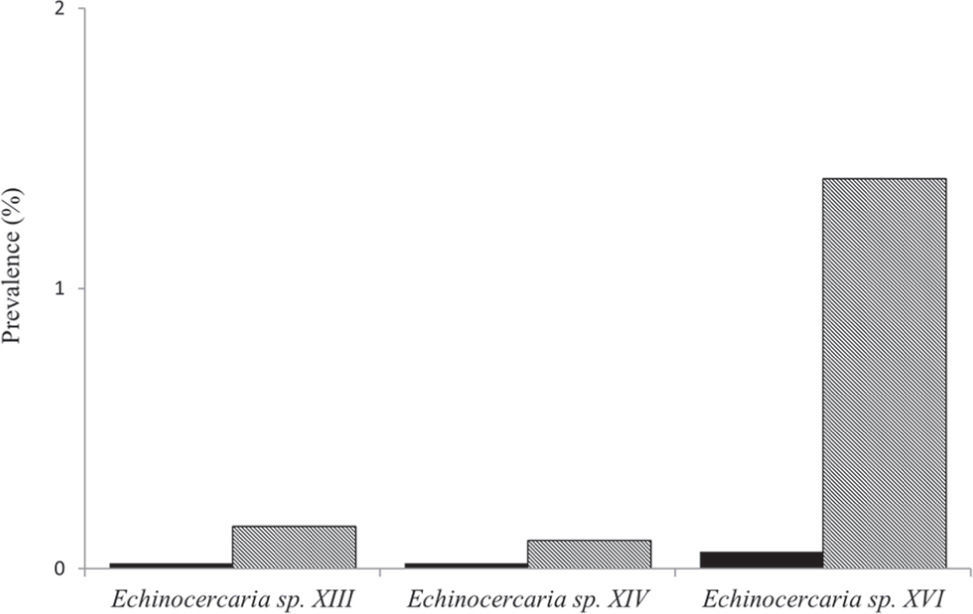
The species of larval Echinostomatidae that infect Biomphalaria straminea (Dunker, 1848) in a ricefield in Corrientes province, Argentina, were studied. Examination of 5 510 snails during 2 rice cultivation cycles, from December 2010 to May 2011 and from December 2011 to April 2012, revealed the presence of 3 new species: Echinocercaria sp. XIII, Echinocercaria sp. XIV and Echinocercaria sp. XVI in 36 snails (0.65%). The most common species was Echinocercaria sp. XVI. Prevalence of 3 species during the first rice cultivation cycle was low (<1%), whereas during the second rice cultivation cycle it was somewhat higher, with prevalence greater than 1% only in Echinocercaria sp. XVI. The species of echinocercariae in B. straminea from the agricultural habitat described in the present study are new additions to the species already reported for the genus Biomphalaria in the region.
Se estudiaron las especies de echinocercarias que infectan a Biomphalaria straminea (Dunker, 1848) en un campo de arroz de la provincia de Corrientes, Argentina. La prospección de 5 510 caracoles durante 2 ciclos de cultivo de arroz, desde diciembre de 2010 a mayo de 2011 y desde diciembre de 2011 a abril de 2012, reveló la presencia de 3 nuevas especies: Echinocercaria sp. XIII, Echinocercaria sp. XIV y Echinocercaria sp. XVI en 36 caracoles (0.65%). La especie más común fue Echinocercaria sp. XVI. Durante el primer ciclo de cultivo de arroz las prevalencias de las 3 especies fueron bajas (<1%), mientras que durante el segundo ciclo de cultivo de arroz las prevalencias fueron algo mayores, con valores superiores al 1% solo en Echinocercaria sp. XVI. Las especies de echinocercarias descritas en el presente estudio parasitando a B. straminea de un ambiente agrícola, se adicionan al registro de especies para el género Biomphalaria en la región.
In South America, some snail species of genus Biomphalaria Preston, 1910 are intermediate hosts of Schistosoma mansoni Sambon, 1907. In Brazil, the American country most affected by this parasite, its natural intermediate hosts are B. glabrata (Say, 1818), B. tenagophila (d'Orbigny, 1835) and B. straminea (Dunker, 1848), in that order of importance (Bezerra et al., 2003; Thiengo and Fernandez, 2007; Lambertucci, 2010).
Although the presence of this parasite has not yet been reported in Argentina, the geographical range of the endemic schistosomiasis areas in Brazil has been expanding to the state of Rio Grande Do Sul, adjacent to northeastern Argentina (Graeff-Teixeira et al., 1999, 2004), an area where 2 of the natural vectors in Brazil, the snails B. tenagophila and B. straminea, are common species (Rumi et al., 2008).
