This study presents a coproparasitological survey of free-ranging jaguars (Panthera onca) and pumas (Puma concolor) inhabiting 2 types of tropical forest in southeastern Mexico. We examined 167 fecal samples (68=jaguar; 33=puma; 66=unidentified large felids), and identified 16 parasitic taxa, 56% of which were nematodes. We compared parasite community composition and prevalence between host species and forest types, and found that parasitic communities of jaguar and puma were more similar between host species in the same forest type than among conspecific hosts inhabiting different forest types. Possible determinants for the observed patterns could include ecosystem differences and host evolutionary history, as well as disparate diet and habitat use of these 2 felines. Further studies are needed for a more accurate estimation of the parasitic community composition of these felines, and for a better comprehension of the effects that habitat perturbation could have on the ecology and transmission of parasitic diseases, and the related consequences for the conservation of wild felines.
Este trabajo presenta un análisis coproparasitológico de jaguares (Panthera onca) y pumas (Puma concolor) que habitan 2 tipos de bosque tropical en México. Examinamos 167 excretas (68=jaguar; 33=puma; 66=grandes felinos sin diferenciar) e identificamos 16 taxones de parásitos, de los cuales el 56% fueron nematodos. Al comparar la composición de las comunidades parasitarias y la prevalencia entre especies de hospederos y entre tipos de bosques, encontramos que las comunidades parasitarias de jaguar y puma son más similares entre especies de hospederos que habitan el mismo bosque, que entre hospederos conespecíficos en distintos tipos de bosque. Los patrones observados pueden deberse a las características propias de cada ecosistema, junto con la historia evolutiva de los hospederos, así como a diferencias en la dieta y uso del hábitat entre ambos felinos. Son necesarios más estudios para lograr estimar de forma certera la composición de las comunidades parasitarias de estos felinos, así como para comprender mejor los efectos que la alteración del hábitat puede tener en la ecología y transmisión de enfermedades parasitarias y sus posibles implicaciones en la conservación de los felinos silvestres.
The jaguar (Panthera onca) and puma (Puma concolor) are the largest felines in the American continent. They are sympatric over much of their distribution, and inhabit a variety of ecosystems including tropical forests and savannas (Ceballos & Oliva, 2005; Nowell & Jackson, 1996). Despite the substantial information available on the biology and ecology of both felid species, little is known about their parasitism and infectious diseases in the wild (Demar, Ajzenberg, Serrurier, Dardé, & Carme, 2008; Fiorello, Robbins, Maffei, & Wade, 2006; Foster, Cunningham, Kinsella, McLaughlin, & Forrester, 2006; García-Prieto, Falcón-Ordaz, & Guzmán-Cornejo, 2012; Patton, Rabinowitz, Randolph, & Johnson, 1986; Rickard & Foreyt, 1992; Srbek-Araujo, Costa-Santos, Medeiros de Almeida, Pezzi-Guimaraes, & Garcia-Chiarello, 2014), and the information is practically null regarding the impact that diseases and parasites have over their fitness, modes of transmission, and the ecological determinants of their parasite community structure.
Knowing which diseases and parasites potentially infect free-ranging populations across their distribution range, as well as their transmission dynamics is particularly relevant for the conservation of these felids, since the effects of parasites and diseases add up to other threats such as habitat loss, hunting, and persecution (Medellín et al., 2002; Nowell & Jackson, 1996). Additionally, as habitat fragmentation and landscape anthropogenization increases, encounters between wild felids and domestic fauna and humans became more common, and diseases and parasitism became of major concern not only for wild feline conservation but also for human health (Brousset & Aguirre, 2007; Laurenson, Mlengeya, Shiferaw, & Cleaveland, 2005; May, 1998).
Here we present the coproparasitological results of 2 intensive sampling periods of jaguar and puma free-ranging populations inhabiting 2 types of tropical forests in 2 regions of southeast Mexico. This study represents the first characterization of the parasite communities of these felines in these 2 regions of southeastern Mexico.
