We report the first record of Litomosoides pardinasi in native and exotic rodents from Chile. Litomosoides pardinasi, described in the Argentine Patagonia parasitizing Phyllotis xanthopygus and Oligoryzomys longicaudatus, was found in Chile parasitizing the peritoneal and thoracic cavities of O. longicaudatus (prevalence (P)= 18.9%, mean intensity (MI)= 57.3) and Phyllotis darwini (P=4.2%, MI=10), and in the peritoneal cavity of Rattus rattus (P=1.24%; MI=22.5). Total P in native rodents from Chile was significantly lower than in Argentina, while the total MI was higher. Prevalence and MI of L. pardinasi in O. longicaudatus from Chile and Argentina showed non-significant differences; prevalence in P. darwini from Chile was significantly lower than in P. xanthopygus from Argentina and than in Chilean O. longicaudatus. Our results, together with those from Argentina, support the hypothesis that L. pardinasi is well established in O. longicaudatus, but seems to be recently acquired by P. darwini and the exotic R. rattus. Considering the known host distribution of Litomosoides species among the sigmodontines, our results also support the hypothesis that L. pardinasi first colonized the Oryzomyini tribe and later, by different phenomena of host-switching, colonized the Phyllotini tribe and the exotic R. rattus.
Litomosoides pardinasi es registrada por primera vez en roedores nativos y exóticos de Chile. La especie Litomosoides pardinasi, parásita de Phyllotis xanthopygus y Oligoryzomys longicaudatus en la Patagonia Argentina, fue encontrada en Chile parasitando la cavidad torácica y abdominal de O. longicaudatus (prevalencia (P)= 18.9%, intensidad media (MI)= 57.3) y de Phyllotis darwini (P=4.2%, MI=10), y la cavidad abdominal del roedor exótico Rattus rattus (P=1.24%; MI=22.5). La P total en roedores nativos de Chile fue significativamente menor que la P de Argentina, en tanto la MI total fue significativamente mayor. La P y MI de L. pardinasi en O. longicaudatus de Chile y Argentina no mostraron diferencias significativas; la P en P. darwini de Chile fue significativamente menor que la de P. xanthopygus de Argentina y que la de O. longicaudatus de Chile. Considerando la distribución de las especies de Litomosoides entre los sigmodontinos, estos resultados apoyan la hipótesis que L. pardinasi está bien establecida en O. longicaudatus y que posiblemente haya sido recientemente adquirida por P. darwini y R. rattus. Así, L. pardinasi colonizó primero a la Tribu Oryzomyini y posteriormente por un fenómeno de cambio de hospedero a la Tribu Phyllotini y a R. rattus.
Worms of the genus Litomosoides Chandler, 1931 are filarioid parasites of the thoracic and abdominal cavities of bats, marsupials, and rodents of the families Ctenomyidae, Echimyidae, Sciuridae, and Cricetidae (Esslinger, 1973; Bain et al., 1989; Notarnicola et al., 2002, 2010). They occur in Neotropical and southern Nearctic regions. Most of the Litomosoides species occur in South America, including Venezuela (6 species), Colombia (10 species), Peru (3 species), Bolivia (5 species), Argentina (8 species), Brazil (11 species), and Uruguay (1 species) (Travassos, 1919; Esslinger, 1973; Bain et al., 1980, 1989; Brant and Gardner, 1997; Moraes Neto et al., 1997; Notarnicola et al., 2000, 2002; Notarnicola and Navone, 2002, 2011; Guerrero et al., 2002, 2003, 2011).
All the records of Litomosoides species have been reported in native host species, with the exception of some specimens of Litomosoides sigmodontis Chandler, 1931 found in one individual of the exotic Rattus norvegicus Berkenhout, 1769 —originally mentioned as Mus decumanus— from 131 specimens trapped in Caracas, Venezuela (Vogel and Gabaldon, 1932).
Among the species of this genus, Litomosoides pardinasiNotarnicola and Navone, 2011 is the southernmost worldwide record described in cricetid rodents from Neuquén, Argentina (Notarnicola and Navone, 2011). These authors found L. pardinasi in Phyllotis xanthopygus Waterhouse, 1837 with a prevalence of 25% and a mean intensity of 7.2, and in Oligoryzomys longicaudatus Bennett, 1832 with 33% and 6, respectively.
