As a general rule, spiders exhibit sexual dimorphism and their populations may differ in size according to season duration and resource availability. However, few studies have focused on dimorphism in tarantulas. Mexican redrump tarantulas, Brachypelma vagans, listed in CITES, have an exceptionally wide distribution. Surprisingly, there are no studies on the possible relationship between the abundance of tarantulas per population and the geographical areas where they are present, or on how the distribution pattern of this spider may affect individual morphological characteristics. Furthermore, there are no studies on sexual dimorphism within the genus Brachypelma. The aim of the study is to determine the existence of sexual and geographical dimorphism in populations of B. vagans. It was observed that the abundance of spiders per population may vary according to the geographical areas where they were recorded. In six localities in southern Mexico, we recorded morphological data on adult tarantulas. Sexual dimorphism was clearly observed at the site that presented numerous spiders characterized by much smaller females. Since the results of this study demonstrate differences in tarantula number of individuals per locality in southern Mexico, they make an important contribution to the conservation of this species.
El dimorfismo sexual es muy común en las arañas, pero también existen diferencias morfológicas entre poblaciones en función de las temporadas y la disponibilidad en los recursos. Pocos estudios han analizado el dimorfismo en tarántulas. La tarántula mexicana de cadera roja Brachypelma vagans, listada en el CITES, presenta una distribución amplia. Sin embargo, se conoce poco sobre sus poblaciones por áreas geográficas y sobre cómo el patrón de distribución de estos organismos, puede afectar las características morfológicas individuales. Tampoco se ha estudiado el dimorfismo sexual en el género Brachypelma. Nuestro estudio se enfoca en determinar si existen dimorfismos sexual y geográfico en poblaciones específicas de B. vagans. Se observó que la abundancia de tarántulas encontradas por población suele ser diferente de acuerdo con las áreas geográficas donde fueron observadas. Registramos datos morfológicos de tarántulas adultas en 6 sitios geográficos distribuidos en el sureste de México. Encontramos dimorfismo sexual únicamente en un sitio que presenta un número de arañas muy alto y donde las hembras son más pequeñas que en otras localidades. Nuestros resultados, considerando la variación en número de individuos por localidades a lo largo del sureste mexicano, tienen consecuencias para la conservación de esta especie.
The large “tarantula” family Theraphosidae Thorell, 1869 (Araneae, Mygalomorphae) consists of 128 genera and 975 species distributed worldwide (World Spider Catalog, 2015). Brachypelma Simon, 1891 comprises 21 species that can be found within the Mesoamerican biological corridor; 14 species occur in Mexico, including 13 endemics and 1 (Brachypelma vagans) that is widely distributed in Mexico and Central America (World Spider Catalog, 2015). All are listed in Appendix II of the CITES convention (Convention on International Trade in Endangered Species), primarily to protect them from the illegal pet trade in the black market, as these highly sought species are very docile, large, and beautifully colored (West, 2005). Species of Brachypelma face other serious problems such as habitat destruction, high juvenile mortality, and late sexual maturity (Machkour-M’Rabet et al., 2007), leading to population decline or local extinction. Due to the vulnerability of the entire genus, some studies have concentrated on understanding their ecology (Criscuolo, Font-Sala, Bouillaud, Poulin, & Trabalon, 2010; Dor, Machkour-M’Rabet, Legal, Williams, & Hénaut, 2008; Machkour-M’Rabet, Hénaut, Rojo, & Calmé, 2005; Machkour-M’Rabet et al., 2007; Vilchis-Nestor, Machkour-M’Rabet, Barriga-Sosa, Winterton, & Hénaut, 2013; Yáñez & Floater, 2000); behavior (Dor, Calmé, & Hénaut, 2011; Dor & Hénaut, 2011, 2012, 2013; Locht, Yáñez, & Vázquez, 1999; Reichling, 2000; Yáñez, Locht, & Macías-Ordóñez, 1999); genetic structure (Longhorn, Nicholas, Chuter, & Volger, 2007; Machkour-M’Rabet, Hénaut, Calmé, & Legal, 2012; Machkour-M’Rabet et al., 2009), and traditional use by local populations (Machkour-M’Rabet, Hénaut, Winterton, & Rojo, 2011). The Brachypelma species are restricted to small and specific areas, with one exception, the Mexican redrump tarantula B. vagans Ausserer, 1875. This is the only species of the genus that is widely distributed and has been subject to several studies during recent years (Dor et al., 2008, 2011; Machkour-M’Rabet et al., 2005, 2007, 2009, 2011, 2012), also B. vagans was identified in Florida (Edwards & Hibbard, 1999) and Cozumel Island (Machkour-M’Rabet et al., 2012) as an invasive exotic tarantula. These studies demonstrate that this tarantula may be present in high densities in rural villages with low levels of anthropogenic disturbance and closed to medium semi-evergreen forests (Machkour-M’Rabet et al., 2005). In these areas, soil structure appears to be an important factor explaining the presence and high density of B. vagans due to their burrower condition (Machkour-M’Rabet et al., 2007). In this context, Hénaut and Machkour-M’Rabet (2005) observed that coexisting females are very aggressive toward congeners and commonly attack other females, which are detected by chemical cues (Dor et al., 2008).
