Mexico is considered a mammal diversity hotspot, and most conservation efforts involving mammals focus on large and charismatic species. Herein, we provide an assessment of the conservation status of species that are often overlooked in conservation programs, Mexican rodents of the families Geomyidae (pocket gophers) and Heteromyidae (pocket mice and kangaroo rats). Based on distributional maps and recent systematic studies, a taxonomic and biogeographical distributional checklist was made. The conservation lists of the International Union for Conservation of Nature and the Mexican Secretaría de Medio Ambiente y Recursos Naturales were used to identify 8 geomyid species, and 8 species and 27 subspecies of heteromyids as endangered. Major threats to their conservation are change in land use, destruction of habitat and a lack of knowledge about their current distribution and population trends.
México es una región del mundo con un elevado nivel de diversidad de mamíferos y la mayoría de los esfuerzos de conservación se concentran en las especies grandes, más carismáticas. Aquí, proveemos una evaluación del estado de conservación de especies que generalmente no son consideradas, los roedores mexicanos de las familias Geomyidae and Heteromyidae. Basados en mapas de distribución y estudios sistemáticos recientes, se preparó una lista taxonómica y de distribución biogeográfica de los roedores geómidos y heterómidos de México. Las listas de conservación de la Unión Internacional para la Conservación de la Naturaleza y la Secretaría de Medio Ambiente y Recursos Naturales de México se utilizaron para identificar 8 taxa de geómidos, 8 especies y 27 subespecies de heterómidos en peligro de extinción. Las principales amenazas para su conservación son: cambio en el uso de suelo, la destrucción del habitat y la falta de conocimiento acerca de su distribución actual y sus tendencias poblacionales.
Rodentia is the most species-rich order in the class Mammalia. Carleton and Musser (2005) listed 2 277 species of rodents, representing more than 42% of the mammal species of the world. Each year the list of rodent species grows as new species are described and taxonomic revisions supported by new evidence, much of it molecular, are published. Based on the list reported by Ramírez-Pulido et al. (2008), 240 (48. 5%) of the 495 species of Mexican terrestrial mammals are rodents, and 169 (34.1%) are endemics. Despite the high diversity of rodents in México, there is a surprising deficit in the number of experts on Mexican rodents, especially those focused on neotropical and tropical rodent faunas (Amori and Gippoliti, 2003), as well as experts focused on Nearctic desert rodents and rodents such as pocket gophers (Geomyidae).
Rodents are an essential part of most natural communities because they often constitute the most important food item of carnivores in food chains. Many rodent species in México are directly important to humans as well because they serve as a food source, potential vector of diseases, or cause economic impact by direct or indirect damage to crops and croplands. Yet despite the ecological and economic importance of rodents in México, basic aspects of rodent biology are still poorly understood, including their life histories, ecological roles in the community, present demographic status of populations, and phylogenetic relationships. This lack of basic knowledge of rodent biology leaves rodent faunas vulnerable to extirpation, even extinction (Lidicker, 2007). Because of the usual negative perception by most people about rodents, it has been difficult to convince the public of the importance of rodent conservation. As a result, more than half of mammalian extinctions worldwide before 1999 were rodents (MacPhee and Flemming, 1999). In the last 20 years, only 2 initiatives (both efforts of the World Conservation Union, IUCN) have called attention to rodent species at risk of extinction in an effort to establish conservation programs worldwide (Lidicker, 1989) and in North America (north of México; Hafner et al., 1998).
Although México is among the top 3 countries in the world in terms of mammalian species diversity (Myers et al., 2000; Ceballos et al., 2002), Mexican rodents were not included in the 2 published surveys of rodent species of conservation concern (Lidicker, 1989; Hafner et al., 1998): no information was available for the first (worldwide) survey, and México was not included within the geographic scope of the latter survey. The current IUCN Red List of Threatened Species (IUCN, 2012), evaluated only at the species level, includes 81 rodent species of México. Fully 60 of these species are in the family Cricetidae, with 4 other families represented: Heteromyidae (8 species), Geomyidae (5), Sciuridae (7), and Dasyproctidae (1). Of the 81 total species, 17 are restricted to islands; several of these currently are considered subspecies of more widely distributed species. By focusing at the species level, the list is biased by uneven taxonomic application. When the IUCN evaluates Mexican rodents at the subspecies level, as done by Hafner et al. (1998) for North America (north of México), many restricted island forms will most likely be added to this growing list of endangered taxa. For example, 23 additional island-restricted heteromyid subspecies currently are included in the list of species at risk by the Mexican Secretaría de Medio Ambiente y Recursos Naturales (Semarnat, 2010). Clearly, the rodents of México need more attention from conservation-oriented scientists.
The natural history of certain rodent species makes them more prone to extinction than others (Rabinowitz, 1981). Species susceptible to extinction are characterized by small, often fragmented, populations located in small geographic areas. The patchy distributions of these species often result from their high habitat specificity. Close proximity of preferred habitat to urban developments or to areas of soil suitable for agricultural conversion increases the likelihood of extinction.
