The presence of the genus Gephyrocharax and the first record of its species G. valencia are confirmed from the island of Trinidad, based on a comparison with all species of the genus. Gephyrocharax valencia is the first species of the genus recorded for an island.
Se confirma la presencia del género Gephyrocharax y el primer registro de su especie G. valencia en la isla de Trinidad, basados en una comparación con todas las especies del género. Gephyrocharax valencia es la primera especie del género documentada para una isla.
Gephyrocharax Eigenmann is a small group of characid fishes no larger than 70mm SL. The genus has 12 valid species that were described, mainly using the diagnosis proposed by Eigenmann (1912) and Eigenmann and Myers (1929): G. atracaudata (Meek and Hildebrand), G. caucanus Eigenman, G. chaparae Fowler, G. chocoensis Eigenmann, G. intermedius Meek and Hildebrand, G. major Myers, G. martae Dahl, G. melanocheir Eigenmann, G. sinuensis Dahl, G. valencia Eigenmann, G. venezuelae Schultz, and G. whaleri Hildebrand. They occur in most basins of southern Central America, and northern and central South America, being distributed in Panama (Cerro-Azul and former Canal Zone drainages, Frijoles and Chame River systems), Colombia (Cauca, San Juan, Atrato, San Jorge, and Magdalena River basins), Venezuela (Lago de Valencia, Lago Maracaibo, and Orinoco River basins) and Bolivia (Beni and Chapare River basins). Until now, Gephyrocharax has not been recorded for Trinidad or other islands in any catalogue of fishes published (Gill, 1858; Price, 1955; Boeseman, 1960; Weitzman, 2003; Eschmeyer, 2012). Additionally, about 7 valid species of Characidae are recorded in Trinidad, a low number for the richest family of Characiformes (Reis et al., 2003).
The senior author examined specimens from 2 lots tentatively identified as Gephyrocharax (USNMN 349208 and UWIZM 2010.14.25), which were collected by the second author in the 1990s from Moriquite and Moruga rivers in Trinidad. Also, a third sample collected by H. Axelrod in 1958 from Piarco (a locality also on Trinidad) was examined (USNM 310578, incomplete locality data; possibly the Caroni River basin). We considered that only 2 of these 3 lots from Trinidad should be assigned to the genus Gephyrocharax according to our findings. Thus, the aim of this note is to confirm the presence of the genus Gephyrocharax and to validate the record of G. valencia for Trinidad, based on adult specimens and a morphological comparison with all species of the genus; ecological notes on the species are provided.
The specimens examined are deposited in the following institutions: AMNH, ANSP, CAS; FMNH; ICNMHN; INHS; MBUCV, MCZ, UF, and USNM. Acronyms of museums are according to Sabaj (2012), except UWIZM (Zoology Museum, Department of Life Sciences, The University of the West Indies, Trinidad and Tobago). Measurements were taken point-to-point with a digital caliper and expressed as percentages of standard (SL) or head length (HL) for subunits of the head. Measurements and counts follow Fink and Weitzman (1974) and Menezes and Weitzman (2009), with the addition of dorsal-fin to pectoral-fin length (from the base of first unbranched dorsal-fin ray to the base of unbranched pectoral-fin ray), dorsal-fin to adipose-fin length (from the base of the first unbranched dorsal-fin ray to the anterior-most point of the base of the adipose fin), pectoral fin to pelvic fin (from the base of the unbranched pectoral-fin ray to the base of the unbranched pelvic-fin ray), pelvic-fin to anal-fin length (from the base of the unbranched pelvic-fin ray to the base of anterior-most externally visible anal-fin ray), and postorbital head length (from the posterior border of the eye to the posterior-most point of the bony opercle). In reporting counts, the mode is enclosed in parenthesis. Specimens were cleared and counterstained (C and S) following Taylor and Van Dyke (1985). Total vertebral counts include the first preural centrum plus first ural centrum (PU1+U1) counted as 1 vertebral element, and separately the 4 vertebrae of the Weberian apparatus. In addition to the comparative material, keys and descriptions of the Gephyrocharax species were used to identify the specimens under study (Eigenmann, 1912; Eigenmann and Myers, 1929; Schultz, 1944).