Many species of animals with wide distribution ranges exhibit geographical variations in growth and life history traits (Stillwell & Fox, 2009). The body size of many animals varies with latitude and altitude (Blanckenhorn & Demont, 2004; Stillwell, Morse, & Fox, 2007), and the most common environmental variable advocated to explain body size variation is temperature (thermocline) (Stillwell & Fox, 2009). The geographic adaptations of animals, which include variations in body size, are generally genetically based (Armbruster, Bradshaw, Ruegg, & Holzapfel, 2001; Karl, Janowitz, & Fisher, 2008). Studies show that a thermocline (or other factors which vary with latitude or altitude) can result in both intra-specific and inter-specific size dimorphism (Blanckenhorn, Stillwell, Young, Fox, & Ashton, 2006; Stillwell & Fox, 2007; Teder & Tammaru, 2005). The body size of animals may also be correlated with population density (Robinson & Redford, 1986). Studies on Nephila clavipes Leach, 1815 (Araneae, Nephilidae) (Higgins, 1992, 1993, 1995) demonstrated that environmental variations result in differences in the size of individuals between spider populations. Higgins (2000) showed that seasonal variations or prey availability result in dissimilarities in spider size; females strategically adapt to these fluctuations by reaching maturity earlier when ecological conditions are degraded or reproduce before the end of the favorable season. Furthermore, Higgins (2000) demonstrated that late maturing N. clavipes females present lower reproductive success in strongly seasonal habitats. In B. vagans, a recent study (Vilchis-Nestor et al., 2013) showed that individuals recently introduced to an island (Cozumel Island, Quintana Roo, Mexico), had larger adults and a lower diversity of body patterns than individuals from mainland populations (e.g., the Yucatán Peninsula, Mexico).
The principal type of dimorphism in spiders is sexual dimorphism, with males usually smaller than females (Hormiga, Scharff, & Coddington, 2000). According to Darwin (1871) and present day authors, the first example of sexual dimorphism in animals was observed in spiders, principally web-building species from various families, but also for non-web-building groups such as the Lycosidae Rabidos rabida (Walker & Rypstra, 2001). Although sexual dimorphism is extreme in web-building spiders, non-web-building spiders generally have a lower degree of dimorphism (Walker & Rypstra, 2001). Sexual dimorphism is also evident in the relative size of body parts, with males having comparatively longer legs than females (Prenter, Montgomery, & Elwood, 1995). In Theraphosidae, males and females appear to be similar sized, but they present sexual dimorphism with respect to metabolic rates (Shillington, 2005). Tarantula females are sit-and-wait predators that remain in the same location during large periods of time. In contrast, males disperse by walking and actively search for females over large distances (Machkour-M’Rabet et al., 2012; Shillington, 2002).
This study compares populations of B. vagans from different geographical locations in Southeast Mexico. First, the study aims to determine if tarantula population size varies according to different geographical locations. Second, we focus on dimorphism among females from different populations to determine if geographical dimorphism occurs in B. vagans.