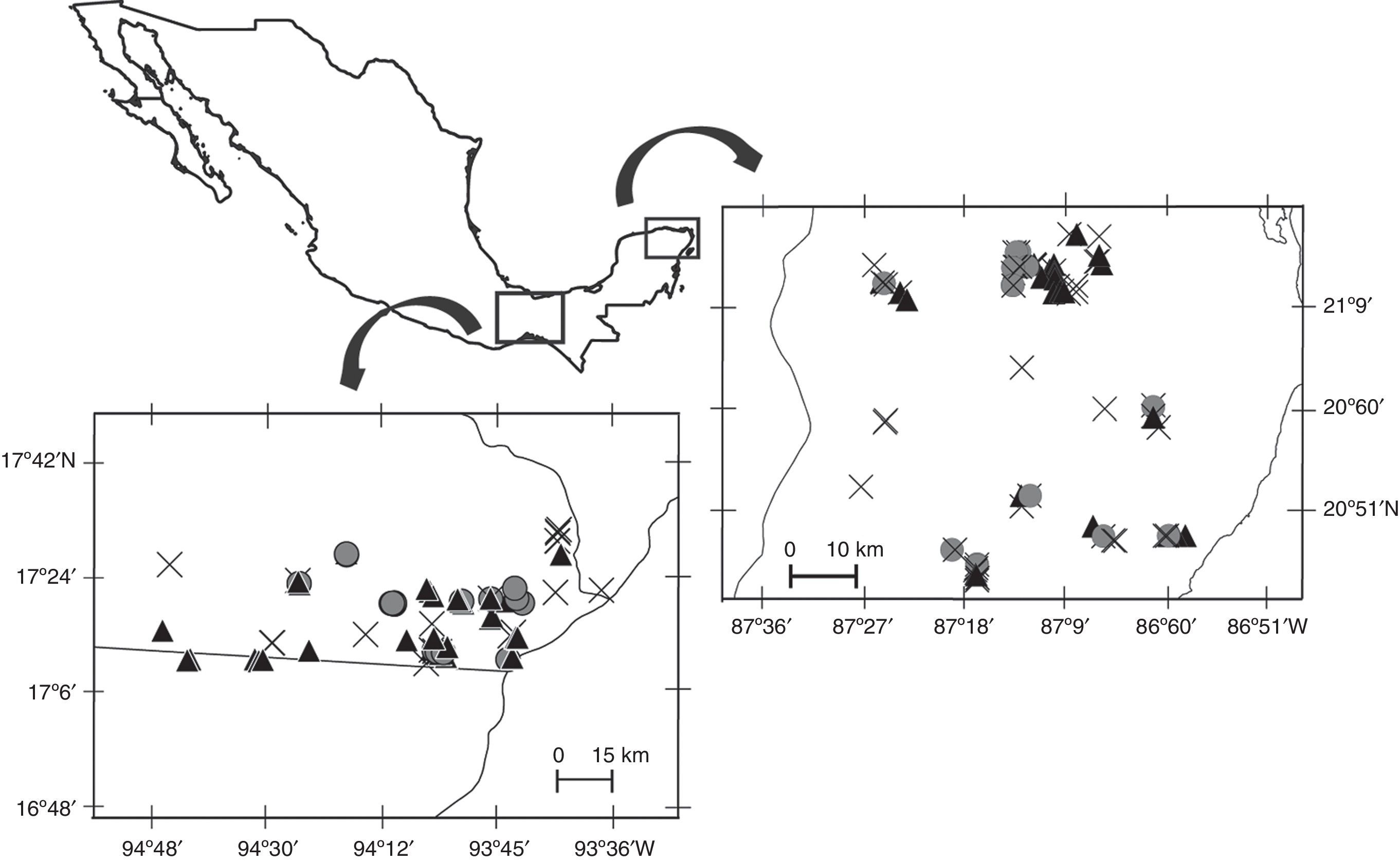
Materials and methodsWe conducted this study between 2008 and 2011 in 2 regions of southeastern Mexico dominated by different tropical forest types: (1) tropical rainforest (TRF) located in Uxpanapa Valley, Veracruz, and (2) semi-deciduous tropical forest (SDF) located in the northeastern portion of the Yucatán Peninsula (Fig. 1). Tropical rainforests are characterized by having exuberant and rich vegetation, with trees taller than 30m height. Semi-deciduous tropical forests are dense ecosystems with trees up to 25m height, and a conspicuous dry season in which many of the trees loss their leaves (Challenger & Soberón, 2008). Both study regions consist of a heterogeneous landscape of forest fragments, secondary vegetation, and anthropogenic land-cover such as agricultural land and pastures for livestock, as well as villages. Mean annual temperature and precipitation in the Uxpanapa valley are 24.4°C and 3640mm, and in the northeastern portion of the Yucatán Peninsula are 25.7°C and 1249mm, where surface water flows are absent, and rain filters through the limestone forming a subterraneous hydrologic net.