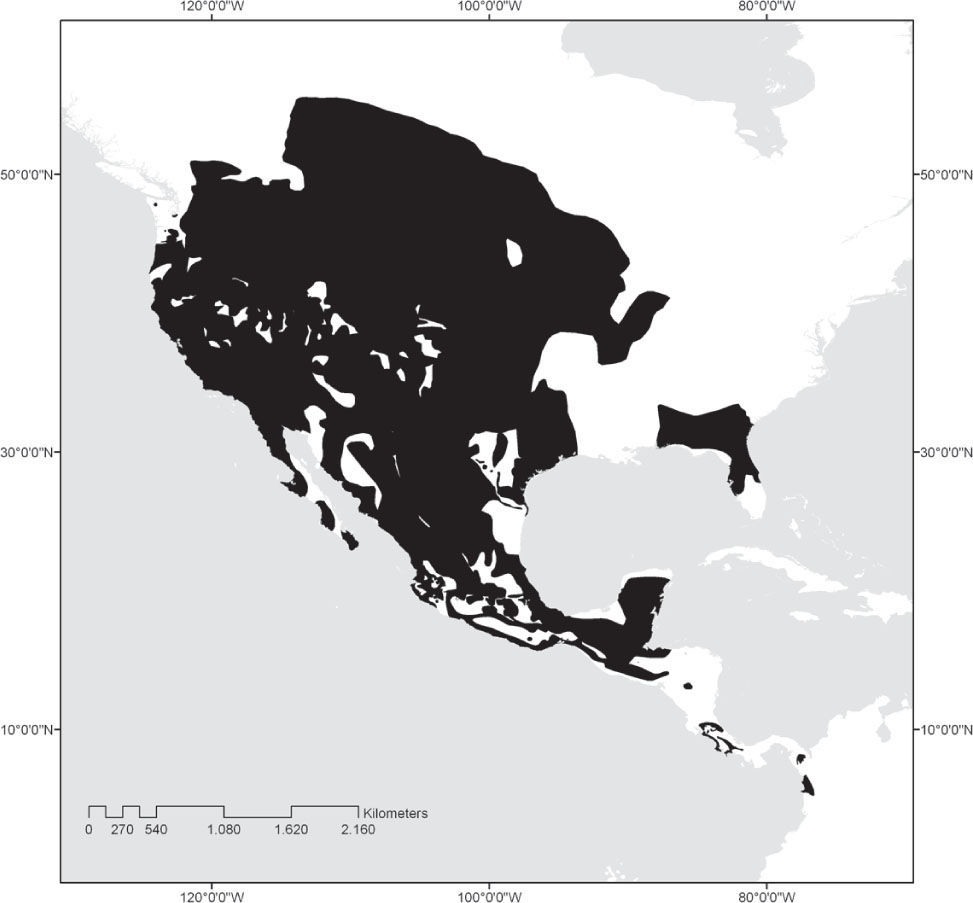
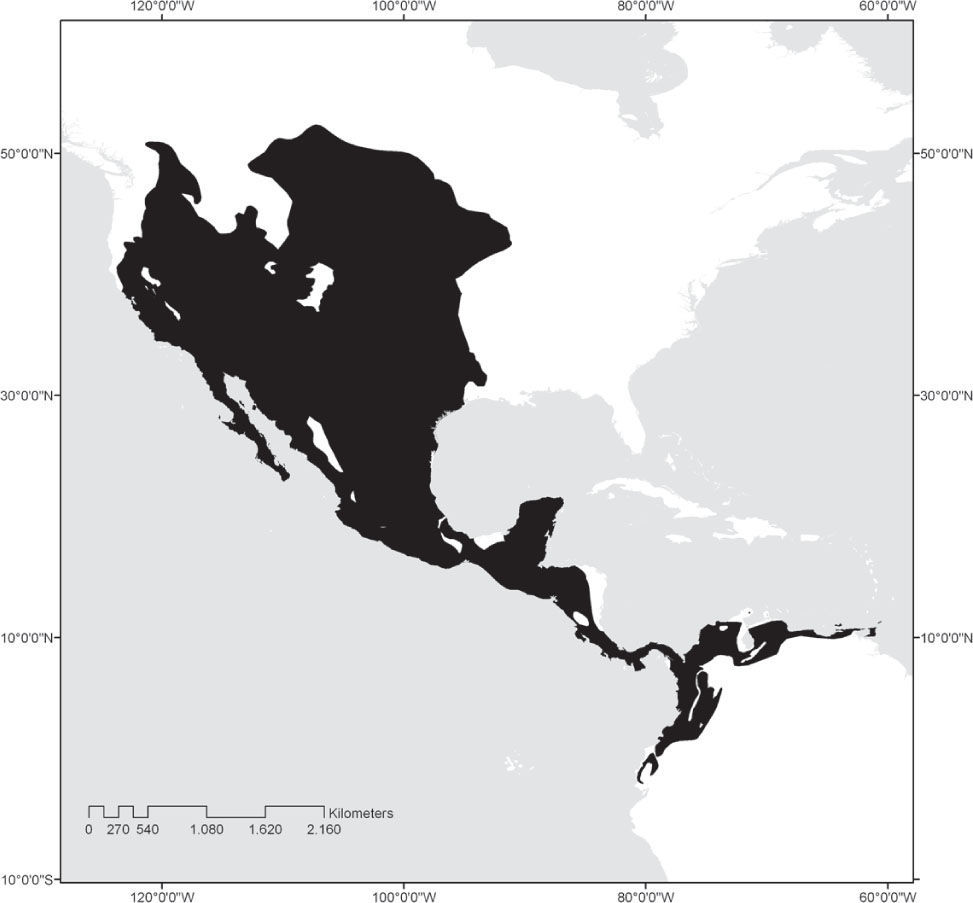
Pocket gophers of the rodent family Geomyidae and pocket mice and kangaroo rats of the family Heteromyidae exhibit natural history characteristics that make rodent species prone to extinction. Pocket gophers live in small, isolated populations often located near areas of urban development or in fertile valleys often converted to agricultural production (Hall, 1981; Hafner et al., 2004, 2005), while arid-adapted heteromyids occupy many small, arid islands near the western coast of México as well as regions that are being subjected to rapid agricultural conversion. Most species in these families are endemic to the southern part of North America, and their distribution is mainly in the United States and México, although some species range into Central America and northern South America (Fig. 1). There are currently 40 named species of pocket gophers (Geomyidae), of which 20 occur in México (Table 1). Thirteen of these 20 species (nearly one-third of all extant pocket gopher species) are endemic to México. The family Heteromyidae shows a similarly high level of endemism in México. The family contains 60 named species, of which 39 (65.0%) occur in México and 12 (one-fifth of all living heteromyids) are endemic to México (Table 2).