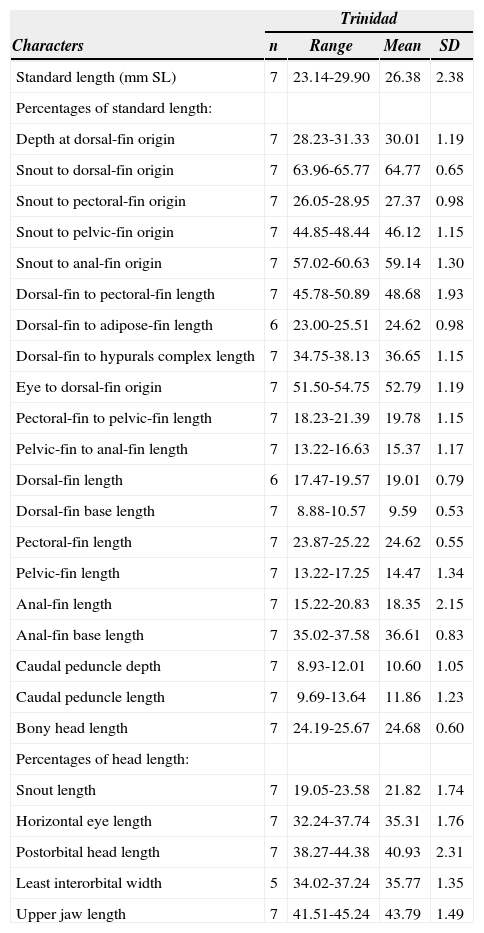
Morphometric data of the specimens examined are presented in Table 1. These specimens correspond to Gephyrocharax valencia (Fig. 1) and are characterized by 38–40 (39) lateral-line scales, 6–7 (6) longitudinal scales between the lateral line and dorsal-fin origin, 5 longitudinal scales between the lateral line and the analfin origin in all specimens, 5 longitudinal scales between the lateral line and the pelvic-fin origin in all specimens, 18–20 (18) predorsal scales, 14–15 (15) scales around the caudal peduncle, ii,8 dorsal-fin rays in all specimens, iv-v,25–27 (iv,27) anal fin-rays, i,6 pelvic-fin rays in all specimens, i,8–9 (i,9) pectoral-fin rays, 1 tooth usually tricuspidate in maxilla, 4–5 (5) teeth tri-to pentacuspidate in the inner row of premaxilla, 4 teeth usually tricuspidate in the outer row of premaxilla in all specimens, 11–13 (13) teeth conical to pentacuspidate in dentary, frontal fontanel and adipose fin developed, humeral spot absent or undifferentiated from chromatophores on the lateral band of body, caudal spot developed on peduncle region and not extending on middle caudal-fin rays, and 38-39 (39) vertebrae in 4 specimens. The males have a claw-shaped structure formed by the ventral and posterior procurrent rays 2 and 3 on the lower lobe of the caudal fin, a pouch scale on the lower lobe of caudal fin, a gill-gland anteriorly developed on the ventral limb of the first branchial arch, and bony hooks on the rays of the pelvic, anal, and caudal fins.