We collected scats from 38 localities in the Uxpanapa Valley (TRF), and from 19 localities in the Yucatán Peninsula (SDF). Surveys of each locality consisted of 1–2 wandering, circular, transects. We surveyed a total of 608.5km in TRF, and 171.52km in SDF. Surveys often followed human or game trails, but also included off-trail and road-side portions. All surveys were conducted with permission of local land owners.
Scat samples were found with the aid of wildlife scat detection dogs from the Conservation Canines program at University of Washington (Wasser et al., 2004). Each fecal sample was divided into 2 pieces, 1 piece was preserved in 4% formalin for parasitological analysis, and the other was kept frozen for genetic identification of the felid species. Given sufficient size of the sample, a portion was left in the field to minimize disruption of any territorial marking behavior.
Host species ID was confirmed using mitochondrial DNA sequencing of sloughed intestinal cells (Chaves, Graeff, Lion, Oliveira, & Eizirik, 2011). The ATP6 region (approximately 175 base pairs) was able to distinguish all sympatric carnivore species (data not shown). Additionally, host hair found in the scats (ingested during auto-grooming) was used to support the host species ID.
The coproparasitological examinations were conducted at the Laboratory of Animal Ecophysiology at the Center of Tropical Research, in the Universidad Veracruzana, Mexico, using flotation in saturated sodium chloride solution, and simple sedimentation techniques (Greiner & McIntosh, 2009; Hendrix, 1998). Both techniques were performed for each collected sample, examined by direct light microscopy (10×, 40×, 100×). The identification of parasites was based on egg morphology, shape, size and color.