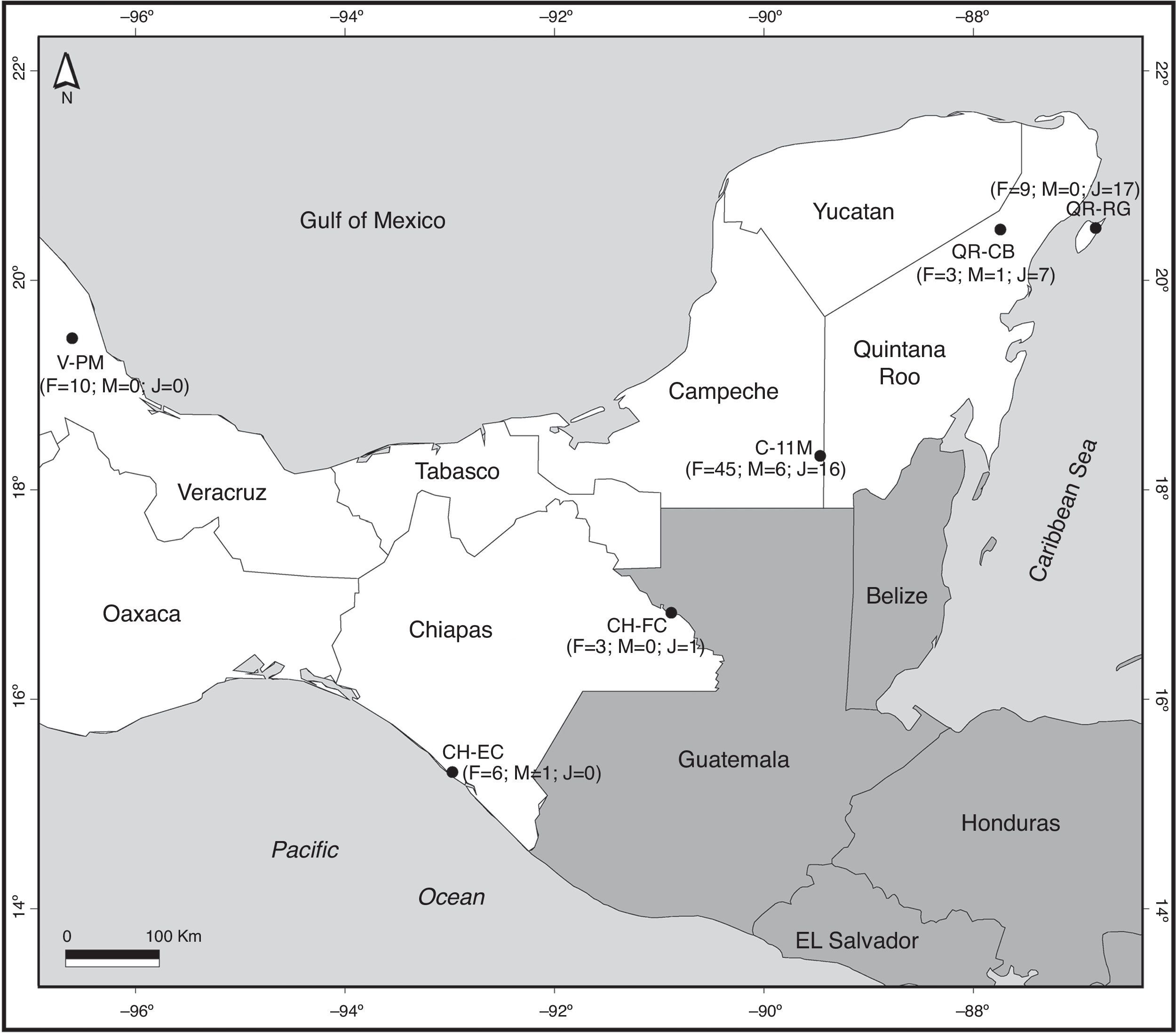
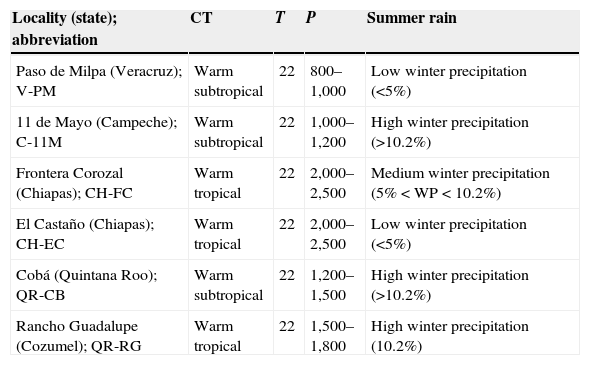
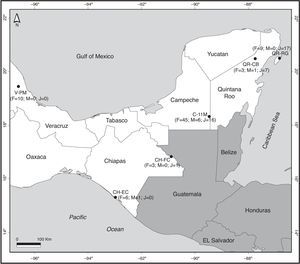
Materials and methodsAll data for the morphology of B. vagans were obtained from a total of 6 sites in four different states of southern Mexico (Table 1, Fig. 1). In Chiapas, we sampled 2 sites, El Castaño (CH-EC; 15°17′N, 92°58′W, 16masl) near the Pacific coast, and in Frontera Corozal (CH-FC; 16°49′N, 90°53′W, 117masl) in the Lacandona rainforest. In Campeche, we visited 1 site (11 de Mayo; C-11M; 18°18′N, 89°27′W, 281masl) close to the Calakmul Biosphere Reserve. In Quintana Roo, we visited 2 sites, 1 on the mainland (Coba; QR-CB; 20°28′N, 87°44′W, 13masl) and 1 on Cozumel Island (Rancho Guadalupe; QR-RG; 20°29′N, 86°50′W, 17masl). We also recorded data from a site in Veracruz (Paso de Milpa; V-PM; 19°26′N, 96°36′W, 260masl). Collections were made at the beginning of the rainy season for Chiapas and Yucatán Peninsula, which is between May and July. The start of the rains coincides with the reproductive season. All the sites studied were rural communities with similar ecological characteristics: deep clay soil with little vegetation, roots, and limestone rocks (Machkour-M’Rabet et al., 2005, 2007). Climatic characteristics were very similar for all study sites (Table 1).
Climatic and seasonal characteristics of the sampling sites where Brachypelma vagans individuals were collected. CT, climate type; T, annual mean temperature in degrees; P, annual precipitation in mm.
| Locality (state); abbreviation | CT | T | P | Summer rain |
|---|---|---|---|---|
| Paso de Milpa (Veracruz); V-PM | Warm subtropical | 22 | 800–1,000 | Low winter precipitation (<5%) |
| 11 de Mayo (Campeche); C-11M | Warm subtropical | 22 | 1,000–1,200 | High winter precipitation (>10.2%) |
| Frontera Corozal (Chiapas); CH-FC | Warm tropical | 22 | 2,000–2,500 | Medium winter precipitation (5%<WP<10.2%) |
| El Castaño (Chiapas); CH-EC | Warm tropical | 22 | 2,000–2,500 | Low winter precipitation (<5%) |
| Cobá (Quintana Roo); QR-CB | Warm subtropical | 22 | 1,200–1,500 | High winter precipitation (>10.2%) |