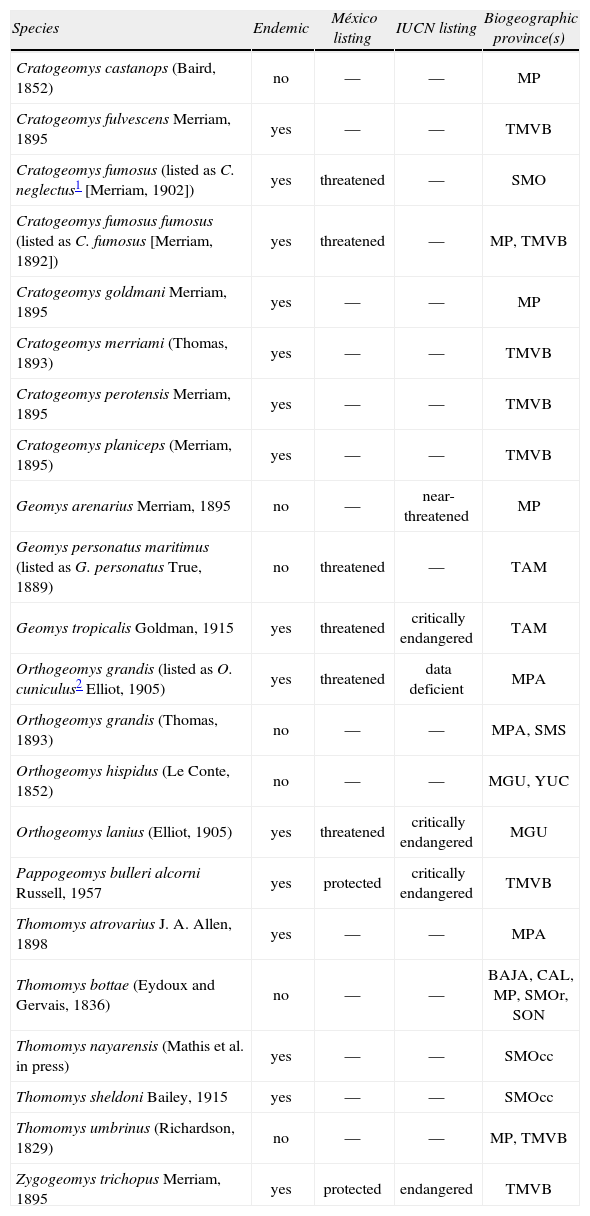
Distribution and conservation status of Mexican mammals of the family Geomyidae. Conservation status in México is based on Semarnat (2010) and on international basis according to the IUCN (2012). Biogeographic provinces are defined following Morrone et al. (2005). The taxonomic list is modified from Patton (2005a) to include 6 additional pocket gopher species endemic to México (Cratogeomys planiceps [Hafner et al., 2004], C. fulvescens and C. perotensis [Hafner et al., 2005], Thomomys atrovarius [Hafner et al., 2011), T. sheldoni [Mathis et al., 2013a], and T. nayarensis [Mathis et al., 2013b]) and excludes 6 endemic taxa now recognized as junior synonyms of other species (C. gymnurus, C. neglectus, C. tylorhinus, and C. zinseri [Hafner et al., 2004], Pappogeomys alcorni [Hafner et al., 2009], and Orthogeomys cuniculus [Hafner et al., in press]). Biogeographic provinces are: Baja California (BAJA), California (CAL), Mexican Plateau (MP), Mexican Gulf (MGU), Mexican Pacific Coast (MPA), Sierra Madre Oriental (SMO), Sierra Madre Occidental (SMOcc), Sonora (SON), Tamaulipas (TAM), Trans-Mexico Volcanic Belt (TMVB), and Yucatán (YUC)
| Species | Endemic | México listing | IUCN listing | Biogeographic province(s) |
| Cratogeomys castanops (Baird, 1852) | no | — | — | MP |
| Cratogeomys fulvescens Merriam, 1895 | yes | — | — | TMVB |
| Cratogeomys fumosus (listed as C. neglectus1 [Merriam, 1902]) | yes | threatened | — | SMO |
| Cratogeomys fumosus fumosus (listed as C. fumosus [Merriam, 1892]) | yes | threatened | — | MP, TMVB |
| Cratogeomys goldmani Merriam, 1895 | yes | — | — | MP |
| Cratogeomys merriami (Thomas, 1893) | yes | — | — | TMVB |
| Cratogeomys perotensis Merriam, 1895 | yes | — | — | TMVB |
| Cratogeomys planiceps (Merriam, 1895) | yes | — | — | TMVB |
| Geomys arenarius Merriam, 1895 | no | — | near-threatened | MP |
| Geomys personatus maritimus (listed as G. personatus True, 1889) | no | threatened | — | TAM |
| Geomys tropicalis Goldman, 1915 | yes | threatened | critically endangered | TAM |
| Orthogeomys grandis (listed as O. cuniculus2 Elliot, 1905) | yes | threatened | data deficient | MPA |
| Orthogeomys grandis (Thomas, 1893) | no | — | — | MPA, SMS |
| Orthogeomys hispidus (Le Conte, 1852) | no | — | — | MGU, YUC |
| Orthogeomys lanius (Elliot, 1905) | yes | threatened | critically endangered | MGU |
| Pappogeomys bulleri alcorni Russell, 1957 | yes | protected | critically endangered | TMVB |
| Thomomys atrovarius J. A. Allen, 1898 | yes | — | — | MPA |
| Thomomys bottae (Eydoux and Gervais, 1836) | no | — | — | BAJA, CAL, MP, SMOr, SON |
| Thomomys nayarensis (Mathis et al. in press) | yes | — | — | SMOcc |
| Thomomys sheldoni Bailey, 1915 | yes | — | — | SMOcc |
| Thomomys umbrinus (Richardson, 1829) | no | — | — | MP, TMVB |
| Zygogeomys trichopus Merriam, 1895 | yes | protected | endangered | TMVB |
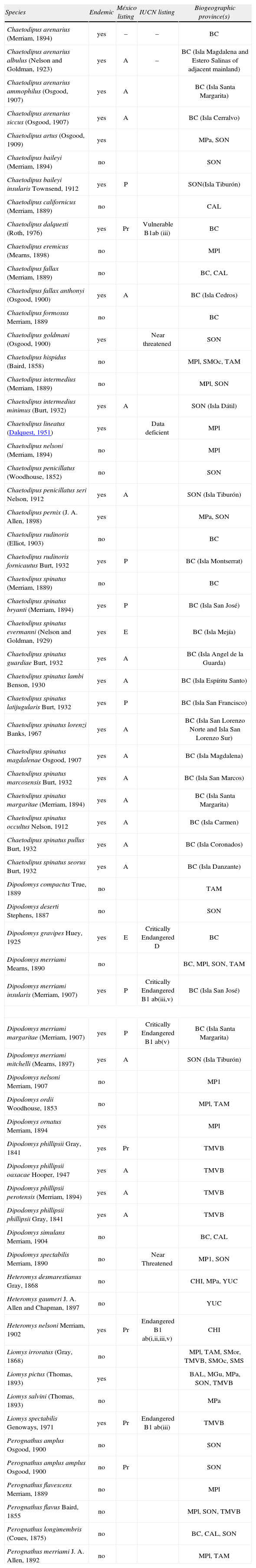
Distribution and conservation status of Mexican mammals of the family Heteromyidae. Conservation status in México is based on Semarnat (2010) and on international basis according to the IUCN (2012). Biogeographic provinces are defined following Morrone et al. (2005). The taxonomic list is modified from Patton (2005b) to include 1 additional species endemic to México (Dipodomys ornatus; Fernández et al., 2012). Biogeographic provinces are: Baja California (BAJA), California (CAL), Mexican Plateau (MP), Mexican Gulf (MGU), Mexican Pacific Coast (MPA), Sierra Madre Oriental (SMO), Sierra Madre Occidental (SMOcc), Sonora (SON), Tamaulipas (TAM), Trans-México Volcanic Belt (TMVB), and Yucatán (YUC)
| Species | Endemic | México listing | IUCN listing | Biogeographic province(s) |
| Chaetodipus arenarius (Merriam, 1894) | yes | – | – | BC |
| Chaetodipus arenarius albulus (Nelson and Goldman, 1923) | yes | A | – | BC (Isla Magdalena and Estero Salinas of adjacent mainland) |
| Chaetodipus arenarius ammophilus (Osgood, 1907) | yes | A | BC (Isla Santa Margarita) | |
| Chaetodipus arenarius siccus (Osgood, 1907) | yes | A | BC (Isla Cerralvo) | |
| Chaetodipus artus (Osgood, 1909) | yes | MPa, SON | ||
| Chaetodipus baileyi (Merriam, 1894) | no | SON | ||
| Chaetodipus baileyi insularis Townsend, 1912 | yes | P | SON(Isla Tiburón) | |
| Chaetodipus californicus (Merriam, 1889) | no | CAL | ||
| Chaetodipus dalquesti (Roth, 1976) | yes | Pr | Vulnerable B1ab (iii) | BC |
| Chaetodipus eremicus (Mearns, 1898) | no | MPl | ||
| Chaetodipus fallax (Merriam, 1889) | no | BC, CAL | ||
| Chaetodipus fallax anthonyi (Osgood, 1900) | yes | A | BC (Isla Cedros) | |
| Chaetodipus formosus Merriam, 1889 | no | BC | ||
| Chaetodipus goldmani (Osgood, 1900) | yes | Near threatened | SON | |