In Chile, several studies have reported helminths in rodents (e.g., Babero and Cattan, 1975; Durette-Desset et al., 1976; Landaeta-Aqueveque et al., 2007a, 2007b), but none have reported filarioid species. Filarioids in Chile include Dipetalonema reconditum Grassi, 1980, D. dracunculoides Cobbold, 1870, and Dirofilaria repens Railliet and Henry, 1911 in dogs (Alcaíno et al., 1984; López et al., 2012), and Setaria equina Abildgaard, 1789 in horses (Alcaíno and Gorman, 1999). Argentina and Chile share several rodent species and some are known to be hosts for this group of nematodes in Argentina.
This study reports the first record of L. pardinasi in rodents of Chile. We also report new hosts and the first record of L. pardinasi in the exotic rodent Rattus rattus Linnaeus, 1758. Finally we discuss the evolution of the host-parasite associations of this nematode.
Materials and methodsWe sampled 1 150 native and exotic rodents between the latitudes 31°30'32” S, 35°08'13” S, and between 0 and 1 100m asl in central Chile, for a total of 19 sampling sites. Localities covered heterogeneous landscapes encompassing several eco-regions, ranging from arid- Mediterranean, to humid-Mediterranean. Rodents were analyzed for the presence of intestinal, peritoneal, and pleural helminths. Trapped native species consisted of 344 Abrothrix olivaceus Waterhouse, 1837, 10 Abrothrix longipilis Waterhouse, 1837, 58 O. longicaudatus, 119 Phyllotis darwini Waterhouse, 1837 (Cricetidae), 183 Octodon degus Molina, 1782 (Octodontidae), and 7 Abrocoma bennetti Waterhouse, 1837 (Abrocomidae), and the exotic rodents were 161 R. rattus, 87 R. norvegicus, and 181 Mus musculus Linnaeus, 1758 (Muridae). Rodents were trapped with Sherman traps baited with rolled oats, and killed using isoflurane anesthesia. Captures followed all ethical guidelines of the Bioethical Committee of the Facultad de Ciencias Veterinarias y Pecuarias, Universidad de Chile, and were performed with the permission of the Chilean Agriculture and Livestock Bureau (Servicio Agrícola y Ganadero of Chile) and the Chilean National Forest Corporation (Corporación Nacional Forestal).
Worms were recovered directly from the peritoneal and pleural cavities and preserved in 70% ethanol. For examination, samples were cleared with ethanol-glycerin solution and observed under light microscope (Olympus BX51); drawings were made with the aid of a drawing tube. Some worms were studied under Scanning Electron Microscope (SEM Hitachi TM 3000, Pontificia Universidad Católica de Chile, Facultad de Ciencias Biológicas). Measurements are given in micrometers unless otherwise stated; mean values follow the range in parentheses; when two specimens are given, measurements are separated by semicolons.
Prevalence (P) and mean intensity (MI) were calculated following Bush et al. (1997). Fisher's exact test was used to compare prevalences and the negative binomial regression methodology (NBR) was used to analyze intensities. Comparisons with Argentinean P and MI correspond to these reported by Notarnicola and Navone (2011). Statistical analyses were performed in R environment (R Core Team, Vienna, Austria, http://www.R-project.org/).
Voucher specimens of worms were deposited at the Museo Nacional de Historia Natural de Chile, Invertebrate Collection (MNHNCL/NEM), and voucher specimen of rodents were deposited at the Museo Nacional de Historia Natural de Chile - Vertebrate Collection (MNHN), and the Patricio Sánchez Reyes Flora and Fauna Collection (UCK) -Pontificia Universidad Católica de Chile.