| Rancho Guadalupe (Cozumel); QR-RG | Warm tropical | 22 | 1,500–1,800 | High winter precipitation (10.2%) |
Map of sites where Brachypelma vagans was observed in Southeast Mexico. F, numbers of female individuals; M, number of male individuals; J, number of juvenile individuals. For codes of sites see Table 1. The number of wandering males found close to but not inside each sampling area is not considered in this figure.
Individual spiders were collected manually throughout the entire area. Despite differences in study area size, most tarantulas were found in their burrows, which were concentrated in particular areas such as gardens or football fields, as observed in previous studies (Machkour-M’Rabet et al., 2005, 2007). In Veracruz and Campeche states, we also collected males found walking close to the sampling area (less than 1km). We also recorded morphological data for these males as they were considered characteristic of the corresponding localities. Males and females were sexed by the tibial apophysis of leg I and the palp embolus in males, and by the females spermathecae. Collected individuals were measured and classified as adults (males and females) or juveniles based on sexual characters. The specimens were collected and measured in the field during the night between 20:30 and 00:30h. A small stick was used to remove the spiders from their burrows and handle them carefully by hand. For each individual, we measured the length and width of the prosoma (Lpro and Wpro, respectively), the length of patella I (PI) and IV (PIV), and tibia I (TI) and IV (TIV) as described by Chamberlin and Ivie (1938), the same methodology used in previous studies (Machkour-M’Rabet et al., 2005). Measurements were taken in millimeters using a digital vernier (Truper). The individuals were weighed (W) to the nearest 0.1g using a spring balance (Pesola), the measurements of the weight were in grams (g). Once all the data had been recorded, each spider was released in front of the entrance to its burrow. Specimens from previous research in the same areas are deposited in the Museum of Zoology, El Colegio de la Frontera Sur (ECOSUR), Chetumal, Quintana Roo, Mexico.