| Chaetodipus hispidus (Baird, 1858) | no | MPl, SMOc, TAM | ||
| Chaetodipus intermedius (Merriam, 1889) | no | MPl, SON | ||
| Chaetodipus intermedius minimus (Burt, 1932) | yes | A | SON (Isla Dátil) | |
| Chaetodipus lineatus (Dalquest, 1951) | yes | Data deficient | MPl | |
| Chaetodipus nelsoni (Merriam, 1894) | no | MPl | ||
| Chaetodipus penicillatus (Woodhouse, 1852) | no | SON | ||
| Chaetodipus penicillatus seri Nelson, 1912 | yes | A | SON (Isla Tiburón) | |
| Chaetodipus pernix (J. A. Allen, 1898) | yes | MPa, SON | ||
| Chaetodipus rudinoris (Elliot, 1903) | no | BC | ||
| Chaetodipus rudinoris fornicautus Burt, 1932 | yes | P | BC (Isla Montserrat) | |
| Chaetodipus spinatus (Merriam, 1889) | no | BC | ||
| Chaetodipus spinatus bryanti (Merriam, 1894) | yes | P | BC (Isla San José) | |
| Chaetodipus spinatus evermanni (Nelson and Goldman, 1929) | yes | E | BC (Isla Mejía) | |
| Chaetodipus spinatus guardiae Burt, 1932 | yes | A | BC (Isla Angel de la Guarda) | |
| Chaetodipus spinatus lambi Benson, 1930 | yes | A | BC (Isla Espíritu Santo) | |
| Chaetodipus spinatus latijugularis Burt, 1932 | yes | P | BC (Isla San Francisco) | |
| Chaetodipus spinatus lorenzi Banks, 1967 | yes | A | BC (Isla San Lorenzo Norte and Isla San Lorenzo Sur) | |
| Chaetodipus spinatus magdalenae Osgood, 1907 | yes | A | BC (Isla Magdalena) | |
| Chaetodipus spinatus marcosensis Burt, 1932 | yes | A | BC (Isla San Marcos) | |
| Chaetodipus spinatus margaritae (Merriam, 1894) | yes | A | BC (Isla Santa Margarita) | |
| Chaetodipus spinatus occultus Nelson, 1912 | yes | A | BC (Isla Carmen) | |
| Chaetodipus spinatus pullus Burt, 1932 | yes | A | BC (Isla Coronados) | |
| Chaetodipus spinatus seorus Burt, 1932 | yes | A | BC (Isla Danzante) | |
| Dipodomys compactus True, 1889 | no | TAM | ||
| Dipodomys deserti Stephens, 1887 | no | SON | ||
| Dipodomys gravipes Huey, 1925 | yes | E | Critically Endangered D | BC |
| Dipodomys merriami Mearns, 1890 | no | BC, MPl, SON, TAM | ||
| Dipodomys merriami insularis (Merriam, 1907) | yes | P | Critically Endangered B1 ab(iii,v) | BC (Isla San José) |
| Dipodomys merriami margaritae (Merriam, 1907) | yes | P | Critically Endangered B1 ab(v) | BC (Isla Santa Margarita) |
| Dipodomys merriami mitchelli (Mearns, 1897) | yes | A | SON (Isla Tiburón) | |
| Dipodomys nelsoni Merriam, 1907 | no | MP1 | ||
| Dipodomys ordii Woodhouse, 1853 | no | MPl, TAM | ||
| Dipodomys ornatus Merriam, 1894 | yes | MPl | ||
| Dipodomys phillipsii Gray, 1841 | yes | Pr | TMVB | |
| Dipodomys phillipsii oaxacae Hooper, 1947 | yes | A | TMVB | |
| Dipodomys phillipsii perotensis (Merriam, 1894) | yes | A | TMVB | |
| Dipodomys phillipsii phillipsii Gray, 1841 | yes | A | TMVB | |
| Dipodomys simulans Merriam, 1904 | no | BC, CAL | ||
| Dipodomys spectabilis Merriam, 1890 | no | Near Threatened | MP1, SON | |
| Heteromys desmarestianus Gray, 1868 | no | CHI, MPa, YUC | ||
| Heteromys gaumeri J. A. Allen and Chapman, 1897 | no | YUC | ||
| Heteromys nelsoni Merriam, 1902 | yes | Pr | Endangered B1 ab(i,ii,iii,v) | CHI |
| Liomys irroratus (Gray, 1868) | no | MPl, TAM, SMor, TMVB, SMOc, SMS | ||
| Liomys pictus (Thomas, 1893) | yes | BAL, MGu, MPa, SON, TMVB | ||
| Liomys salvini (Thomas, 1893) | no | MPa | ||
| Liomys spectabilis Genoways, 1971 | yes | Pr | Endangered B1 ab(iii) | TMVB |
| Perognathus amplus Osgood, 1900 | no | SON | ||
| Perognathus amplus amplus Osgood, 1900 | no | Pr | SON | |
| Perognathus flavescens Merriam, 1889 | no | MPl | ||
| Perognathus flavus Baird, 1855 | no | MPl, SON, TMVB | ||
| Perognathus longimembris (Coues, 1875) | no | BC, CAL, SON | ||
| Perognathus merriami J. A. Allen, 1892 | no | MPl, TAM | ||
Hafner and Hafner (2009) recognized central México as the probable center of diversification of the family Geomyidae. Most Mexican species inhabit tropical, subtropical, or forested temperate regions in the Trans-México Volcanic Belt (TMVB), and the Sierra Madre del Sur, although a few species live in the shrub-covered deserts of the Mexican Plateau. The most speciose genera are Cratogeomys and Thomomys with 7 and 5 species, respectively, followed by Orthogeomys (4 species), Geomys (3 species), and Pappogeomys and Zygogeomys with 1 species each. Pocket gophers are well equipped for their subterranean lifestyle, having cylindrical bodies, reduced eyes, and strong and well-developed forearms for digging. They possess external, fur-lined cheek pouches in which they transport food items, particularly roots. They prefer habitats with deep soils, which allow them to dig deep, spacious burrow systems (Stein, 2000). All geomyid species are solitary and agressive, coming together only to mate.