Previous studies on the fauna of larval trematodes in planorbid molluscs of genus Biomphalaria (B. occidentalis Paraense, 1981, B. tenagophila, B. orbignyi Paraense, 1975, B. peregrina (d'Orbigny, 1835) and B. straminea) have been carried out in natural environments of Corrientes province, northeastern Argentina (Ostrowski-de Núñez et al., 1990, 1991, 1997; Hamann et al., 1991) but there is little information concerning agroecosystems such as ricefields (Rumi and Hamann, 1990; Fernández et al., 2013). These latter environments, which provide favorable conditions for the development of dense populations of planorbids, are important from the health perspective due to frequent human direct contact with the water (Rumi, 1986). In turn, Corrientes province is the main rice producer of Argentina, with more than half of its cultivated area occupied by rice crops (Aacrea, 2003).
On the other hand, several studies have demonstrated that some species of echinostome and amphistome larvae may interfere with the natural resistance of the snail to S. mansoni infection (Lie et al., 1977a, b; Adema et al., 2000; Silva Garcia et al., 2010; Spatz et al., 2012). In this sense, it is essential to continue the study of trematode larval species that infect freshwater snails of genus Biomphalaria, especially those echinostome species that may affect the interaction between S. mansoni and its host before the possible introduction of S. mansoni in the area. Therefore, the goals of the present paper are to report and describe new species of echinostome cercariae from the freshwater molluscs B. straminea in a ricefield from the Corrientes, Argentina.
Materials and methodsStudy area. The study site was an agricultural area of 25 ha, with 4 cultivated rice parcels connected or associated to the Paraná river basin; the area is located approximately 30km south from Corrientes city, in Corrientes province, Argentina (27°40'23.5” S; 58°48'21.6” W). During the sampling months, water depth ranged between 5 and 10cm in the cultivated parcels, and between 10 and 50cm in the irrigation canals. Water temperature ranged between 17°C and 28°C in the first rice cultivation cycle and between 18°C and 30.5°C in the second rice cultivation cycle.
In the initial phase of flooding, no vegetation was observed in the irrigation canals; later on, the predominant hydrophilic vegetation consisted of Sagittaria montevidensis Cham. and Schlecht, Ludwigia peploides (Kunt) P.H. Raven, Hydrocotyle ranunculoides L.fil., and Limnobium sp. During the months of sampling several waterbird species were observed: Egretta thula (Molina, 1782), Ardea alba Linnaeus, 1758, Nomonyx dominicus (Linnaeus, 1766), Jacana jacana (Linnaeus, 1766), Vanellus chilensis (Molina, 1782), Himantopus mexicanus (Statius Müller, 1776), Aramus guarauna (Linnaeus, 1766), Mycteria americana Linnaeus, 1758, Tringa flavipes Gmelin, 1789 and Plegadis chihi (Vieillot, 1817).
Sampling and laboratory procedure: snails were collected during 2 rice cultivation cycles in the flooding period, from the time of sowing to soon after harvesting of the rice, between December 2010 and May 2011, and between December 2011 and April 2012. Five samplings were carried out in each rice cultivation cycle. The samples were taken manually by 2 persons who sampled during 1 hour and a half from the cultivated parcels and irrigation canals, using simple mesh nets locally known as “copos” (25cm frame diameter). In the laboratory the snails were kept individually in vials with 20ml of tap water, and were observed for the emergence of cercariae. Apparently uninfected snails were dissected to check for other larval intramolluscan stages (e. g., immature infections and metacercariae). Cercariae were studied alive, with and without vital dyes. Drawings were made using a camera lucida attached to a Carl Zeiss Jena microscope. Cercariae fixed in hot 4% formalin were preserved in vials with 70% ethanol, and deposited in the Helminthological Collection of the Centro de Ecología Aplicada del Litoral, Corrientes, Argentina. Photographs were taken with a Leica DFC 295 camera mounted on a Leica DM 2500 microscope. Specimens studied by scanning electron microscopy were dehydrated in an ethanol series, dried using the critical point technique, coated with gold-palladium and examined with a Jeol 5800 LV Scanning Electron Microscope. Measurements of heat-killed and formalin-fixed specimens are expressed in micrometers (μm), with range followed by the mean ± SD in parentheses. The “open nomenclature” recommended by Odening (1971) was adopted for new species of cercariae. For counts of the number of collar spines the criteria given by Kanev et al. (2009) was followed.
To determine the second intermediate hosts, 3 laboratory-reared larval specimens of Physalaemus albonotatus (Steindachner, 1864) and 4 laboratory-reared specimens of Serrapinnus piaba (Lütken, 1875), collected from an artificial tank were exposed to emerged cercariae. The amphibian larvae and fishes were maintained in small aquaria under controlled conditions until dissection, which was carried out 12–69 hours post-exposure (PE).