Morphometric data for Gephyrocharax valencia. UWIZM 2010.14.253 and USNM 349208. Seven specimens from the Moriquite River, southern coast of Trinidad. SD= standard deviation
| Characters | Trinidad | |||
|---|---|---|---|---|
| n | Range | Mean | SD | |
| Standard length (mm SL) | 7 | 23.14-29.90 | 26.38 | 2.38 |
| Percentages of standard length: | ||||
| Depth at dorsal-fin origin | 7 | 28.23-31.33 | 30.01 | 1.19 |
| Snout to dorsal-fin origin | 7 | 63.96-65.77 | 64.77 | 0.65 |
| Snout to pectoral-fin origin | 7 | 26.05-28.95 | 27.37 | 0.98 |
| Snout to pelvic-fin origin | 7 | 44.85-48.44 | 46.12 | 1.15 |
| Snout to anal-fin origin | 7 | 57.02-60.63 | 59.14 | 1.30 |
| Dorsal-fin to pectoral-fin length | 7 | 45.78-50.89 | 48.68 | 1.93 |
| Dorsal-fin to adipose-fin length | 6 | 23.00-25.51 | 24.62 | 0.98 |
| Dorsal-fin to hypurals complex length | 7 | 34.75-38.13 | 36.65 | 1.15 |
| Eye to dorsal-fin origin | 7 | 51.50-54.75 | 52.79 | 1.19 |
| Pectoral-fin to pelvic-fin length | 7 | 18.23-21.39 | 19.78 | 1.15 |
| Pelvic-fin to anal-fin length | 7 | 13.22-16.63 | 15.37 | 1.17 |
| Dorsal-fin length | 6 | 17.47-19.57 | 19.01 | 0.79 |
| Dorsal-fin base length | 7 | 8.88-10.57 | 9.59 | 0.53 |
| Pectoral-fin length | 7 | 23.87-25.22 | 24.62 | 0.55 |
| Pelvic-fin length | 7 | 13.22-17.25 | 14.47 | 1.34 |
| Anal-fin length | 7 | 15.22-20.83 | 18.35 | 2.15 |
| Anal-fin base length | 7 | 35.02-37.58 | 36.61 | 0.83 |
| Caudal peduncle depth | 7 | 8.93-12.01 | 10.60 | 1.05 |
| Caudal peduncle length | 7 | 9.69-13.64 | 11.86 | 1.23 |
| Bony head length | 7 | 24.19-25.67 | 24.68 | 0.60 |
| Percentages of head length: | ||||
| Snout length | 7 | 19.05-23.58 | 21.82 | 1.74 |
| Horizontal eye length | 7 | 32.24-37.74 | 35.31 | 1.76 |
| Postorbital head length | 7 | 38.27-44.38 | 40.93 | 2.31 |
| Least interorbital width | 5 | 34.02-37.24 | 35.77 | 1.35 |
| Upper jaw length | 7 | 41.51-45.24 | 43.79 | 1.49 |
Three valid species of Gephyrocharax have been recorded in cis-and trans-Andean basins from Venezuela (Maracaibo, Caribbean, and Orinoco basins): G. melanocheir, G. valencia, and G. venezuelae (Bonilla et al., 2002). The cis-Andean basin, where G. valencia occurs, is the area geographically closest to the island of Trinidad. Gephyrocharax valencia is readily distinguished from G. venezuelae and G. melanocheir by the absence of a humeral spot (vs. such spot present). Also, G. valencia differs from G. venezuelae by the absence of a black or dark brown pigmentation along the middle caudal-fin rays (vs. such pigmentation present) and from G. melanocheir by the absence of a dark pigmentation strongly concentrated along the base of the anterior dorsal-fin rays (vs. such pigmentation present). Based on these differences and others stated by Eigenmann and Myers (1929) and Schultz (1944), we propose that the best identification for the specimens from Trinidad is Gephyrocharax valencia. We consider that this identification also is supported by the following reasons: the specimens have the diagnostic characters of the genus and the species proposed by Eigenmann (1912, 1920), Eigenmann and Myers (1929), and Schultz (1944); the comparison with type and non-type specimens of all species of the genus confirmed our assignation (n=52); and the few minimal deviations in morphometric data and none relevant in meristic and/or osteological data of the specimens studied compared with the type and non-type specimens of G. valencia examined (snout to pelvic-fin origin length [44.85–48.44% SL, mean= 46.12% SL vs. 41.53 47.03% SL, mean= 44.04% SL], dorsal-fin to pectoral-fin length [45.78–50.89% SL, mean= 48.68% SL vs. 38.96–48.74% SL, mean= 46.49% SL], and dorsal-fin to hypurals complex length [34.75–38.13% SL, mean= 36.65% SL vs. 36.48–43.62% SL, mean= 39.38% SL]). We conclude that these deviations in the morphometric data are possibly associated with a populational variation within the species.