Family HeteromyidaeHeteromyid rodents originated and evolved for 35 million years in North America, spreading into South America only during the last 3 million years (Schmidly et al., 1993). Although the family ranges from southern Canada to northern Colombia and Venezuela (Hall, 1981; Fig. 1), the most speciose genera (kangaroo rats, Dipodomys, 20 species; coarse-haired pocket mice, Chaetodipus, 17 species; and silky pocket mice, Perognathus, 9 species) reach their greatest diversity in the regional deserts of North America, the core of which are in northern México. The 2 species of kangaroo mice are similarly restricted to arid regions (Great Basin of western United States), as is the most widespread species of spiny pocket mouse, Liomys irroratus. The other 4 species of Liomys extend from xeric thorn-scrub habitat into tropical interior basins and savannah. While the 7 species of forest spiny pocket mice (Heteromys) inhabit mesic situations in lowland rainforest, lower montane wet forest and cloud forest, and lower montane dry forests of southern México, Central America, and northern South America (Genoways, 1973; Hafner et al. 2007 found Liomys paraphyletic relative to Heteromys, and placed Liomys in synonymy with Heteromys. However, we herein continue to employ Liomys pending inclusive analysis of all species within the 2 genera).
Most heteromyids retain a conservative scansorial body form (pocket mice of the genera Perognathus, Chaetodipus, Liomys, and Heteromys), in marked contrast to the richochetal forms of kangaroo rats and mice (Dipodomys and Microdipodops; Hafner, 1993). Body size ranges from the diminutive Perognathus (8 g) to the largest kangaroo rats (180g; Brylski, 1993). All species are nocturnal and solitary, have small ears, and possess external, fur-lined cheek pouches (unique to the Heteromyidae and Geomyidae) for transport of seeds, which for nearly all heteromyid species makes up the bulk of their diet (Jones, 1993; Reichman and Price, 1993).
As noted elsewhere (Hafner et al., 1998), conservation of species far more charismatic than rodents often depends first and foremost on preservation of the rodent community that sustains them. Numerous and widespread mammals, rodents often are highly adapted to narrowly defined habitats, such that the diversity of rodents reflects the diversity of available habitats, and the status of a specific rodent species often provides a measure of the health of a specific habitat. Therefore, to highlight the importance of this component of biodiversity, herein we list the taxa of geomyid and heteromyid rodents of México, indicate those that have been recognized as threatened or endangered, note possible modifications to this list, and summarize the nature of the threat to each taxon.
Materials and methodsA list of species of geomyid and heteromyid rodents occurring in México was developed based on a review of the literature and web-based databases, in-press papers, and personal communications with colleagues. Taxonomy follows Patton (2005a, 2005b), with modifications made according to subsequent publications (Fernández et al, 2012; Hafner et al., 2004, 2005, 2008, 2009, 2011; Mathis et al., 2013a, 2013b; Rios and Álvarez-Castañeda, 2010; Rogers and Gonzalez, 2010). Species distributions are based on maps and records from Hall (1981) and Patton (2005a, 2005b); endemism and conservation status for each species are based on Semarnat (2010) and IUCN (2012); biogeographic provinces are as defined by Morrone et al. (2002) and Morrone (2005).
ResultsIn Table 1, a current checklist of Mexican species of pocket gophers is provided, it includes their distributions within the biogeographic provinces defined by Morrone et al. (2002) and Morrone (2005). Twelve of the 20 species of Mexican geomyids occur in only a single biogeographic province, and 19 of 20 occur in only 1 or 2 biogeographic provinces. The single exception to this trend is Thomomys bottae, which is distributed predominantly north of México, where it likewise occurs in a wide variety of habitats. The highest diversity of geomyids is in central México (8 of 20 species occur in the TMVB biogeographic province; Table 1), and 7 of the 13 pocket gopher species endemic to México live in the TMVB biogeographic province.
The Mexican government lists 8 Mexican pocket gopher species as either threatened or protected (Semarnat, 2010), and 6 of these also are listed as either threatened or endangered on the IUCN red list (IUCN, 2012).
México is the geographic center of diversity for the family Heteromyidae (39 of 60 recognized species) and particularly of the genera Chaetodipus (all 17 species) and Liomys (4 of 5 species). The highest diversity of heteromyid rodents in México occurs in the northern deserts (BC, SON, and MPl provinces; Table 2). Of the 39 species of Heteromyidae that occur in México (Table 2), 24 occur in a single biogeographic province, and 32 occur in only 1 or 2 provinces. Some exceptions must be highlighted like the widespread D. merriami (4 provinces), L. pictus (5 provinces), and L. irroratus (6 provinces).
Currently, 8 species and 27 subspecies of heteromyid rodents are considered to be of conservation concern by either the IUCN (2012) or Semarnat (2010; Table 2).
DiscussionAlthough both heteromyid and geomyid rodents are conspicuous and widespread components of the rodent fauna of México, many of the endemic species and subspecies occur only in small, isolated distributions, including both figurative islands of preferred habitat (Geomyidae) and literal islands near the Pacific coast and in the Gulf of California. As mentioned earlier, many species are endemic to México, and present their largest distribution within the country. A few species have only a small distribution within the Mexican Republic, but occupy extensive areas outside of the country. As evidenced by recent publications and ongoing studies, it is likely that further investigation into the phylogeography of particularly widespread species will reveal cryptic evolutionary lineages, and therefore possibly alter conservation priorities.