ResultsA total of 5 510 snails were examined (2010–2011: n=3 494; 2011–2012: n=2 016), 36 of which (0.65%) were infected with echinostome species. During the first rice cultivation cycle the prevalence of infection ranged between 0.02% (Echinocercaria sp. XIII) and 0.06% (Echinocercaria sp. XVI), and during the second rice cultivation cycle the prevalence of infection ranged between 0.10% (Echinocercaria sp. XIV) and 1.39% (Echinocercaria sp. XVI) (Fig. 1). The most common species was Echinocercaria sp. XVI. The shell size of infected snails ranged from 5.00 to 12.50 (mean=8.12±SD=1.58) in the first rice cultivation cycle and from 5.50 to 12.40 (8.30±1.64) in the second rice cultivation cycle.
The tadpoles of P. albonotatus and fishes of S. piaba were exposed only to Echinocercaria sp. XVI because the infected snails with Echinocercaria sp. XIII and Echinocercaria sp. XIV died before that cercariae could be used for experimental infections. All the tadpoles exposed (n=3), harbored metacercariae after 12 hours; while none of the fishes exposed (n=4), harbored metacercariae after 24, 48 and 69 hours.
Family Echinostomatidae (Looss, 1899) Poche, 1926
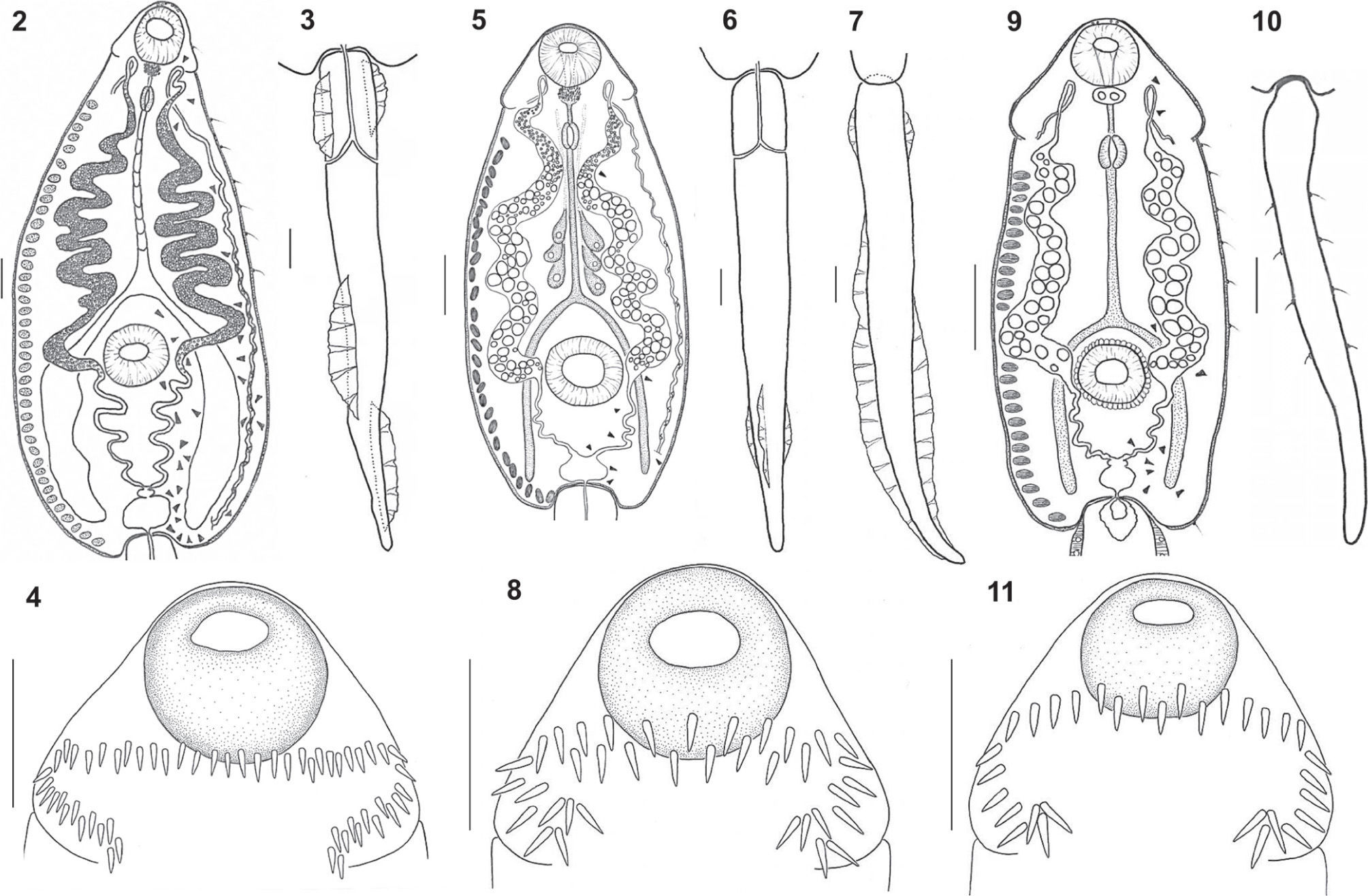
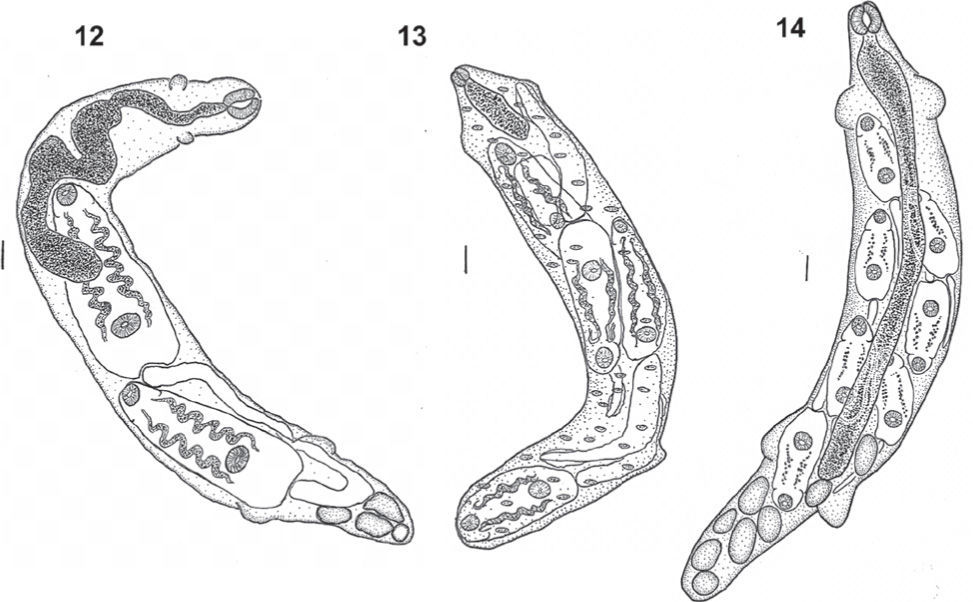