The G-test was used to compare the number of males, females, and juveniles collected among sites. Morphological measurements for males and females recorded at the sites were compared using the nonparametric Mann–Whitney U-test.
In Veracruz and Campeche state, walking males that were found close to but outside the sampling area were considered for means of analysis, representative of males from within the study area. To compare morphological measurements of females between sites, a nonparametric Kruskal–Wallis test was carried out, followed by a post hoc comparison of mean rank. All statistical analyses were performed using Statistica v7.0.
ResultsWe collected a total of 126 B. vagans individuals from the 6 sites: 41 juveniles and 85 adults. The number of females (n=77) was significantly higher (G=64, d.f.=1, p=0.000) than males (n=8), giving an overall sex ratio of approximately 10 females per male. In Campeche and Veracruz we found 6 and 11 males, respectively, outside the sampling area on the village perimeter.
The number of individuals observed presents a high degree of variation according to location. In Veracruz, 10 females were observed at the Paso de Milpa site. In Chiapas, 6 females and 1 male were observed in El Castaño and 3 females and 1 juvenile in Frontera Corozal. In Quintana Roo, 11 individuals (3 females, 1 male, and 7 juveniles) were observed in Cobá and 26 in Cozumel (9 females and 17 juveniles). Sixty-seven tarantulas were observed at the 11 de Mayo site in the state of Campeche (Fig. 1). The number of collected individuals at each site varied considerably (Fig. 1), varying between 4 and 11 individuals at the sites in Chiapas, Veracruz, and mainland Quintana Roo. At Rancho Guadalupe (Cozumel Island) we observed 23 individuals, while the 11 de Mayo site, with 67 individuals, accounted for the largest proportion (53%) of the total number of spiders collected at the 6 sites.
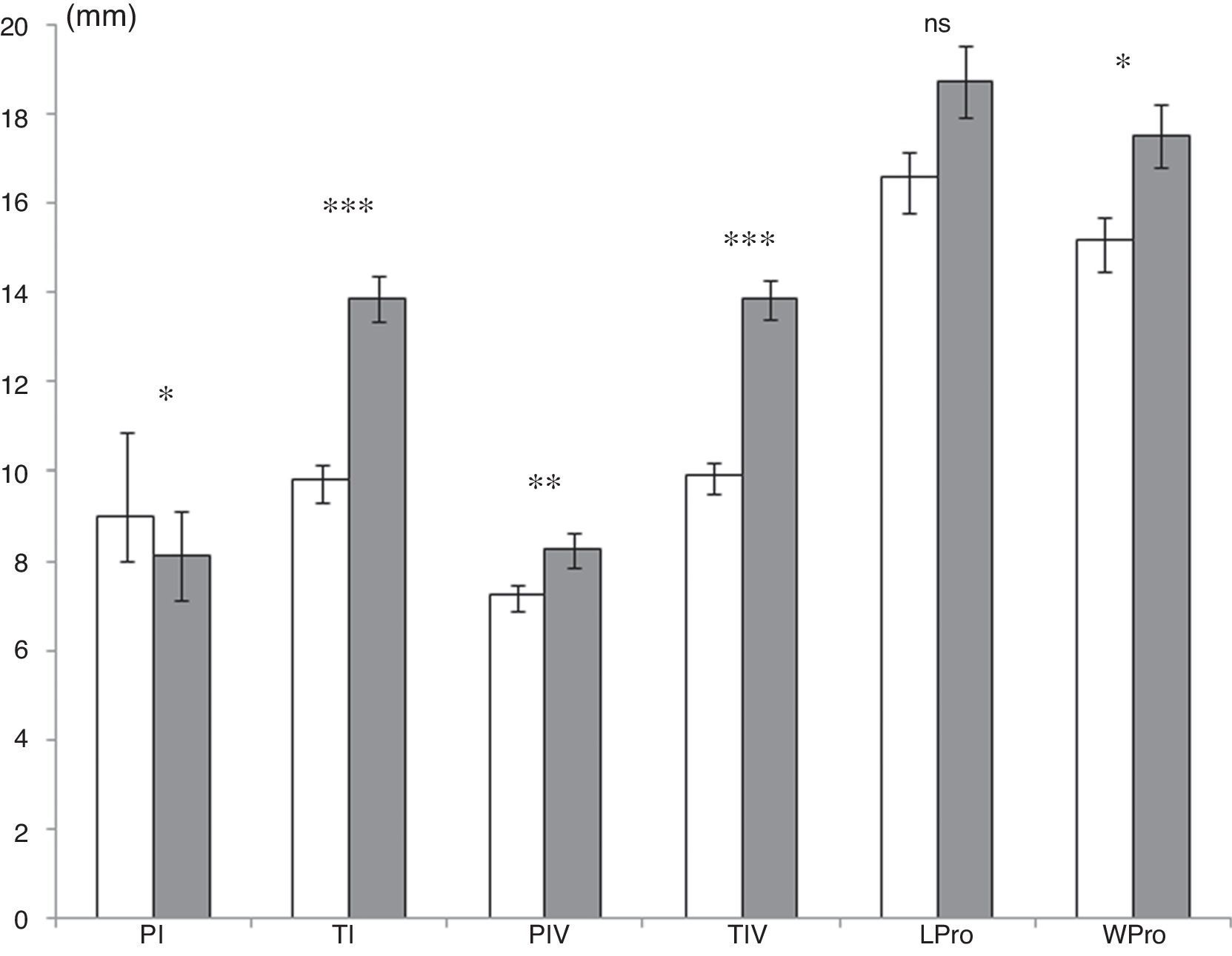
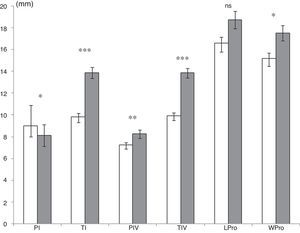