Family GeomyidaeMost people in México have little or no knowledge of pocket gophers, and those familiar with “tuzas” (local name in spanish for pocket gophers) seem to view them as a single, widespread species distributed throughout México. Most human encounters with pocket gophers occur when pocket gophers consume family crops, so it is not surprising that support for pocket gopher conservation in México is not a priority. In their survey of threatened rodents of North America (north of México), Hafner et al. (1998) included United States populations of 3 species of pocket gophers that also occur in México: Thomomys umbrinus, Geomys arenarius, and G. personatus. The current ICUN Red List of Threatened Species (2012) includes G. arenarius and 4 additional species of Mexican geomyids, G. tropicalis, Orthogeomys lanius, Pappogeomys alcorni, and the monotypic genus Zygogeomys.
More than 50% of the Mexican geomyids are confined to a single biogeographic province, and almost a 100% are distributed in only 1 or 2 biogeographic provinces. The high diversity of geomyids in central México (Table 1) appears to be the result of the complex topography of central México combined with the confluence of 3 major habitat types (boreal forest, high desert, and tropical forest) in this region where the Nearctic and Neotropical biogeographical provinces meet (Corona et al., 2007). The high ecological and physiographic diversity of the TMVB undoubtedly has created many opportunities for isolation and diversification of geomyid clades. Not surprisingly, 7 of the 13 pocket gopher species endemic to México live in the TMVB biogeographic province, where high levels of endemism have been observed in many different animal and plant groups (Ramamoorthy et al., 1993; Strattersfield et al., 1998; Luna et al., 2007).
The following accounts present the current status of each of the 8 pocket gopher species listed by the Mexican government.
Zygogeomys trichopus (the Michoacán pocket gopher), endemic monotypic genus, is listed as “protected” by the Mexican government and “endangered” by the IUCN. Once an abundant species, Z. trichopus now has a very limited and fragmented distribution (less than 5 000km2) in the mountains of Michoacán. Reduction of the range of this species is believed to result from a combination of agricultural encroachment and elimination of the forest buffer separating Z. trichopus from its lower-elevation competitor, Cratogeomys fumosus (Hafner and Barkley, 1984). The planting of vast orchards of avocado (Persea americana) trees near known populations of Z. trichopus over the past 30 years has had an unknown impact on the pocket gophers. MSH and DJH visited 1 Z. trichopus locality on the side of Mt. Tancítaro in 1993 and noted abundant gopher activity in nearby avocado orchards. Studies of the continuing effects of agricultural encroachment and deforestation are critically needed to ensure the future of this endemic genus.
Geomys tropicalis (the tropical pocket gopher), endemic species, is listed as “threatened” by the Mexican government and “critically endangered” by the IUCN. Geomys tropicalis is restricted to a tiny region (<100km2) near the town of Altamira, Tamaulipas. Only small fragments of suitable pocket gopher habitat were found in the vicinity of Altamira in 1993 (MSH, personal observation). This species is, indeed, critically endangered, and studies of the density and distribution of G. tropicalis populations are urgently needed to protect it from extinction.
Geomys arenarius (the desert pocket gopher) is not listed by the Mexican government but is listed as “near threatened” by the IUCN. We have observed that this species is still widespread in south-central New México (DJH, personal observation) but its dependence on sandy bottomlands subject to grazing and prolonged fire suppression may soon pose the threat of extinction. Populations are reportedly dense along the Rio Grande in southern New Mexico and in the vicinity of El Paso, Texas (IUCN, 2012), but prolonged periods of warming and drying threatens their continued existence. The species is known in México only along the Río Grande (= Río Bravo) in the vicinity of Júarez, Chihuahua, and the nearby Samalayuca sand dunes (Anderson, 1972; Ceballos and Oliva, 2005). Ceballos and Navarro (1991) considered these populations to be endangered based on their limited distribution in which they have suffered from overgrazing, industrial development, and urban growth. Populations in the Samalayuca dunes are afforded protection in a 632km2 portion of the dune field that was declared a Natural Protected Area by the Mexican government in 2009. The status of populations of G. arenarius in northern Chihuahua should be investigated to ascertain the level of risk faced by these populations.
Geomys personatus personatus (the Texas pocket gopher), subspecies of G. personatus, occurs along the coast of northern Tamaulipas and is listed as “threatened” by the Mexican government. Hafner et al. (1989) considered G. personatus to be “Lower Risk, near threatened” based on its restricted distribution in southern Texas and adjacent Tamaulipas, and 3 subspecies (G. p. fuscus, G. p. maritimus, and G. p. streckeri) to be threatened based on their restricted distributions in Texas. Although G. personatus is not currently listed by the IUCN, the report on this species states that at least 3 subspecies of G. personatus, including the Mexican subspecies, are threatened by continued habitat loss within their restricted ranges (IUCN, 2012). Given the extremely limited distribution of G. p. personatus in northern Tamaulipas and its dependence on loose, sandy soil that is being used for agricultural purposes, its continued listing by the Mexican government is appropriate. As with G. arenarius in northern Chihuahua, the status of populations of G. personatus in northern Chihuahua should be investigated to ascertain the level of risk faced by these populations.
Pappogeomys bulleri alcorni (Alcorn's pocket gopher), endemic subspecies, is listed as “protected” by the Mexican government and “critically endangered” by the IUCN. It occurs in a small region near the town of Mazamitla, Michoacán, where it comes into contact (or near contact) with the much larger and behaviorally dominant Cratogeomys fumosus. As with other threatened or endangered species of pocket gophers, the major threats to P. b. alcorni are fragmentation of its habitat for agricultural use, habitat loss through deforestation, and targeted killing by farmers. Demastes et al. (2002) trapped in agricultural fields near the type locality and captured only C. fumosus. In a more recent visit to this region (2009), specimens of P. b. alcorni were absent in agricultural fields but were abundant in the forested hillsides near the type locality (MSH and DJH, personal observation). P. b. alcorni, unlike most pocket gophers, appears to be a forest specialist (as are the other subspecies of P. bulleri;Hafner et al., 2009) and may be more widespread in this region than previously thought. A survey of the status of P. b. alcorni populations in the forests near its type locality may lead to reevaluate its risk status.
Cratogeomys fumosus neglectus (the Querétaro pocket gopher), endemic subspecies of pocket gopher, is listed as “threatened” by the Mexican government (as C. neglectus), but is not listed by the IUCN because it is a subspecies of a widespread species (C. fumosus) that is relatively common throughout its range (Hafner, et al., 2004; IUCN, 2012). The IUCN account for C. fumosus, however, refers to the subspecies neglectus and states that the isolated population in Querétaro is at risk. Cratogeomys fumosus neglectus is known from a very restricted area near the towns of Pinal de Amoles and La Cañada, Querétaro, and although it is often locally abundant where it occurs (León et al., 2001), its restricted distribution may necessitate continued protection by the Mexican government. A survey of the status of C. f. neglectus populations in the mountains surrounding its type locality is needed to clarify its current risk status.