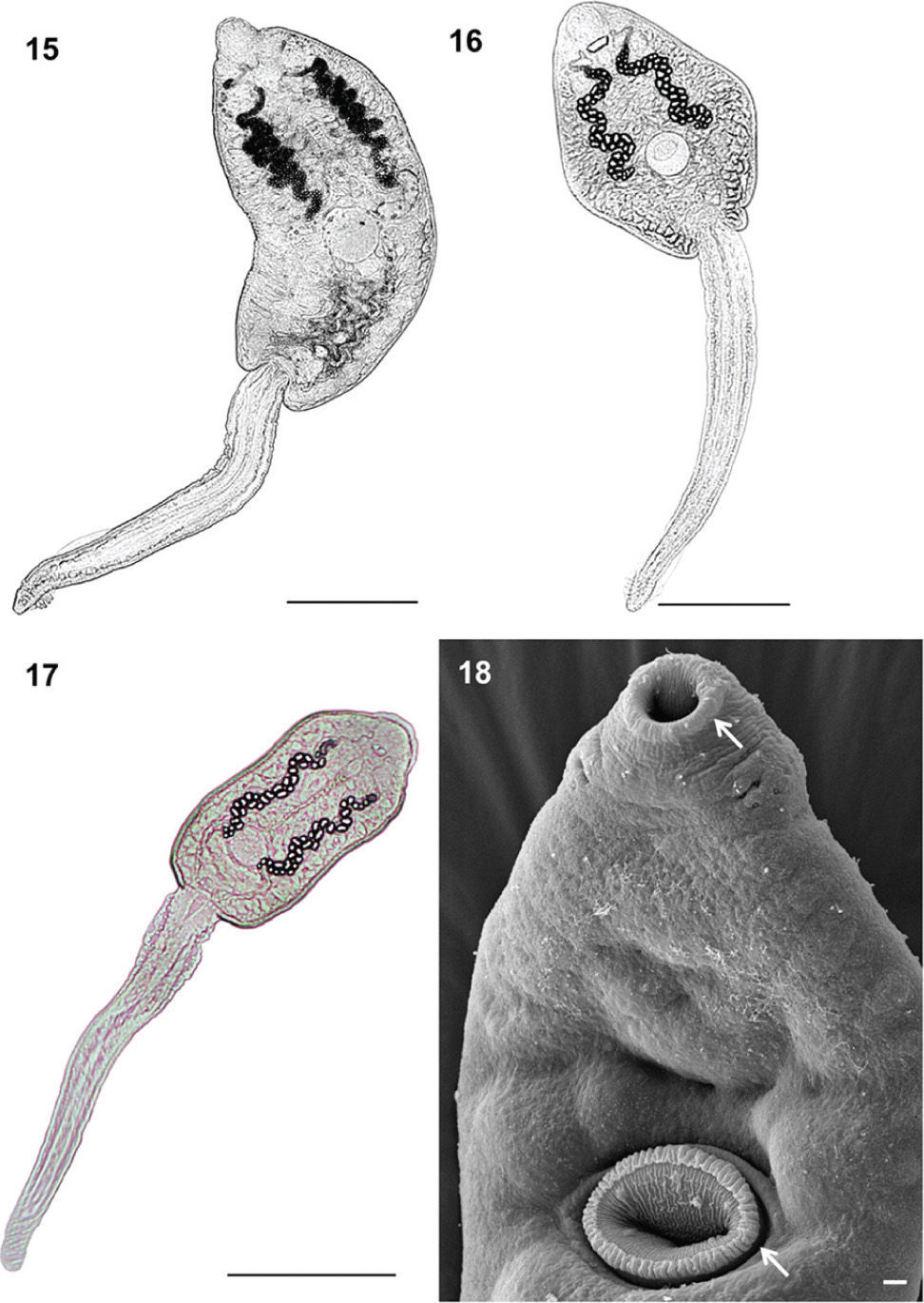
Echinocercaria sp. XIII (Figs. 2–4, 12, 15)
Echinocercaria sp. XIII, 2: cercarial body; 3: tail; 4: detail of head collar with collar spines. Echinocercaria sp. XIV, 5: cercarial body; 6: tail ventral view; 7: tail lateral view; 8: detail of head collar with collar spines. Echinocercaria sp. XVI, 9: cercarial body; 10: tail; 11: detail of head collar with collar spines. Bars= 50μm.
Light micrographs of cercarial bodies. 15: Echinocercaria sp. XIII; 16: Echinocercaria sp. XIV; 17: Echinocercaria sp. XVI, and scanning electron micrograph, 18: Echinocercaria sp. XVI ventral view with detail of trasparent tegumental rim in oral sucker and ventral sucker (arrows). 15–17: Bars= 200μm; 18: bars= 5μm.
Measurements based on 20 formalin-fixed specimens. Body 540–780 (650±79) long by 150–220 (166±18) wide, tegument without spines. Sensory hairs on lateral margins of body: 1 pair at level of oral sucker, and 6 pairs between pharynx and intestinal bifurcation. Head collar developed, with 53–54 spines, arranged as 45–46 dorsal and lateral spines in 2 rows, and 4 corner spines on each side. Oral sucker 46–67 (47±4) long by 41–57 (47±4) wide, prepharyngeal body present, 15–17 (16±1) long by 13–16 (14±1) wide, with numerous spines. Prepharynx short, pharynx muscular, 23–32 (28±2) long by 16–18 (18±1) wide. Esophagus long. Intestine bifurcates shortly anterior to ventral sucker. Ceca broad, reaching level of excretory vesicle. Ventral sucker 62–73 (67±3) long by 57–74 (66±4) wide, slightly larger than oral sucker, situated posterior to midbody. Numerous cystogenous cells with granular contents between main tubes of excretory system and body wall, and with bar and granular-shaped contents between main tubes of excretory system and esophagus. Penetration gland cells not conspicuous, but 6 gland duct openings present on dorsal lip of oral sucker. Excretory system stenostomate, with main tubes dilated between pharynx and posterior border of ventral sucker, filled with small refractile granules, 3–5 in diameter. Flame cells present in at least 29 pairs, probably arranged in groups of 3 flame cells, up to 18 ciliary patches in common collecting ducts. Excretory vesicle divided into 2 unequal chambers. Caudal duct of excretory system enters anterior portion of tail, bifurcates into 2 branches ending on lateral body margins. Tail 490–712 (595±53) long by 30–58 (49±6) wide, with 4 dorsoventral fin-folds, 2 in the proximal end of tail and 2 in the posterior end of tail.