In order to characterize parasite communities by host and by region, we estimated the following parameters for the 2 felid species, as well as for the 2 forest types: 1, percentage of positive samples (number of samples containing any parasite species divided by the total number of examined samples); 2, prevalence (defined by [Bush, Lafferty, Lotz, & Shostak, 1997]), and 3, parasite richness (number of parasitic taxa identified). We compared species richness between hosts and forest types using randomized (100×) sample-based rarefaction curves (Dove & Cribb, 2006) and Chao2 richness estimator calculated using EstimateS v.9.1 (Colwell, 2013). We also assessed the similarity of parasite communities between host species and between forest types by applying the Jaccard index, which considers the species richness of the communities being compared and the number of species that they have in common.
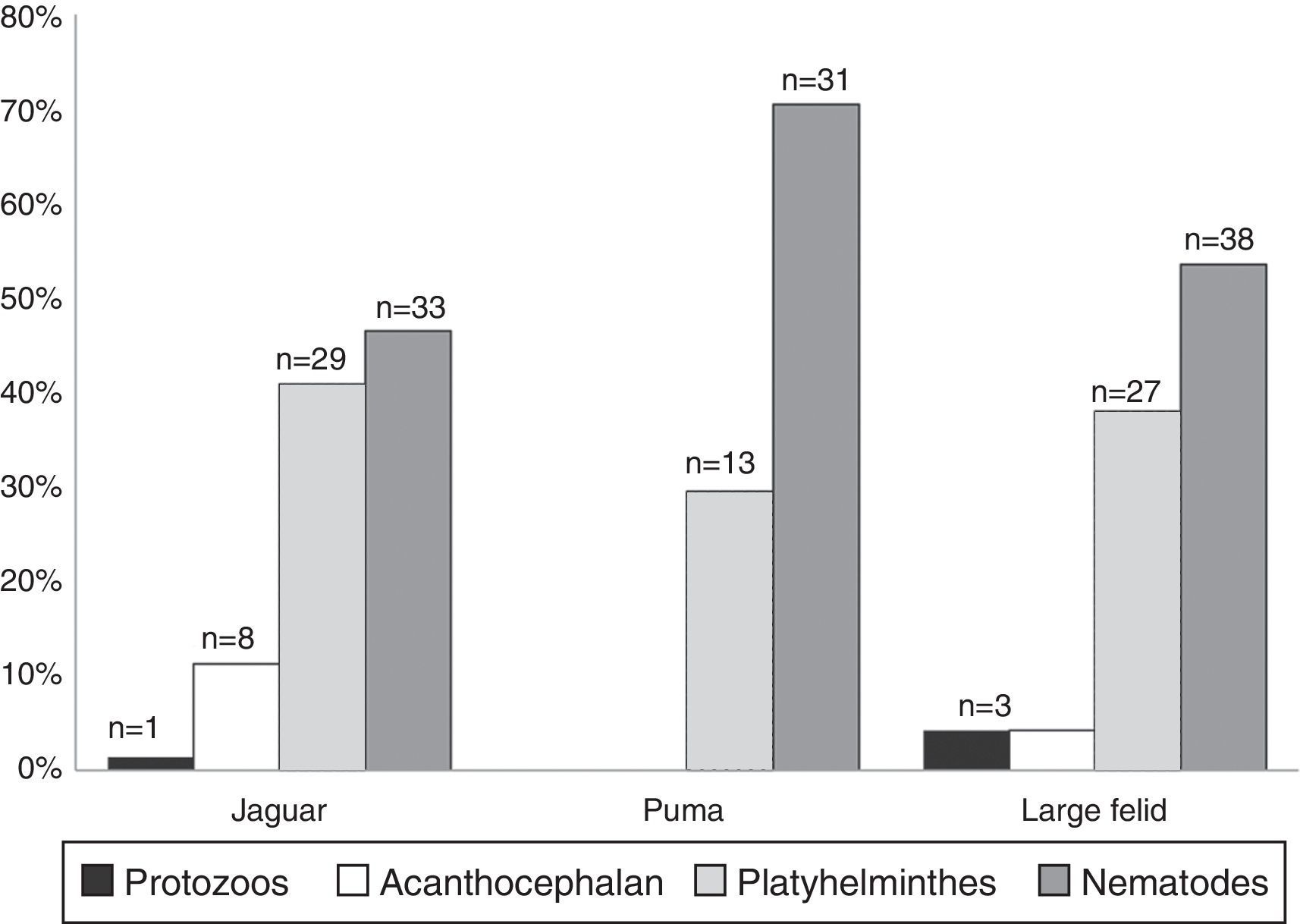
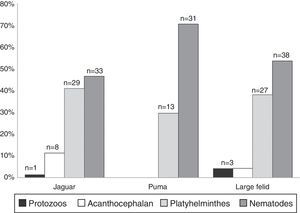
ResultsWe examined a total of 167 fecal samples from free-ranging felines; 68 were confirmed as jaguar samples, 33 were confirmed as puma samples, while 66 were confirmed as large felid scats but we could not discriminate between jaguar and puma (hereinafter referred as large felid samples). The number of positive samples was slightly higher in jaguar (43/68) than in puma (18/33) with over half of all samples containing at least 1 identifiable parasite (Table 1). The number of positive samples was also higher in SDF than in TRF for both felines (Table 1). We identified 16 parasite taxa for the total samples (Table 1, Fig. 2). In general, nematodes had the highest prevalence followed by platyhelminthes, acanthocephalans, and coccidians (Fig. 3). We found 4 forms of eggs of Strongyloides but we could not identify them to species level.
Prevalence and richness of parasites from jaguar and puma inhabiting tropical rainforest and semi-deciduous tropical forest in southeastern Mexico. Mean egg size (μm±s.d.) showed inside brackets below parasite's name.