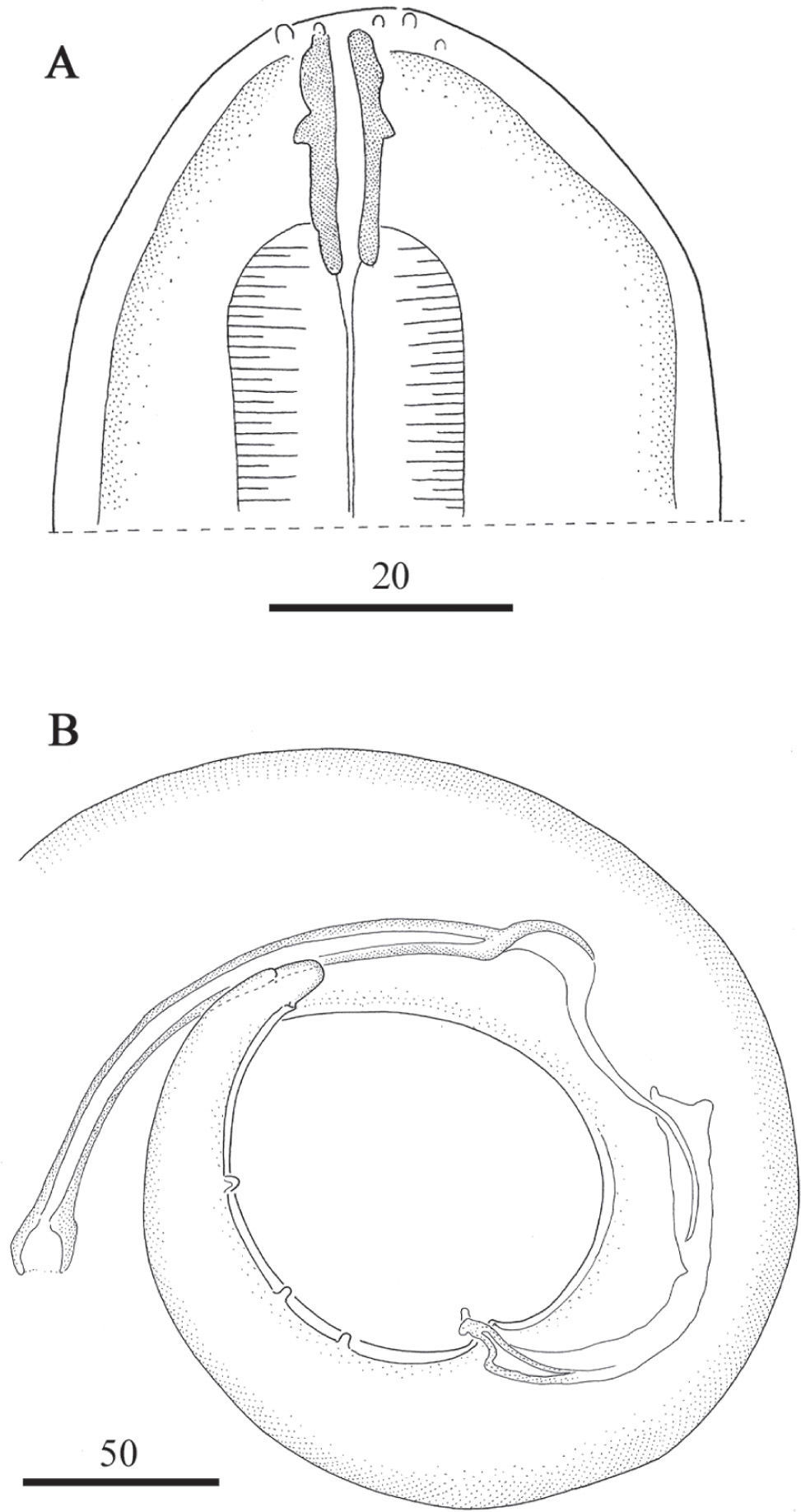
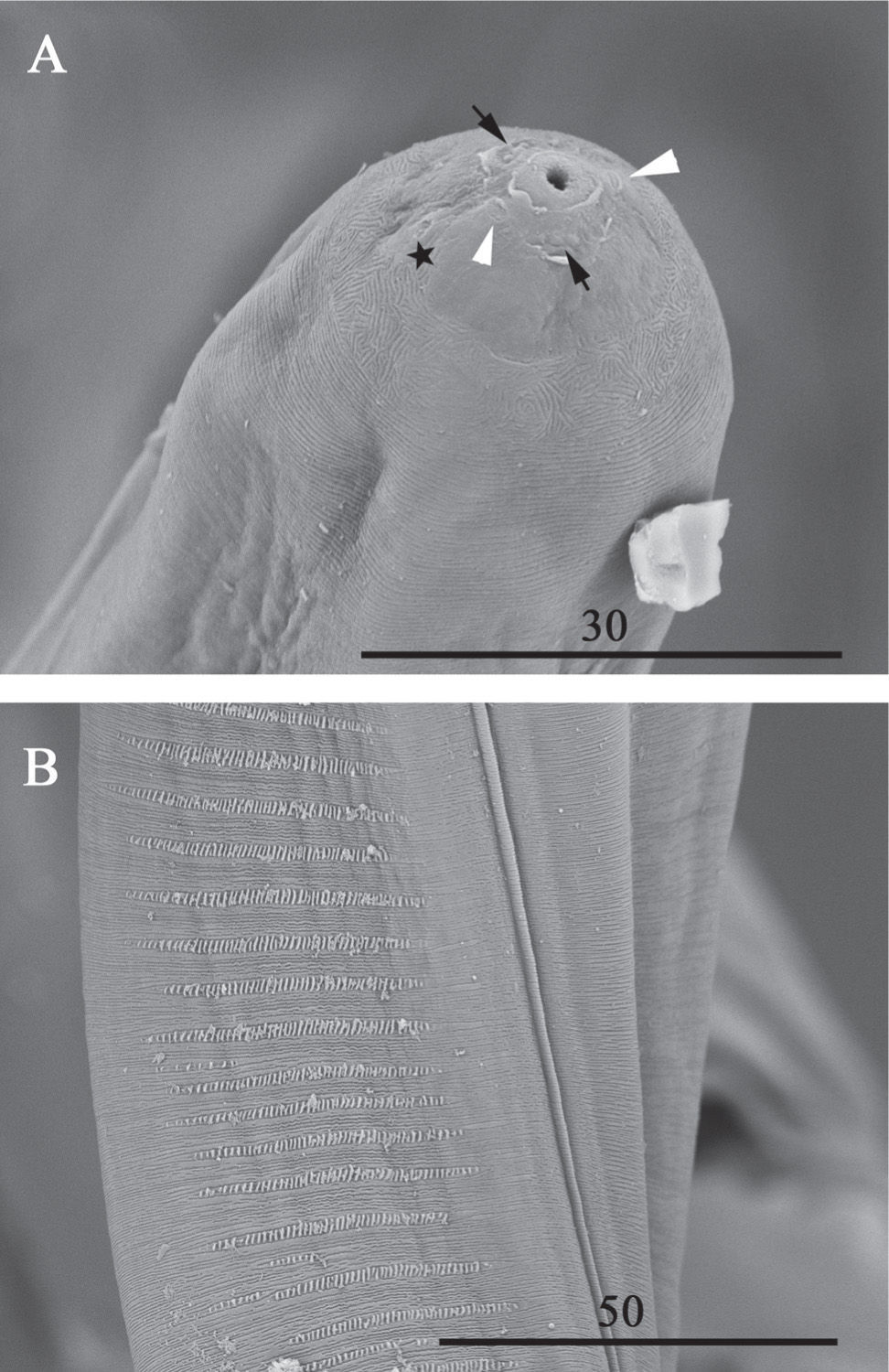
ResultsThe filarioids were identified as L. pardinasi according to the morphological and morphometric characters stated by Notarnicola and Navone (2011). Specimens show the following characters: the shape of the buccal capsule displays an anterior enlargement in the cuticularized segment (Fig. 1A); the left spicule is constituted with a handle longer than the blade; blade consisting of a filament; the right spicule is well cuticularized, with a prominent dorsal heel and a terminal cap; the tail is attenuated with one pair of ad-cloacal papillae and three to four pairs of slightly asymmetric postcloacal papillae (Fig. 1B); the arrangement of the head papillae consists of two dorsal cephalic papillae near the amphids and four labial papillae (Fig. 2A); the area rugosa constituted by transverse ridges made of small longitudinal crests (Fig. 2B); and the position of the vulva, posterior to the esophago-intestinal junction. Measurements of the specimens are included in Table 1.