Cratogeomys fumosus fumosus (the smoky pocket gopher), as with C. f. neglectus (see preceding account), C. f. fumosus is listed as “threatened” by the Mexican government (as C. fumosus), but is not listed by the IUCN because it is a subspecies of a widespread species that is not threatened over most of its range. However, the IUCN report for C. fumosus refers to the subspecies fumosus and states that populations of C. fumosus in the state of Colima are at risk. Hafner et al. (2004) used molecular evidence to show that C. f. fumosus is much more widespread in México that previously thought (e.g., Hall, 1981). Rather than being restricted to the vicinity of Colima city, C. f. fumosus is patchily distributed over much of western Michoacán and the eastern slopes of the Sierra Madre del Sur in Jalisco and Colima (Hafner et al., 2004). Where it occurs, populations of C. f. fumosus appear to be in good condition (MSH, personal observation), so continued listing of this subspecies by the Mexican government probably is unnecessary.
Orthogeomys lanius (the big pocket gopher), endemic species of pocket gopher, is listed as “threatened” by the Mexican government and “critically endangered” by the IUCN. Prior to 2013, O. lanius was known only from the type locality of Xuchil, Veracruz, where it was originally collected in 1904. However, the species was recently rediscovered in central Veracruz by M. S. Hafner, D. J. Hafner, and E. E. Gonzáles (manuscript in preparation) and mitochondrial DNA sampled from the newly discovered individuals is nearly identical to DNA extracted from the skin of 1 of the 109-year-old specimens. Phylogenetic analyses show O. lanius to be a valid species sister to O. hispidus. Observations in the field suggest that O. lanius is reasonably abundant in a roughly 1 000 km2 region of central Veracruz, where it persists in forested refugia often too steep and rugged for cultivation by humans. The actual distribution and abundance of O. lanius should be investigated through fieldwork in the area of its type locality.
Orthogeomys cuniculus (the Oaxacan pocket gopher), endemic pocket gopher, is listed as “threatened” by the Mexican government but “data deficient” by the IUCN. Recent molecular evidence based on DNA extracted from the skin of a paratype of O. cuniculus shows this taxon to be nested phylogenetically within specimens of O. grandis (M. S. Hafner, unpublished data). In January 2010, we found abundant pocket gophers activity in the vicinity of the type locality of O. cuniculus (Zanatepec, Oaxaca; MSH and DJH, personal observation), therefore, if future research confirms that O. cuniculus is a junior synonym of O. grandis, then there is no need to list this population of O. grandis as threatened or endangered.
Family HeteromyidaeThe rodents of nocturnal nature, mostly in sparsely populated desert regions, results in their generally being regarded as “mice,” although most people in rural communities recognize the “rata canguro” (local name in spanish) based on a hopping, long-tailed rodent caught in a light at night. If regarded at all, most heteromyid rodents are generally considered pests in a wasteland. As species that are tightly adapted to the North American regional deserts, however, the arid-adapted heteromyid rodents signal the state of health and threats to these unique biotic zones, the richest cores of which occur in México. Similarly, because of their ability to survive on arid islands along the Pacific coast and in the Gulf of California, the status of heteromyid rodents reflects the impacts of human-induced threats including exotic plant and animal introductions and habitat depletion. Moreover, the long evolutionary history of heteromyid rodents, particularly of the genus Chaetodipus in North American regional deserts has made them exceptional taxa in which to reconstruct the dynamic geological and biogeographic history of regional deserts (Fernández et al., 2012; Hafner and Riddle, 2011; Neiswenter and Riddle, 2010; Riddle and Hafner, 2006a,b; Riddle et al., 2000a,b). Similarly, phylogeographic studies of the genus Heteromys, spanning the Central American land bridge between North America and South America, should provide additional detail to the biogeographic history of that region.
México is the center of diversity for heteromyid rodents and since most of the members of this family are arid-adapted, it is not surprising that their highest diversity occurs in the deserts (Table 2), where our trapping efforts have shown that a single trap-line sampling local microhabitats often will yield 2 species of Dipodomys, 2–3 species of Chaetodipus, and 1 species of Perognathus (DJH, personal observation). More than 50% of Heteromyid rodents in México inhabit only 1 biogeographic province with a few widespread species.
In general, agricultural conversion of desert habitat poses the greatest threat to heteromyid species, whereas the majority of threatened subspecies occur on small desert islands off the Pacific coast and in the Gulf of California, where they have been subjected to a variety of human-induced threats.
Species of conservation concernAs summarized by Hafner and Riddle (2005), desert regions of North America have disappeared at an alarming rate in the face of agricultural conversion and urban expansion. In the United States, the San Joaquin Valley of California with its 22 endemic species and subspecies of arid-adapted mammals had suffered 98% conversion to agriculture by 1976 (Williams and Kilburn, 1992), while the Mojave Desert, Pacific coastal sage desert, and the northern periphery of the Sonoran Desert have retreated in face of explosive expansion and related agricultural conversion of Los Angeles (California), Las Vegas (Nevada), and Phoenix (Arizona). In México, agricultural conversion has caused the probable extinction of D. gravipes in the coastal sage desert of northern Baja California, and threatens the fog-desert Magdalena Plains of the Baja California Peninsula's Cape Region (C. dalquesti), the coastal thorn-scrub desert of Sinaloa and Nayarit (C. goldmani), desert grassland habitat of the Mexican Plateau (D. spectabilis), and the Oriental Basin of Puebla, Tlaxcala, and Veracruz (D. phillipsii). Elsewhere in México, entire tracts of native desert have disappeared under agricultural conversion (the Altiplano of Zacatecas, Desierto Mayrán of Coahuila). In Sonora, the core region of the Sonoran Desert is severely threatened by the simultaneous targeted removal of mesquite woodland (Hafner and Riddle, 2005; Hoffmeister, 1986) and the more insidious and widespread introduction of buffelgrass (Pennisetum ciliare), which competes with native plants and promotes disastrous wildfires (Arizona-Sonora Desert Museum, 2012; Hafner, 2012). In addition to restricting already limited distributions of some species, the twin threats of agricultural conversion and urban expansion have destroyed areas of contact between species and faunas. For example, the Mexicali-El Centro-Yuma-San Luis development area has nearly eradicated a region in northern Baja California where the Mojave, Sonoran, and Peninsular regional deserts intermix, including contact of 9 species of Chaetodipus. On a more local level, we (DJH, personal observation) revisited in 2005 a site of contact between C. dalquesti and C. arenarius on the Magdalena Plains near Insurgentes, Baja California Sur that we had first detected in 1999. Six years later, heavy equipment was plowing under desert for new agricultural fields within a few kilometers of this formerly remote, undisturbed site.