| Phylum | Parasite | Tropical rainforest | Semi-deciduous tropical forest | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Jaguar (%) | Puma (%) | Large felids (%) | Jaguar (%) | Puma (%) | Large felids (%) | Jaguar (%) | Puma (%) | Large felids (%) | ||
| Apicomplexa | Coccidiasina gen. sp. (25±7.2×16±5) | 10 | 3.9 | 2.2 | 1.5 | 4.5 | ||||
| Platyhelminthes | Anoplocephalidae gen. sp. (56.8×50.4) | 2.4 | 1.5 | |||||||
| Spirometra sp. (60±5.7×35±2.3) | 19 | 53.9 | 66.7 | 52.2 | 32.4 | 30.3 | 36.4 | |||
| Taeniidae gen. sp. (35±1.9×29±1.9) | 4.8 | 5.6 | 15.4 | 13.3 | 6.5 | 8.8 | 9.1 | 4.5 | ||
| Acanthocephala | Acanthocephala gen. sp. (69±3.5×48±2.3) | 14.3 | 7.7 | 6.5 | 11.8 | 4.5 | ||||
| Nematoda | Ancylostoma sp. (62±5.3×35±3.8) | 2.4 | 5.6 | 15 | 3.9 | 20 | 4.4 | 2.9 | 12.1 | 7.6 |
| Ascarid-like egg (70±4.4×44±8.8) | 4.8 | 5.6 | 6.7 | 2.2 | 2.9 | 6.1 | 1.5 | |||
| Capillaria sp. (53±7.7×24±3.4) | 5 | 7.7 | 2.9 | 1.5 | ||||||
| Gnathostoma sp. (77±2.3×38±4.7) | 6.5 | 4.5 | ||||||||
| Nematoda gen. sp. (40.5×22.8) | 5.6 | 3 | ||||||||
| Physaloptera sp. (41±3.2×28±1.2) | 19.2 | 40 | 17.4 | 7.4 | 18.2 | 12.2 | ||||
| Spirocerca sp. (37±2.5×16±1.4) | 7.1 | 5.6 | 7.7 | 20 | 13 | 7.4 | 12.1 | 9.1 | ||
| Strongyloides sp. (54±7.2×30±7.4) | 9.5 | 11.1 | 15 | 19.2 | 46.7 | 19.6 | 13.2 | 27.3 | 18.2 | |
| Toxocara sp. (59±1.5×53±2.4) | 5 | 19.2 | 26.7 | 17.4 | 7.4 | 12.1 | 1.5 | |||
| Trichuris sp. (60±1.8×28±2.1) | 2.4 | 2.2 | 1.5 | 1.5 | ||||||
| Uncinaria sp. (84±7.7×48±2) | 4.8 | 6.7 | 2.9 | 3 | ||||||