Emergence of cercariae was monitored during 4 days in 2 infected snails. At 19.6–37.5°C cercariae emerged during light hours, with an emergency-peak between 7am and 1pm.
Redia. Body 1 780–2 370 long by 325–350 wide, with muscular pharynx 110–152/80–90 long/wide and intestinal caecum of 1 385–1 500 long occupying more than ½ redial lengths. Two pairs of appendages, 1 anterior and 1 posterior.
Taxonomic summaryPrevalence: 0.02% (2010–2011); 0.15% (2011–2012). Specimen deposited: accession number CECOAL 11050506.
Remarks. The collar with 2 rows of 53–54 (4 corner spines), is similar to those of the genus Hypoderaeum Dietz, 1909. This genus has 43–82 spines arranged in 2 rows in the cephalic collar with 4–5 corner spines, and their adults parasitize birds, with reports mainly in Asia, and also in Europe, North America and Mexico (Yamaguti, 1971; Jones et al., 2005). The cercaría of Hypoderaeum conoideum (Bloch, 1782) Dietz, 1909 is similar to Echinocercaria sp. XIII, regarding the number and arrangement of collar spines (50–54 in 2 alternating rows) and the numerous small refractile granules in the main tubes of the excretory system, but differs by its larger body size (630–980/100–280 in Hypoderaeum conoideum vs. 540–780/150–220 in Echinocercaria sp. XIII), smaller tail size (300–500/50 vs. 490–712/30–58) and by the absence of fin-folds in the tail (Yamaguti, 1975). Hypoderaeum conoideum uses snails of species Lymnaea stagnalis (Linnaeus, 1758) and L. (Radix) limosa (Linnaeus, 1758) as first intermediate hosts, which are distributed mainly in Europe and Asia (Yamaguti, 1971), hence the presence of this parasite in Argentina is unlikely.
In Argentina, Ostrowski-de Núñez et al. (1997) described Echinocercaria sp. V from B. orbignyi in Corrientes province with more than 50 collar spines (although only 48 were figured), a prepharyngeal body with spines in rosette, similar measurements of body, tail and refractile granules in the excretory system, but differs from the present Echinocercaria sp. XIII in its the branched excretory canals, which contain the refractile granules. Unfortunately, the exact number of collar spines was not established. Martorelli (2003) described Cercaria Echinostoma sp. 3 from B. tenagophila in Timboy Stream, Corrientes province with 58 collar spines, branched excretory canals and considerably smaller measurements.
Adult digenetic trematodes with 53–54 spines in the cephalic collar have not been reported for Argentina (Lunaschi et al., 2007).
Echinocercaria sp. XIV (Figs. 5-8, 13, 16).
Measurements based on 30 formalin-fixed specimens. Body 270–366 (310±25) long by 138–222 (187±22) wide, tegument without spines. Head collar well developed, with 37 spines, arranged as 29 dorsal and lateral spines in 2 rows and 4 corner spines on each side. Oral sucker subterminal, 34–46 (41±4) long by 32–46 (39±5) wide. Prepharyngeal body present, 14–21 (15±2) long by 18–23 (22±1) wide, with numerous spines forming rosette. Pharynx muscular, 18–23 (21±2) long by 11–18 (15±2) wide, esophagus long, intestine bifurcate just anterior to ventral sucker, ceca dorsal to excretory tubes, reaching level of excretory vesicle. Ventral sucker 44–62 (49±5) long by 46–69 (52±6) wide, larger than oral sucker, situated posterior to midbody. Numerous cystogenous cells with bar-shaped contents, between pharynx and end of the body. At least 3 pairs of penetration glands observed in the central body between main collecting tubes and esophagus. Excretory system stenostomate, with main tubes extending from anterior wall of excretory vesicle to prepharyngeal level; main collecting tubes dilated between pharyngeal and ventral sucker levels and filled with 84–112 refractile granules ranging in size between 1.5–10 in diameter, the smaller ones near pharynx. Flame cells arranged in at least 8 pairs, up to 9 ciliary patches in common collecting ducts. Caudal duct of excretory system enters anterior portion of tail, bifurcates into 2 branches ending on lateral body margins. Tail 480–642 (566±37) long by 42–60 (49±5) wide, with 7 finfolds: 2 dorsal, 2 ventral, 2 very small lateral and 1 ventral small lobulated at level of 2 lateral.
Emergence of cercariae was monitored during 4 days in 2 infected snails. At 19.5–36.7 °C cercariae emerged during the night and early morning hours (0 am - 8 am). Redia. Orange-brown pigmented body 1 170–2 669 long by 272–372 wide, with muscular pharynx 47–57/41–54 long/wide, and intestinal cecum 236–570 long occupying less than ⅓ of redial length. Two pairs of appendages, 1 anterior and 1 posterior.