Males and females were compared in populations with a sufficient number of individuals of each sex, fixed at a minimum of 6. Only the sites in Campeche and Veracruz met this minimum number. Male and female spiders presented significant differences in size in Campeche (Fig. 2). Males were significantly larger than females with longer legs (tibias from the I and IV legs, and patella from the IV leg) and prosoma. Female weight (mean±SE: 7.7±0.6g) was also significantly lower (Mann–Whitney U-test: U=132, p=0.008) than male weight (mean±SE: 11.3±1g). This was not the case in Veracruz, especially with respect to the females and males found in Paso de Milpa. At this site, males were significantly larger than females in two measurements: TI male: 13.2±0.3mm, TI female: 10.8±0.4mm (Mann–Whitney U-test: U=4, p=0.000), and TIV male: 13.2±0.4mm, TIV female: 11.4±0.3mm (Mann–Whitney U-test: U=17.5, p=0.008). Males were significantly lighter than females (males: 8.9±0.4g vs. females: 21.3±1.5g; U=0.00, p=0.000). In this area no differences between sexes were observed for other morphological characters: PI (males: 9.5±0.1mm vs. females: 9±0.4mm; U=55, p=0.4), PIV (males: 8.6±0.1mm vs. females: 8.6±0.2mm; U=50, p=0.3), prosoma length (males: 19.7±0.4mm vs. females: 20.8±0.6mm; U=52, p=0.4), and prosoma width (males: 18±0.4mm vs. females: 19.8±0.6mm; U=55, p=0.5).
Mean (±standard error) of morphological measurements (mm) among Brachypelma vagans males (gray) and females (white) for the site in Campeche State. For abbreviations of morphological measurements see Table 2. Mann–Whitney U-test: ns, not significant; *p<0.05; **p<0.01; ***p<0.001.
Comparing males found in different geographical areas, no significant differences were observed between Campeche and Veracruz: PI (Campeche 8.12±1mm; Veracruz: 9.5±0.4mm; U=55, p=0.4), TI (Campeche 13.8±0.4mm; Veracruz: 13.2±0.3mm; U=46, p=0.2), PIV (Campeche 8.2±0.3mm; Veracruz: 8.6±0.1mm; U=50, p=0.3), TIV (Campeche 13.8±0.4mm; Veracruz: 13.2±0.4mm; U=44, p=0.17), prosoma length (Campeche 18.7±0.7mm; Veracruz: 19.7±0.4mm; U=52, p=0.4), prosoma width (Campeche 17.5±0.7mm; Veracruz: 18±0.4mm; U=55, p=0.5), and weight (Campeche 11.3±1mm; Veracruz: 8.9±0.4mm; U=21, p=0.1).