| Sample size | 42 | 18 | 20 | 26 | 15 | 46 | 68 | 33 | 66 | |
| Positive samples (%) | 50 | 27.8 | 30 | 84.6 | 86.7 | 65.2 | 63.2 | 54.5 | 54.5 | |
| Richness | 10 | 6 | 10 | 9 | 14 | 10 | ||||
| Taxa per sample | 1–4 | 1–2 | 1–4 | 1–6 | 1–4 | 1–6 | ||||
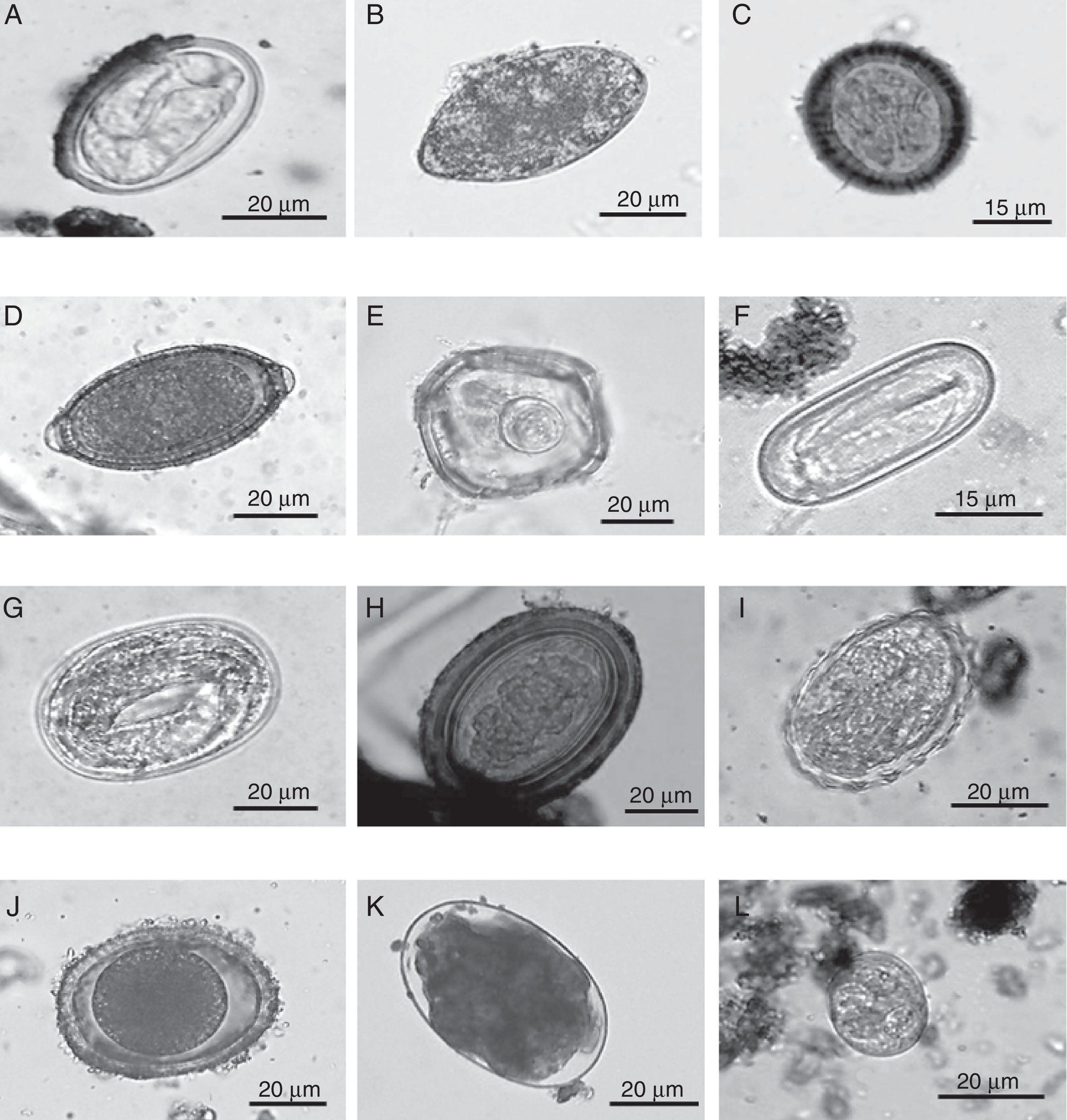
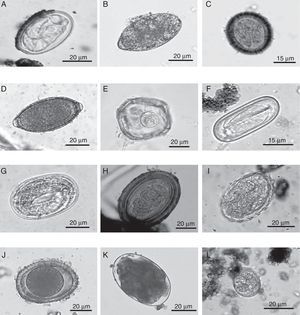
Eggs of intestinal parasites identified in jaguar and puma fecal samples collected in southeastern Mexico: A=Physaloptera sp.; B=Spirometra sp.; C=Taeniidae; D=Trichuris sp.; E=Anoplocephalid; F=Spirocerca sp.; G=Strongyloides sp.; H=Acanthocephalan; I=Ascarid-like egg; J=Toxocara sp.; K=Ancylostoma sp.; L=coccidian.
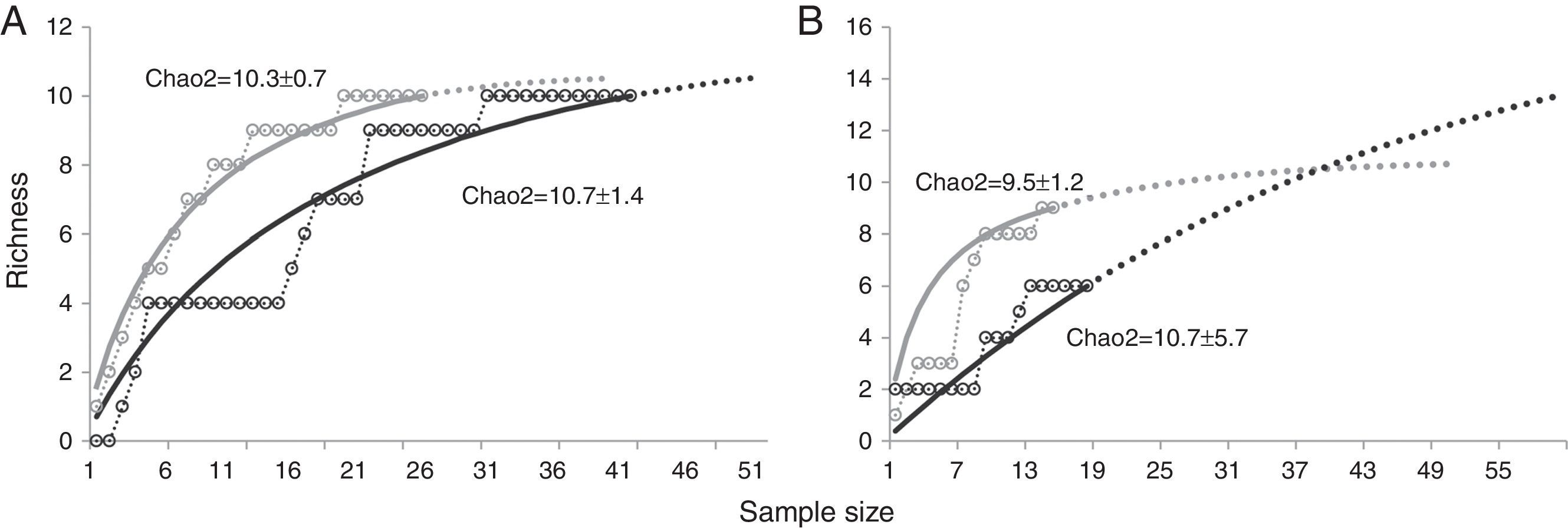
Parasitic species richness was similar in both jaguar and puma, with 14 taxa versus 10 taxa respectively; also the number of taxa per sample was relatively equal between species (Table 1). We observed the same number of parasite taxa in both forest types for jaguar, while puma presented more taxa in SDF than in TRF. Species accumulation curves and Chao2 richness estimator confirmed equal parasite species richness between forest types for jaguar, but showed higher parasitic richness in TRF compared with SDF for puma (Fig. 4). Jaccard values show that jaguar and puma share 37% of their parasitic fauna. In addition, similarity in parasitic communities between these 2 felines is higher in SDF than in TRF (Table 2). Notably, the parasitic communities are more similar within the same type of forest between host species than within host species between forest types (Table 2).