On Trinidad, Gephyrocharax valencia was collected from the middle reaches of the Moruga and Moriquite drainages, the largest river systems draining the south coast of the island (Fig. 2). The topography in this region is hilly, and both rivers run through muddy to sandy mud substrates, which they have eroded to a depth of 2m and width of 3–4m. Both rivers have similar water characteristics: depth varies from 0.3m in the dry season to 2m in the wet; current speed can be stagnant or sluggish to slow (0.3m/s); water temperature 24−25° C; dissolved oxygen, 4.2−6mg/l; conductivity, 265–370μS/cm; pH 6.7. The sites differed in levels of total suspended solids (16mg/l at the Moruga site vs. 380mg/l at the Moriquite), biochemical oxygen demand (2.8mg/l at the Moruga, and 5.6mg/l in the Moriquite), and conductivity (368mg/l at the Moruga, and 265mg/l at the Moriquite). In the Moruga River, G. valencia was collected with 8 other species of fishes, including the characid Corynopoma riisei Gill, whereas in the Moriquite River, it was collected with 5 species of fishes, but with the only other characid being Astyanax bimaculatus (Linnaeus).
Price (1955), Boeseman (1960), and Kenny (1995) proposed that most of the freshwater fishes on Trinidad colonized the island from the South American mainland to the south, at the time when Trinidad was still part of the mainland. In fact, Diaz de Camero (1996) proposed a paleogeographical association between the Orinoco River, the Columbus Channel, and the southern portion of Trinidad during the Neogene. Kenny (1988) provided evidence that the separation of Trinidad from the mainland occurred as recently as 1000-1600 years ago, when the remaining land bridge located at the extreme end of the south western peninsula of Trinidad was breached. Kenny (1995) believed that post-separation colonization was achieved by fishes crossing the Columbus Channel during the rainy season, when the discharge of freshwater from the Orinoco River decreases the salinity in the sea surrounding the island. Although detailed information on the capacities of migration and tolerance to lower concentrations of salinity are unknown in Gephyrocharax valencia, we suggest that the species could colonize the island via the Columbus Channel. Phylogenetic, ecophysiological, and biogeographical studies on Gephyrocharax are needed to test this dispersal hypothesis.
Material examined. Gephyrocharax valencia:Trinidad, West Indies: UWIZM 2010.14.253, 5, 23.14–29.90mm SL (2 C and S 25.22–27.99mm SL), Moriquite River, approximately 10°6'51.54” N, 61°17'39.78” W at 24m a.s.l. USNM 349208, 2 of 9 (2 x-rays), 24.78–28.27mm SL, Moruga, Moruga River, Basse Terre Village, approximately 10°07.904’ N 61°15.436’ W at 46m asl. Comparative material. Corynopoma riisei:Venezuela: MBUCV 285, 3, 27.43–35.40mm SL (2 C and S, 27.43–34.06mm SL). USNM 310578, 2 of 9 (2 x-rays), 23.20–31.86mm SL. Colombia: FMNH 56400, holotype (x-ray) of Stevardia aliata Eigenmann, 45.35mm SL. Gephyrocharax atracaudatus:Panama: AMNH 37808, 20 of 82, 29.13–38.16mm SL. FMNH 7573, holotype (x-ray) of Deuterodon atracaudata