /m3 | 0.045±0.020 | 0.026±0.013 | 0.813 | 0.427 | |
| Oct-12-02 | No. caterpillar /ind. | 0.500±0.154 | 0.400±0.152 | 0.424 | 0.676 |
| No. caterpillar /m2 | 0.050±0.015 | 0.038±0.015 | 0.802 | 0.432 | |
| No. caterpillar /m3 | 0.032±0.010 | 0.026±0.012 | 0.603 | 0.554 | |
| Nov-09-02 | No. caterpillar /ind. | 0.050±0.050 | 0.100±0.069 | 1.000 | 0.330 |
| No. caterpillar /m2 | 0.002±0.002 | 0.007±0.005 | 1.416 | 0.173 | |
| No. caterpillar /m3 | 0.001±0.001 | 0.005±0.003 | 1.453 | 0.163 | |
Much still remains to be explored in the field of plant-insect interactions and their ecological consequences. One of these issues is the effect that foraging on male or female plants has on an insect's performance. We found that caterpillars of A. mexicanariaseem to prefer feeding on female plants rather than on male plants of B. cordata,under laboratory conditions.It is thus possible that the nutritional quality of the leaves differs between trees of either sex. If the nutritional quality of leaves is indeed different depending on the sex of the plant, one would expect higher metabolic efficiencies in insects fed with leaves of higher nutritional quality (higher content of nitrogen, proteins, carbohydrates, lipids, and other nutrients) (Slansky Jr., 1993). We found higher nutritional efficiencies (efficiency of conversion of ingested food to biomass and efficiency of conversion of digested food to biomass; ECIand ECDrespectively) in caterpillars fed with female tree leaves, thus suggesting that the nutritional quality of female tree leaves may indeed be higher than that of male trees. Similarly, the relative consumption rate in fresh weight (RCRfw) was higher in caterpillars fed with male tree leaves, as would be expected from a poorer nutrient content in leaves of male trees, to which caterpillars respond by consuming more food to compensate the difference. This feeding behavior has been well documented for several caterpillar species (Cano- Santana & Oyama, 1992; Simpson & Simpson, 1990; Slansky Jr., 1993). We also found that the relative consumption rate in dry weight (RCRdw) was the same for caterpillars fed with either male or female tree leaves. It is thus likely that if the nutritional quality of leaves differs in a sex-dependent fashion, this difference is caused by a higher nutrient concentration in female leaves. Similarly, we found that fifth instar caterpillars fed with female tree leaves had a higher relative growth rate (RGRdw) than those fed with male tree leaves. This could also reflect the post-metabolic advantage of better nutritional efficiencies (ECIand ECD) when caterpillars feed on female tree leaves.
In order to optimize food use, some insects can regulate their metabolism or feeding behavior, in a non-reversible fashion, at a given developmental stage. If sex-based feeding experience modifies the feeding behavior of A. mexicanaria,a preference towards the tree leaves of the same sex the caterpillar was feeding on before collection, would be expected, in a feeding preference assay. However, we found that caterpillars preferred feeding on female tree leaves, no matter the sex of the tree from which they were collected. In addition, we found that caterpillars gained weight equally well when fed with tree leaves of the same or different sex than that of the trees from which they were collected. This result thus suggests that previous feeding experiences of A. mexicanariado not alter the capacity of the caterpillars to convert ingested food to biomass. In contrast, we found that fifth, but not fourth, instar caterpillars collected from female trees grew faster than instars collected from male plants, no matter the sex of the tree leaves used for feeding. It is thus possible that female trees possess compounds perceived by fifth instar caterpillars, capable of accelerating their growth independently of a caterpillar's final biomass.Slansky Jr.(1993)has reviewed the existence of such instar-specific metabolic differences.
We found that the density of caterpillars on male and female trees was the same. It thus appears that the oviposition preferences of A. mexicanariado not depend on the sex of the tree. However, the effects of other factors, such as the presence of other herbivores, predators and parasitoids, or differences in temperature, levels of light, humidity, and nutrient concentration, etc., on oviposition preferences cannot be ruled out (Pilson, 1992). It is consequently unclear whether dioecism affects the selection of the tree for oviposition. This issue warrants further investigation.
Male plants tend to allocate more resources to growth than to defense against herbivores, whereas female plants assign more resources to self-defense than to growth (Ågren et al., 1999; Cepeda & Dirzo, 2002, 2010; Cornelissen & Stiling, 2005). This difference in resource allocation may explain, at least in part, the fact that herbivores usually prefer to feed on male plants, since they can be more nutritious (reviewed by Ågren et al., 1999; Cepeda & Dirzo, 2010). In contrast, we found that A. mexicanariacaterpillars preferred female plants of B. cordata. Since the caterpillars appear during the flowering season of B. cordata, it is possible that resource assignment to nutritional reserves could be greater in female trees because they will eventually produce fruit. In fact, Cepeda and Dirzo (2010)found that depending on the reproductive season it is the reproductive allocation (biomass and nutrients) in dioecious plants. Overall, the results presented in this report indicate that the sex of B. cordataaffects the performance of A. mexicanaria.Therefore, differences in the nutritional quality of female and male trees of B. cordatamay explain differences in caterpillar performance. A direct assessment of nutrient content in either sex should be conducted. The study of the causes and effects of sex-biased herbivory will surely bring forth many exciting findings in the future.
We thank Julio Arriaga Romero, Alejandro Hunab Molina Vázquez and Esteban Zamorán Pineda for their invaluable help in the field. We thank Xóchitl Ponce Wainer for the insightful English revision and observations. We thank Erick García-García for his valuable observations. We thank Paolo Fontana for his priceless suggestions. Thanks to Marco Antonio Romero-Romero for the technical support. We thank professors Jorge Meave del Castillo, Ma. Teresa Valverde Valdés, Consuelo Bonfil Sanders and Silvia Castillo Arguero for their comments on this study, and Claudia González Cortés, Gabriela Castaño Meneses, Adolfo Ibarra, Ernesto Velázquez Montes, Rosa María Fonseca Juárez, and Eduardo Cuevas Miranda for their suggestions.