Parasite species richness for jaguar and puma in southeastern Mexico. A, Jaguar; B, puma. Tropical rainforest=black; semi-deciduous tropical forest=gray. Species accumulation curves (open circles); randomized curves (solid lines); extrapolation of accumulation curves (dotted lines).
Pumas and jaguars have a rich parasitic fauna with at least 10 and 14 parasite species, respectively. Most of the parasites found in puma samples in this study coincide with previous reports (Foster et al., 2006; García-Prieto et al., 2012); however, we found a greater number of parasitic taxa than what have been published so far for free-ranging jaguar populations (Patton et al., 1986; Srbek-Araujo et al., 2014; Vieira, Luque, & Muniz-Pereira, 2008). This is the first report of Ancylostoma sp., Physaloptera sp., Spirocerca sp., Strongyloides sp., and Uncinaria sp. in jaguars from Mexico (see García-Prieto et al., 2012).
Also, this is the first report of an acanthocephalan for both jaguar and puma in Mexico. Acanthocephalans of the genera Oligacanthorhynchus and Oncicola, have been reported for wild felids in the Neotropics (Patton et al., 1986; Vieira et al., 2008); however the eggs that we found rather resemble that of Echinopardalis, also a parasite of wild felids in the American continent (Noronha, Ferreira, Rangel, Araujo, & Correa-Gomes, 1994; Schmidt, 1972).
The occurrence of anoplocephalid cestodes in the jaguar scats might be due to an accidental ingestion. These types of tapeworms have been reported in horses, but also in collared pecari and in white-lipped pecari (García-Prieto et al., 2012), both common preys of large felids in Mexico (Aranda & Sánchez-Cordero, 1996; Nuñez, Miller, & Lindzey, 2000). Since we only found 1 sample to be positive for this parasite, this is a potential case of pseudoparasitism, where a parasite was consumed along with its definitive host but it did not establish within the feline host.
Strongyloides spp. were among the most common parasites found in this study with 4 forms of eggs; unfortunately we were not able to identify the species neither by eggs shape nor by size. Strongyloides is a highly diverse genus of common parasitic nematodes of vertebrates (Dorris, Viney, & Blaxter, 2002). Three species have been reported to infect felines: S. felis, S. planiceps and S. tumefaciens; but only the last one has been reported for America as a parasite of pumas in Florida (Forrester, 1992). It has also been observed that S. stercolaris, a parasite of humans and dogs, can infect felines under laboratory conditions (Bowman, Hendrix, Lindsay, & Barr, 2002); thus the possibility of a potential cross-transmission of this parasite to wild felines via humans or domestic fauna could not be discarded.
Toxocara cati is a recurrent parasite of wild felines in the Neotropics (Foster et al., 2006; García-Prieto et al., 2012; Srbek-Araujo et al., 2014), and it is also a common parasite of domestic cats. This parasite can be transmitted directly by egg ingestion or through paratenic hosts, such as mice (Bowman et al., 2002). Moreover, Toxocara canis the dog roundworm, has been reported to be found also in cats (Bowman et al., 2002). Given the conditions of forest fragmentation and constant human presence, it is possible that an interchange of Toxocara sp. between wild felines and domestic dogs and cats via paratenic hosts might be occurring in the study sites.