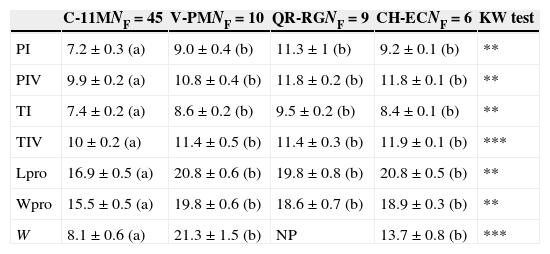
Due to the low number of female individuals observed at most sites, we could only compare females from 4 of them: Paso de Milpa in Veracruz with 10 females, El Castaño in Chiapas with 6 females, Rancho Guadalupe in Quintana Roo with 9 females, and 11 de Mayo Campeche with 45 females. Despite the low number of individuals, differences in size between females from the area with a high number of individuals (11 de Mayo) and areas with few individuals (the remaining localities), demonstrate a significant difference between 11 de Mayo and the other sites. Leg and prosoma size is significantly lower in female tarantulas from Campeche when compared to the other 3 sites. Weight is higher in tarantulas from Chiapas, Cozumel (Rancho Guadalupe), and Veracruz. Despite their geographical distance, the morphology of tarantulas from populations with low number of individuals appears to be similar (Table 2).
Mean (±standard error) for all morphological measures at sites with different number of females tarantulas. C-11M, 11 de Mayo site in Campeche, V-PM Paso de Milpa in Veracruz; CH-EC, El Castaño in Chiapas; QR-RG, Rancho Guadalupe in Cozumel Island Quintana Roo. NF, number of female individuals; morphological measurements in mm; PI, and PIV, length of patella I and IV respectively; TI and TIV, length of tibia I and IV, respectively; Lpro and Wpro, length and width of the prosoma, respectively. W=weight (in g). Kruskal–Wallis test: **p<0.01; ***p<0.001; NP, not possible. Letters following means represent intergroup differences (post hoc test) for each morphometric measurement.
| C-11MNF=45 | V-PMNF=10 | QR-RGNF=9 | CH-ECNF=6 | KW test | |
|---|---|---|---|---|---|
| PI | 7.2±0.3 (a) | 9.0±0.4 (b) | 11.3±1 (b) | 9.2±0.1 (b) | ** |
| PIV | 9.9±0.2 (a) | 10.8±0.4 (b) | 11.8±0.2 (b) | 11.8±0.1 (b) | ** |
| TI | 7.4±0.2 (a) | 8.6±0.2 (b) | 9.5±0.2 (b) | 8.4±0.1 (b) | ** |
| TIV | 10±0.2 (a) | 11.4±0.5 (b) | 11.4±0.3 (b) | 11.9±0.1 (b) | *** |
| Lpro | 16.9±0.5 (a) | 20.8±0.6 (b) | 19.8±0.8 (b) | 20.8±0.5 (b) | ** |
| Wpro | 15.5±0.5 (a) | 19.8±0.6 (b) | 18.6±0.7 (b) | 18.9±0.3 (b) | ** |
| W | 8.1±0.6 (a) | 21.3±1.5 (b) | NP | 13.7±0.8 (b) | *** |
Some studies of tarantulas show different aspects of sexual dimorphism. For example, Shillington (2002, 2005) reported the significance of life history sexual dimorphism for Aphonopelma anax Chamberlin, 1940 (Araneae: Theraphosidae). Mature male tarantulas lead a very active life that revolves around searching for females for reproduction. This implies higher energy demands, leading to a higher resting metabolic rate (RMR) than females. Pérez-Miles (2002) described sexual dimorphism related to abdominal urticating hairs for different species of tarantula of the Theraphosinae subfamily; however, few formal studies have reported sexual size dimorphism in tarantulas. Shillington observed that males of A. anax have a smaller abdomen and longer legs than females (Shillington & Peterson, 2002). Pérez-Miles (1989) observed variation in somatic characters between males and females, particularly for legs and prosoma. This study presents, for the first time, clear evidence of sexual size dimorphism in tibia length for Brachypelma. Males of B. vagans have a significantly longer tibia than females, probably a result of selective pressure related to the reproductive role of wandering males. For many species of tarantula, sexually mature males abandon their burrow and actively search for females to mate with over a large geographical area (Shillington, 2002), which makes it more difficult to find males than females. This capacity of males increases their reproductive success and gene dispersal. A recent genetic study of B. vagans (Machkour-M’Rabet et al., 2012) reported that the males of this species have a very high potential for dispersal. As the males cover long distances while seeking a mate (Shillington, 2002; Machkour-M’Rabet et al., 2012), this could have led to the phenotypic adaptation of a longer tibia. Another explanation could be that during reproduction the male hooks the female fangs to elevate the female body and insert the palpal organ into the female genitalia (Shillington & Verrell, 1997); consequently, access to female genitalia could be favored by longer legs in males, particularly pair I, to facilitate the reproduction process. Therefore, the evolutionary process may favor longer legs in males. Male size may also be an adaptation for protection against females that may not predate organisms of an equivalent or larger size, given the risk of injuries.