Measurements of Litomosoides pardinasi parasitizing 3 different hosts in Chile
| P. darwini | O. longicaudatus | R. rattus | |
|---|---|---|---|
| Females | (n=2) | (n=6) | (n=2) |
| Body length (mm) | 55.4* | 56.1 (36.2–72.2) | 53.3 † |
| Maximum width | – | 244 (190–325) | 310† |
| Width at vulva | 186; 207.7 | 177.1 (160–200) | 170.5 |
| Buccal capsule (L×W) | 19×9; 22×9 | 18 (15–20)×8 (7–10) | 19×8; 25×12 |
| Esophagus length | 415; 533 | 529 (432–645) | 496; 567 |
| Tail length | – | 260 (225–312) | 855 † |
| Vulva to apex | 772; 961 | 1 132 (925–1 335) | 1 271; 1 559 |
| Males | (n=3) | (n=5) | (n=6) |
| Body length (mm) | 21.5 (20.3–22.7) | 15.5 (10.2–18.7) | 18.1 (15–22.2) |
| Maximum width | 99 (83–108) | 127 (100–140) | 111 (105–120) |
| Buccal capsule (L×W) | 19 (16–22) × 8 (6–9) | 17 (16–20)×7 (5–8) | 18 (13–20) × 7 (6–9) |
| Esophagus length | 423 (418–427) | 405 (350–462) | 452 (424–475) |
| Tail length | 139 (99–186) | 132 (115–160) | 146 (125–170) |
| Left spicule | 199 (180–217) | 222 (170–241) | 210 (153–232) |
| Handle | 112 (93–130) | 132 (115–150) | 107 (93–124) |
| Right spicule | 101 (96–108) | 105 (87–117) | 116 (85–197) |
| Area rugosa length | 1 622 (1 333–2 046) | 1 514 (1 350–1 700) | 1 467 (1 162–1 725) |
Hosts and localities: Oligoryzomys longicaudatus Bennett, 1832 from Los Queñes (35°08'13” S, 70°45'19” W), UCK 423; from Polpaico (33°09' S, 70°53' W), MNHN 1598; from Rinconada de Maipú (33°29' S, 70°49' W), PM84,
PM183, PM184, PM204, PM207, PM233, PM264, PM266; from Calera de Tango (33°38' S, 70°47' W), PL186. Phyllotis darwini Waterhouse, 1837 from Las Chinchillas National Reserve (31°30' S, 71°06' W), MNHN 1599, Dn56, T66, L56, K56. Rattus rattus from Fundo El Bosque (33°32' S, 70°48' W), M180; from La Pintana (33°34' S, 70°37' W), M145, Chile.
Site of infection: abdominal and peritoneal cavities.
Specimens deposited: voucher specimens at the Museo Nacional de Historia Natural de Chile, Invertebrates Collection numbers MNHNCL/NEM 11853; 11854; 11855.
Remarks. Litomosoides pardinasi was found in the peritoneal and thoracic cavities of 11 O. longicaudatus (P=18.9%; MI=57.3) and 5 P. darwini (P=4.2%; MI=10), and in the peritoneal cavity of 2 R. rattus (P=1.24%; MI=22.5) captured in 7 areas located in Chile. Total prevalence and total mean intensity in native host species were 9% and 42.5, respectively. Phyllotis darwini showed significantly lower prevalence than O. longicaudatus (p=0.003), and non-significantly higher prevalence than R. rattus.
When comparing our data with that from Argentina, total prevalence in Chile, excluding R. rattus (9%, n=177), was significantly lower than in Argentina (25.5%, n=47) (p=0.005). When comparing prevalence from the same host genus, in Chilean P. darwini it was significantly lower (4.2%, n=119) than in the Argentinean P. xanthopygus (25%, n=44) (p<0.001), while prevalence in Chilean O. longicaudatus (19%, n=58) was not significantly different from that of the Argentinean O. longicaudatus (33.3%, n=3) (p=0.490). The total mean intensity in Chile excluding R. rattus (42.5) was significantly higher than in Argentina (7.08) (Likelihood ratio test (LRt): p=0.001). Analyzing by host species or genus, there were no significant differences between the mean intensities of O. longicaudatus from Chile (57.3) and Argentina (6) (LRt: p=0.150); the same was observed between the Phyllotis spp. (P. darwini: 10 vs. P. xanthopygus: 7.2; LRt: p=0.560).