Taxonomic summaryPrevalence: 0.02% (2010–2011); 0.10% (2011–2012). Specimen deposited: accession number CECOAL 12022907.
Remarks. The only cercaria with 37 collar spines described from the area is Cercaria Echinostoma sp. 2 Martorelli, 2003 from B. tenagophila in Boquerón de Franquía, Uruguay (adjacent to Corrientes province). It differs from Echinocercaria sp. XIV by its larger body (413×197 vs. 310×187), smaller tail (337×46 vs. 566×49), fewer refractile granules in main tubes of excretory system (59–65 vs. 84–112) and the arrangement in a single row of collar spines.
The present cercaria is similar to those of the genus Echinostoma Rudolphi, 1809 in the number of collar spines within the 31–51 range, and the possession of findfolds in the tail, but differs in the arrangement of the lateral collar spines in 2 rows. Several cercariae of Echinostoma species with 37 spines have been cited in Brazil. Echinostoma lindoense Sandground and Bonne, 1940, Echinostoma barbosai Lie and Basch, 1966 and Echinostoma paraensei Lie and Basch, 1967 were reported in snails of the genera Biomphalaria Preston, 1910, Echinostoma erraticum Lutz, 1924 in snails of genera Biomphalaria and Drepanotrema Fischer and Crosse, 1880, Echinostoma luisreyi Maldonado, Vieira and Lanfredi, 2003 and Echinostoma rodriguesi in snails of the genera Physa Draparnaud, 1801 (Komma, 1972; Lie, 1968; Pinto and Melo, 2013). The definitive hosts of E. lindoense, E. rodriguesi and E. erraticum are birds and mammals, those of E. barbosai and E. luisreyi are birds, and those of E. paraensei are mammals (Pinto and Melo, 2013).
Two adult of Echinostoma species with 37 spines in the cephalic collar (Echinostoma chloephagae Sutton and Lunaschi, 1980; Echinostoma mendax Dietz, 1909) have been reported for Argentina paraziting anseriform birds (Anatidae) and 2 species, Echinostoma revolutum (Frölich, 1802) Rudolphi, 1809 and Echinostoma rodriguensi Hsu, Lie and Bash, 1968, paraziting rodent mammals (Lunaschi and Drago, 2007; Lunaschi et al., 2007). In Brazil adults of E. mendax, with an arrangement of collar spines similar to Echinocercaria sp. XIV (dorsal and lateral spines in 2 rows), have been described parasitizing anseriform birds of the family Anatidae (e. g., Cairina moschata (Linnaeus, 1758)) (Travassos et al., 1969).
Echinocercaria sp. XVI (Figs. 9-11, 14, 17-18).
Measurements based on 20 formalin-fixed specimens. Body 116–227 (198) long by 92–122 (110) wide. Tegument without spines, with 6 pairs of short lateral sensory setae between pharynx and ventral sucker and 1 pair at excretory vesicle level. Head collar well developed, with 31 spines arranged as 7 dorsal spines in 2 rows, 8 lateral spines and 4 corner spines on each side. Oral sucker subterminal, 25–32 (29) long by 23–34 (30) wide, with transparent tegumental rim. Prepharyngeal body present with 2 globular inclusions. Prepharynx short, pharynx muscular, 14–18 (16) long by 11–14 (12) wide. Esophagus long, intestine bifurcates just anterior to ventral sucker, ceca dorsal to excretory tubes, reaching level of excretory vesicle. Ventral sucker 21–34 (30) long by 28–41 (34) wide, situated posterior to midbody, with conspicuous, transparent tegumental rim. Numerous cystogenous cells with bar-shaped contents, between pharynx and end of body. Penetration gland cells not conspicuous, but 4 gland duct-openings visible on dorsal lip of oral sucker. Excretory system stenostomate, with main tubes extending from anterior wall of excretory vesicle to prepharyngeal level; main collecting tubes dilated between pharyngeal and ventral sucker levels and filled with 26–42 (32) refractile granules, 8-16μm in diameter. Flame cells difficult to see, arranged in at least 10 pairs, excretory vesicle divided into 2 unequal chambers. Caudal branch of excretory system enters anterior part of tail, where it becomes reduced to a triangular sac, with no visible pores. Tail 306–448 (398) long by 30–48 (42) wide, without fin-folds and with 4 pairs of sensory setae.