In non-web building spiders, males had longer legs than females (Walker & Rypstra, 2001), but not bigger prosoma. At the Campeche site females are numerous and smaller than in other populations. In addition, they are smaller than males found within the same population. Few studies have reported cases of geographical variation in sexual dimorphism in arthropods (Bidau & Martí, 2007; Blanckenhorn & Demont, 2004; Stillwell & Fox, 2009). For spiders, Pekár and Vañhara (2006) described geographical sexual size dimorphism in Zodarion rubidum Simon, 1914 (Araneae: Zodariidae), an ant-eating spider. Regions with higher temperatures provide optimal conditions for higher prey availability resulting in larger females (Pekár & Vañhara, 2006). In addition to variations in resource availability, the quality of prey can also influence intraspecific variation in body size (Amarello et al., 2010). However, in Campeche where females are smaller, male size does not appear to be affected. Data were collected at sites with human presence that are frequently inhabited by B. vagans (Machkour-M’Rabet et al., 2005); therefore, there is little pressure from natural predators such as the mammal “coatí”, Nasua narica L., 1766 (Carnivora: Procyonidae) (Hirsch, 2009), since wild mammals do not normally venture into human settlements. The Pepsi wasps (Pepsis spp.; Hymenoptera, Pompilidae), also known as tarantula-hawks, as described by Cazier and Mortenson (1964), may also predate on B. vagans. However, during our time in the communities of Campeche (Hénaut & Machkour-M’Rabet, 2005; Machkour-M’Rabet et al., 2005, 2007, 2011), this species of wasp was rarely observed and does not appear to be an important predator of tarantula populations. The number of individuals per population may explain the morphological differences between females from different populations and, between males and females in the Campeche population. Machkour-M’Rabet et al. (2005, 2007) describe the relationship between traditional human activity, soil characteristics, and the presence of B. vagans in villages. Using the characteristics from these initial studies, it was easy to find B. vagans individuals at other sites, even though they were geographically distant. However, the number of tarantulas varied considerably with more individuals found at the Campeche site. Although, the causes are still unknown, the high number of tarantulas in 11 de Mayo site may indicate that ecological factors are particularly favorable for these spiders in this particular area, although female tarantulas were smaller than those found in other geographical areas. The latter may be associated with a high degree of intraspecific competition, with large numbers of individuals competing for the same resources. Moreover, cannibalism behavior was commonly observed between females at this site (Hénaut & Machkour-M’Rabet, 2005).
The number of spiders collected is a good indicator of the abundance of tarantulas at each locality. The high number of tarantulas observed in Campeche State and the low number of B. vagans observed in others sites of southern Mexico has to be seriously considered when developing conservation strategies for this species. As B. vagans may rapidly go extinct in states where their numbers are so low, there is a clear need for this species to receive adequate protection. Furthermore, large populations of this tarantula in Campeche need to be provided special protection as they represent important centers of dispersal and resilience for this species. Moreover, the population from Campeche, close to the Calakmul Biosphere Reserve, had more individuals than the remaining populations. This may be a result of its proximity to a large protected area, and further research may help us understand the importance of these areas for the conservation of the Mexican redrump Tarantula.
We would like to thank the people from the villages visited during sampling for granting us access to their land and for their hospitality during our stay. Thanks to the reviewers and to the associate editor of the Revista Mexicana de Biodiversidad for the suggestions and comments that improved our work. Thanks to Claudia Vilchis-Nestor for the information on the Cozumel population of Brachypelma vagans. The research was financed by Conacyt project 000000000158138. This study was conducted according to relevant national and international guidelines. Permit # FAUT-0241 was granted to Dr. Yann Hénaut, issued by the Secretaría de Medio Ambiente y Recursos Naturales, according to the Norma Oficial Mexicana NOM-126-ECOL-2000.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.