DiscussionHere we report the first record of L. pardinasi in Chile and expand the host record to P. darwini and R. rattus. Identification was based on morphological attributes and supported by morphometric characters. Most of the measurements of Chilean L. pardinasi averaged between the minimum and maximum measurements of Argentinean samples (Notarnicola and Navone, 2011). However some differences were observed: female tails of L. pardinasi found in O. longicaudatus from Chile are shorter than those obtained from Argentina, while the female tail from the specimen found in R. rattus is similar to the specimen of O. longicaudatus from Argentina (855 versus 800μm; Table 1). Male worms from the three host species found in Chile presented shorter tails than those found in Argentina. The Chilean specimens also had shorter left spicules (153–241μm versus 210–270μm). However, the spicular ratio from Chilean specimens fit within the range of the specimens from Argentina (i. e., 1:1.96 in P. darwini; 1:2.12 in O. longicaudatus, and 1:1.91 in R. rattus from Chile versus a range of 1:1.8 to 1:2.4 in P. xanthopygus and 1:2.09 in O. longicaudatus from Argentina). Morphometric variability was reported within other Litomosoides species and these observations were attributed to differences in the host species and/or geographic distribution (Esslinger, 1973; Bain et al., 1989; Guerrero et al., 2002; Notarnicola 2005; Notarnicola et al., 2010).
Prevalences and mean intensities of L. pardinasi in O. longicaudatus from Chile and Argentina showed non-significant differences, while in Phyllotis spp. the prevalence from the Chilean specimens was significantly lower than in Argentina. Thus, it appears that L. pardinasi is well established in O. longicaudatus based on the similarities in their prevalences and mean intensities in Chile and Argentina. In contrast, this filarioid species seems to be recently acquired by P. darwini and the exotic R. rattus in Chile, based on the significantly lower prevalence, suggesting that this parasitism is a result of a recent host-switching phenomenon. Moreover, of the 16 Litomosoides species registered in Sigmodontinae rodents, 6 are present in Akodontini, 9 in Oryzomyini —including L. pardinasi—, and 1 in both Akodontini and Oryzomyini. Litomosoides pardinasi is the unique species registered in Phyllotini. This result supports the hypothesis that L. pardinasi primarily colonized the Oryzomyini tribe and later, by different phenomena of host-switching, colonized the Phyllotini (P. xanthopygus in Argentina and P. darwini in Chile) and the exotic host R. rattus. The host-switching phenomenon was frequently reported between native rodents living in sympatry (Guerrero et al., 2002; Notarnicola, 2005; Notarnicola and Navone, 2011).
Vogel and Gabaldon (1932) reported the presence of adults and microfilariae of L. sigmodontis in R. norvegicus from Caracas, Venezuela. This filarioid species was also found to parasitize the cotton rat Sigmodon hispidus Say and Ord, 1825 from Mexico and USA (Ochoterena and Caballero, 1932; Forrester and Kinsella, 1973). Female specimens of L. pardinasi found in R. rattus presented microfilariae in the uterus, indicating that its life cycle can be completed in the host and can be transmitted to other hosts. All these results confirm that filarioid species are able to colonize allochthonous rodents when they share microhabitats with parasitized native rodents.
The mite Ornithonyssus bacoti Hirst, 1913 (Acari, Macronyssidae) has been reported as a vector for several species of Litomosoides in rodents as well as in bats (Bain et al., 1980, 2002). Another study showed that Hoplopleura travassosi Werneck, 1932 (Anoplura, Hoplopleuridae) presented positive association with Litomosoides bonaerensis Notarnicola, Bain and Navone 2000 (Lareschi et al., 2003). In Chile, O. bacoti has been recorded in several native and allochthonous rodents (Jofré et al., 2009; Peña Oyarce, 2009); whereas H. travassosi and Hoplopleura aitkeni Johnson, 1972 have been found in O. longicaudatus and P. darwini, respectively, in a geographic range that encompasses this work (González-Acuña et al., 2003; 2005). The presence of these arthropods in native and allochthonous rodents provide more information to the hypothesis that filarioids are transmitted by these vectors in rodents living in sympatry.
The authors wish to thank Maria Cristina Estivariz from CEPAVE for the drawings and Eileen Smith for editorial support. This work was supported by the Conicyt Ph. D. scholarships awarded to C.L. (number 24110058) and J.P.C. (AT-24100028), and Fondecyt grants awarded to P.E.C (1070960), C.B. (11090086) and F.T.P. (1110664). JN is a member of Conicet.