Meek and Hildebrand, 43.71mm SL.Gephyrocharax caucanus: Colombia: FMNH 56012, holotype (x-ray), 49.98mm SL. MCZ 35811, 1 (x-ray), 37.53mm SL. MCZ 35872, 1 of 2 (x-ray), 43.20mm SL. USNM 81921, 3 paratypes (3 x-rays), 42.51–48.05mm SL. Gephyrocharax chaparae: Bolivia: ANSP 68967, holotype (x-ray), 32.46mm SL. ANSP 68968, 6 of 11 paratypes (3 x-rays), 30.95–44.71mm SL. ANPS 68979, 1 paratype, 31.01mm SL. ANSP 69195, holotype (x-ray) of Corynopomops opisthopterus Fowler, 29.76mm SL. Gephyrocharax chocoensis:Colombia: CAS 44278, 1 of 9 paratypes. FMNH 56016, holotype (x-ray), 48.57mm SL. MCZ 30956, 1 of 8 (x-ray), 44.33mm SL. USNM 79208, 2 paratypes (2 x-rays), 44.71–47.75mm SL. Gephyrocharax intermedius:Panama: FMNH 8945, holotype (x-ray), 43.92mm SL. FMNH 12511, 1 paratype, 33.08mm SL. FMNH 12512, 1 paratype, 31.00mm SL. USNM 78556, 2 of 26 (2 x-rays), 33.38–36.51mm SL. Gephyrocharax major: CAS 44286, 9 syntypes (9 x-rays), 35.87–56.44mm SL. Gephyrocharax martae:Colombia: ZMUL 3703, 34.51mm SL. Gephyrocharax melanocheir:Colombia: CAS 44292, 1 of 6 paratypes. CAS 44293, 1 of 4 paratypes, 35.42mm SL. FMNH 69554, 3 of 9 paratypes (3 x-rays), 32.50–39.30mm SL. USNM 79209, 2 paratypes (2 x-rays), 30.89–34.50mm SL.Venezuela: UF 23806, 5, 22.17-28.17mm SL. Gephyrocharaxsinuensis:Colombia: ICNMHN 6843, 9, 30.73–39.56mm SL. Gephyrocharax valencia:Venezuela: ANSP 134924, 2 of 6 C and S, 24.51–32.71mm SL. CAS 44295, 1 of 2 paratypes, 30.37mm SL. CAS 44297, holotype (x-ray), 27.68mm SL. INHS 60438, 8, 28.18–39.83mm SL. UF 80511, 45, 18.38–36.25mm SL. Gephyrocharax venezuelae:Venezuela: MCZ 37269 (Ex USNM 121366), 5 paratypes (5 x-rays), 28.97–33.84mm SL. USNM 121369, holotype (x-ray), 30.87mm SL. Gephyrocharax whaleri:Panama: FMNH 36760, 1 paratype, 48.75mm SL. FMNH 36761, 1 paratype, 45.14mm SL. USNM 106513, holotype (x-ray), 38.08mm SL. USNM 235926 (Ex USNM 109276), 2 paratypes (2 x-rays), 37.17–38.80mm SL.
We thank María de las Mercedes Azpelicueta and Juan M. Mirande who provided constructive comments that improved an initial version of the manuscript. We also thank the following individuals and institutions for permission to visit collections under their care, loan, exchange, and gift of specimens, photographs and radiographs: John Lundberg, Mark Sabaj, and Kyle Luckenbill (ANSP); Barbara Brown (AMNH); Jon Fong and Dave Catania (CAS); Mary Rogers, Philip Willink, Kevin Swagel, and Chris Jones (FMNH); Jose I. Mojica, Ofelia Mejía, and Gustavo Ballen (ICNMHN); Daniel Wylie and Chrys (INHS); Francisco Provenzano (MBUCV); Karsten Hartel and Andrew Williston (MCZ); Larry Page and Rob Robins (UF); Richard Vari and Sandra Raredon (USNM); M. Rutherford (UWIZM), and Lars Lundquist (ZMUL). Bruno Pianzola helped with the figure 1. Financial support was provided by the Project 2814, Fundación para Promoción de la Investigación y la Tecnología, Banco de la República, Colombia (JAVR), Latin-American grant CONICET-Argentina (JAVR), the PhD program funding (Expte: 1000-007351/2011), Facultad de Ciencias Naturales y Museo, UNLP (JAVR), and the Darwin Initiative for the Survival of Species.