Three other species are considered to be of conservation concern because of their restricted distributions: C. lineatus (southwestern San Luis Potosí and southeastern Zacatecas); H. nelsoni (southern Chiapas and western Guatemala); and L. spectabilis (southeastern Jalisco). Specific threats to any of the 3 restricted regions are not known. The validity of C. lineatus (Dalquest, 1951) has been questioned (Williams et al., 1993) because of its similarity to C. nelsoni (with which it is supposedly sympatric), subtle distinguishing characteristics (color and texture of pelage, variable characters associated with age and molt), and inability of subsequent workers to capture specimens clearly referable to the species. Nowhere else in the distribution of Chaetodipus are 2 coarse-haired members of the genus widely sympatric, and genetic samples gathered from known localities have rendered only C. nelsoni (DJH, personal observation).
Subspecies of conservation concernThere are 23 out of 27 subspecies considered to be of conservation concern are restricted to islands near the Pacific coast or in the Gulf of California (Table 2). These populations, many of which previously were considered as species distinct from their mainland counterparts, have been subjected to a variety of human-induced impacts, including introduction of non-native predators and competitors (e.g., cats, goats, Rattus, Mus), introduction of congeneric competitors from the mainland, habitat modification (e.g., clearing of ironwood; introduction of iceplant), and direct poisoning campaigns (summarized in Hafner and Riddle, 2005; Lawlor et al., 2002). Native mammals are known from 36 islands, based largely on a single (or rarely a few) trapping efforts during the first half of the twentieth century. Subsequent resampling efforts during the last 50 years have generally been at the same accessible sites, and 14 of the native forms are considered to be extinct, possibly extinct, or in danger of extinction (Hafner and Riddle, 2005). As noted by Hafner and Riddle, 2005:239), “Species may be disappearing that have never been detected.” More extensive and intensive sampling is necessary to confirm extinction and provide baseline data with which to evaluate future change, including introduction of mainland forms from both natural over-water dispersal (e.g., rafting) or human-related activities (e.g., transport on fishing vessels). Although many of the islands are included in the Islas del Golfo de California natural protected area (http://www.conanp.gob.mx), 4 of the larger islands are privately or communally owned (Tiburón, del Carmen, Cerralvo, and San José). Adverse human impact associated with increased tourism (e.g., development; introduction of exotic species) likely will increase with government-backed projects such as Escalera Náutica, a chain of 22 marinas (10 existing, 12 new) around the Baja California Peninsula (http://bajaquest.com/escaleranautica/; Boletín Oficial del Gobierno del Estado de Baja California Sur, 2000; Hafner and Riddle 2005).
Three other subspecies of conservation concern, all subspecies of D. phillipsii, are restricted to the southeastern peripheral deserts of eastern TMVB (Fernández et al., 2012), which are subjected to extensive agricultural conversion. A molecular analysis by Fernández et al. (2012) recognized a northern clade of D. phillipsi (formerly D. p. ornatus) to be specifically distinct. The remaining subspecies of conservation concern, P. a. amplus (previously P. a. rotundus), enjoys an extensive distribution in Arizona and only marginally occurs across the border in México.
Suggested modifications to conservation prioritiesAmori and Gippoliti (2003) have emphasized the importance of considering taxonomic rank in rodent conservation, and Amori et al. (2011) have supported the generally accepted importance of biodiversity hotspots (regions of high species diversity) in setting conservation priorities. From the perspective of rodent conservation, we argue instead for a habitat-based approach that focuses on the conservation of functional ecosystems, as advocated by Hafner et al. (1998). Although we agree that endangered genera (e.g., the endangered, endemic genus Zygogeomys) merit special consideration, the application of species versus subspecific distinction in the case of disjunct isolates (e.g., island populations) often is subjective. Similarly, emphasis on biodiversity hotspots may misdirect conservation efforts, as hotspots often indicate regions of contact between adjoining biotas and as such may represent peripheral, and potentially ephemeral, habitats for both biotas. The ubiquitous nature of rodents, and the often close matching between a taxon and a specific habitat, makes them ideal as indicators of habitat and ecosystem health, and as early warnings of adverse impact. An ecosystem approach would consider first, the number and diversity of threatened taxa (regardless of taxonomic rank) in a particular habitat, and only then prioritize based on taxonomic rank and degree of threat. It is likely that an ecosystem with a variety of threatened rodents is likely to contain a variety of other living organisms that also are threatened. Because of limited funds, conservation agencies are forced to make pragmatic decisions about conservation priorities, and we argue that those funds should be targeted on preservation of functional ecosystems under the greatest threat, rather than on individual species.
Following these guidelines, we would suggest the following modifications to the list of threatened geomyid and heteromyid rodents of México. No taxon with a limited distribution in México but with a widespread distribution elsewhere should be included (e.g., P. a. amplus). Protected status should be accorded to threatened populations and subspecies, just as it is to threatened species (e.g., all threatened island forms), and protected status should be restricted to only those subspecies or populations that actually are threatened, rather than to the entire species (e.g., D. s. cratodon in the Chihuahuan Desert, and D. s. intermedius of the Sonoran Desert, instead of the entire species, D. spectabilis).
In addition to protecting taxa that currently are threatened, it remains necessary to adopt a proactive approach to mitigate adverse human impact before it results in a threatened status for species. Probably the most difficult task facing rodent conservation efforts in México is the common perception of a uniform, widespread rodent community over extensive areas, which cannot seriously be threatened by human impact. Thus, the Oriental Basin of the eastern TMVB has been reduced to marginal, peripheral pockets of a formerly widespread arid-adapted community, with at least 4 threatened rodent species (D. phillipsii, Neotoma nelsoni, Peromyscus bullatus, and Xerospermophilus perotensis), while the vast Sonoran Desert region of Sonora and the Sinaloan thorn-scrub are being dramatically altered by active habitat conversion and introduced exotics. Surveys of indicators of these broad arid regions (e.g., Peromyscus merriami in the mesquite forests of the Sonoran Desert; C. goldmani and Neotoma phenax in the Sinaloan thorn-scrub) are necessary to assess the impact of current human-mitigated threats to each region. As sensitive indicators of habitat health and as important components of the food chain of most terrestrial ecosystems, rodents can provide those critical early warnings of adverse human impact to ecosystems, and public education should emphasize these important roles of rodents.
Thanks to Juan Carlos Windfield Pérez who kindly prepared the figures, David Valenzuela and 2 anonymous reviewers for their critical evaluations of this manuscript.