We found ascarid-like eggs in jaguar and puma samples from both forest types. Lagochilascaris is a genus of parasites that infects mostly carnivores, by preying on mammalian intermediate hosts infected with encysted L3 in their muscle tissue (Campos, Freire-Filha, Vieira, Paco, & Maia, 1992). However, the ascarid-like egg that we found in the present study differs in size and shape from Lagochilascaris, and rather resembles the Ascaris egg, a common parasite of pigs and humans. However, the information obtained in this study was not enough to confirm the identity of these eggs.
Parasitic prevalence and parasite community richness were relatively equal between jaguar and puma samples. However, the shared gastrointestinal parasitic fauna was only 37%, even though they both are top predators, and they coexist in the majority of their distribution range in Mexico. Differences in their intestinal parasitic fauna could be related to diet differences or segregation in habitat use (de Oliveira, 2002; Estrada-Hernández, 2008; Harmsen, Foster, Silver, Ostro, & Doncaster, 2009; Scognamillo, Maxit, Sunquist, & Polisar, 2003). For example, jaguars are thought to be relative forest specialists in comparison to the more generalist habitat associations of pumas. Additionally, differences could be influenced by distinct host–parasite evolutionary histories of each felid, driving host specificity and sensitivity, but also limiting the shared parasitic taxa because of divergent ancestry (Adamson & Caira, 1994; Paterson & Banks, 2001; Poulin, 1995).
Parasitic communities of jaguar and puma are more similar between host species in the same forest type than among conspecifics inhabiting different forest types. Differences in parasite communities within host species have been previously observed in mammals and fish, where similarities decay with geographic and environmental distances between host populations (Krasnov, Mouillot, Shenbrot, Khokhlova, & Poulin, 2010; Poulin, 2003; Warburton, Kohler, & Vonhof, 2015). The 2 types of forest sampled in the present study are located in 2 disparate regions of southeastern Mexico, each of them characterized by presenting different landscape composition, as well as climatic conditions such as temperature and precipitation regimes, and humidity. Hence the dissimilarities found in parasitic community composition among conspecifics could be strongly related to the ecosystem differences of the 2 regions, such as flora and fauna assemblages and abiotic conditions associated with each forest type, influencing which parasite species are more likely to infect these felines in each region (Holt, Dobson, Begon, Bowers, & Schauber, 2003).
The presence of several specimens of parasites that resemble species typically found in humans or domestic animals, and that have not been reported for jaguar or puma (such as the ascarid-like eggs, and a high variety of Strongyloides eggs) suggests a close and continuous contact between wild felines, human villages and livestock, and a possible cross-transmission of parasites.
The increase in the wildlife-livestock contact is of major concern because the potential that it represents for the emergence of diseases, and the spread of parasites leading to both wildlife and human health issues (Daszak, Cunningham, & Hyatt, 2001). More studies increasing sampling efforts throughout their distribution are needed to better estimate parasite richness in free-ranging populations of these 2 felines. Studies in areas with different habitat perturbation degrees could help reveal the effects of human–wildlife contact on the host–parasite ecology.
Most of the wild felids in Mexico are considered as threatened species by the environmental authorities (Semarnat, 2010). Given their conservation status, the majority of the parasitological studies rely only on the information that we can get from the scats samples. Non-invasive fecal sampling proved to be an effective technique for studying the parasitic fauna of threatened species, and hence their health status. However, fecal examination has strong limitations, especially at the moment of parasite species identification and burden estimates. Molecular techniques and DNA extraction from parasite eggs found during fecal examinations would help to overcome these difficulties, and could also be a useful tool for assessing the risk of cross-transmission between human/domestic fauna and wildlife, and its implications for the persistence of threatened taxa.
We acknowledge the individual working dogs, Sadie May and Scooby, of the UW Conservation Canines, for their tireless energy and contribution to a good natured field team. We also thank all the local people for their hospitality and help during field work, along with the staff of El Eden nature preserve, especially the director, Marco Lazcano. We sincerely thank Arturo Gómez Pompa and Cristina Mac Swiney for their valuable support. We also thank Martín García Varela for his help and comments. The CNHE aided us with the parasite identification. This study was partially funded by Conacyt in Mexico and the Veracruz State Government (Grant Number 108990). The UW Department of Biology Riddiford-Truman graduate–student award used to partially fund travel of the CK9 team.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.