Emergence of cercariae was monitored during 2 days in 3 infected snails. At 24.6–36.6°C cercariae have emergency peaks between 0 am and 7 am. They penetrated in exposed tadpoles of Physalaemus albonotatus, and encysted in the body musculature, especially in the mouth region. Oval cysts with double wall measured 114–132 (127±5) long by 78–90 (83±5) wide at 12 hours PE. The morphology of metacercariae is very similar to that of the cercariae.
Redia. Orange-brown pigmented rediae vary considerably in size of body and pharynx; larger rediae were found in low numbers in each infected snail (3–5). Body 510–1 650 long by 130–410 wide, muscular pharynx 40–210 /40–175 long/wide, intestinal cecum 380–1 520 long reaching end of the body in the larger rediae and more than ½ of redial length in smaller ones. Two pairs of appendages, 1 anterior and 1 posterior.
Taxonomic summaryPrevalence: 0.06% (2010–2011); 1.39% (2011–2012).
Specimen deposited: accession number CECOAL 11022108.
Remarks. The number and arrangement of collar spines of this cercaria is similar to one of the 11 models of collar spine arrangements described for the genus Echinostoma by Kanev et al. (2009), but differs in the position of the first and second lateral spines, which in Echinocercaria sp. XVI correspond to the double row of dorsal spines. Two species of this genus with 31 collar spines parasitize birds: Echinostoma anseris Yamaguti, 1939 in Japan and China and Echinostoma sudanense Odhner, 1911 in Africa (Yamaguti, 1971; Kanev et al., 2009). In America, Dietziella egregia (Dietz, 1909) with 31 collar spines was reported paraziting Harpiprion caerulescens (Vieillot, 1817) in Brazil and paraziting Plegadis chihi in Argentina, both threskiornithid birds (Travassos et al., 1969; Digiani, 2000). In Argentina, Echinocercaria sp. II Ostrowski-de Núñez et al. 1990 from B. straminea and B. orbignyi, and Echinocercaria sp. III Ostrowski-de Núñez et al. 1991 from B. occidentalis in Corrientes province, are similar to this new species in the number (7) and arrangement of dorsal spines, the presence of a prepharyngeal body with 2 globular inclusions, cystogenous cells with bar- shaped contents, 4 gland duct-openings on the dorsal lip of the oral sucker, tail without fin-folds, caudal branch of the excretory system reduced to a triangular sac, and similar emergence of cercariae, but they differ by having 27 instead of 31 spines in the head collar.
DiscussionThe present study describes 3 echinostome cercariae parasitizing B. straminea in a rice field from Corrientes, Argentina, which can now be added to the 8 echinocercaria species previously reported from Biomphalaria spp. in this region (Ostrowski-de Núñez et al., 1990, 1991, 1997; Martorelli, 2003).
The prevalence of the 3 species was low during the first rice cultivation cycle (<1%), whereas it was somewhat higher during the second cycle, with a prevalence greater than 1% only in Echinocercaria sp. XVI. The higher prevalence of Echinocercaria sp. XVI in the second rice cultivation cycle could be related to the increased presence of the definitive hosts in the environment. Low levels of prevalence have been observed in previous studies from field collections when the number of snails collected was high (Ostrowski-de Núñez et al., 1990, 1991, 1997; Fernández et al., 2013). Generally, prevalence level seems to be related with sample size: when the number of snails collected is high, the prevalence of infection is relatively low and vice versa (Ewers, 1964; Ostrowski-de Núñez et al., 1991).
Agricultural wetlands such as rice fields can harbor numerous species of aquatic invertebrates and vertebrates including fishes, amphibians and birds (Czech and Parson, 2002; Elphick and Oring, 2003; Stenert et al., 2009; Machado and Maltchik, 2010; Maltchik et al., 2011). In turn, with the increase of rice fields and other similar agricultural activities throughout the world, these agroenvironments have become important refuges for water birds (Czech and Parson, 2002), which may be reflected in the number of species recorded during samplings. Although the life cycles of the cercariae described herein are not known, birds could possibly be their definitive hosts, based on the known life cycles of similar echinocercariae. Further, the positive results of experimental infections in Echinocercaria sp. XVI in tadpoles but not in fish, have suggested that its second intermediate host should be amphibian larvae, which are included in the diet of several species of birds recorded in this agricultural environment. In this sense, the study of larval trematodes parasitiziting snails provides information about the biodiversity of both the parasites and their hosts, harbored in agricultural wetlands.
Finally, given the importance of B. straminea as an intermediate host of S. mansoni in endemic regions adjacent to northeastern Argentina, and of rice fields as environments with which humans have frequent direct contact, the study of larval trematodes in agricultural habitats of Corrientes province should be encouraged to obtain further information about larval trematode species, especially those that may affect the interaction between S. mansoni and its host, before the possible introduction of S. mansoni in the area.