Immature stages of Mycomya chilensis were laboratory-reared from material associated with Trametes versicolor, from a Nothofagus forest in Argentinean Patagonia (Chubut province). The adult male is redescribed and the larva and pupa are described for the first time. This is the first association between Mycomya and its host in South America, and the first description of immature Mycetophilidae in Patagonia. Notes on the biology of M. chilensis are also provided.
Se criaron en laboratorio los estadios inmaduros de Mycomya chilensis, provenientes de material asociado a Trametes versicolor, que fueron colectados en un bosque de Nothofagus de la Patagonia Argentina (provincia de Chubut). Se redescribe el macho, en tanto la larva y pupa son descritas por primera vez. Ésta es la primera asociación entre una especie de Mycomya y su huésped para América del Sur, y la primera descripción de un estadio inmaduro de Mycetophilidae de la Patagonia. Se incluyen además notas sobre la biología de M. chilensis.
Mycetophilidae, commonly known as “fungus gnats”, are a diverse and cosmopolitan family of Diptera containing more than 4,000 species described in more than 150 extant genera (Oliveira & Amorim, 2014). In the Neotropical region, including the Subantarctic forests of Patagonia (these forests actually belong to the Andean region, not the Neotropical region, see Morrone, 2015), Mycetophilidae are one of the most diverse dipteran families, with 1,145 species described in 54 genera (Oliveira & Amorim, 2014). Taxonomic knowledge of the family in South America is mainly due to the work of Duret (e.g., 1976, 1978), Lane (e.g., 1956, 1962), Freeman (1951, 1954), and Edwards (e.g., 1931, 1932, 1933, 1934, 1940). Recent additions to the taxonomy and phylogeny of the group include, among others, Amorim, Oliveira, and Balbi (2008), Amorim and Oliveira (2008), Oliveira (2013), and Oliveira and Amorim (2010a, 2010b). Knowledge of the biology and immature stages of the group, however, is almost nonexistent in South America, being restricted to larval description and biological observations of Neoempheria puncticoxa Edwards in Brazil (Oliveira, Albertoni, Borkent, & Amorim, 2015).
Mycomya Rondani and Noempheria Osten Sacken are the largest genera and possess the widest distribution in the subfamily Mycomyinae (Väisänen, 1984). For the Neotropical region, 86 Mycomya species are recognized of which 31 are known from the Patagonian Subantarctic forest and the Malvinas Islands in Argentina and Chile. The fauna of this region was mainly described by Freeman (1951). A number of papers have been published on the biology of immature stages of Mycomya larvae but most are from the Nearctic, Palearctic, or Holarctic regions (e.g., Krivosheina & Zaitzev, 2008; Madwar, 1937; Ševčík, 2010). For the Neotropical region, larval stages of Mycomya are unknown, as are its biological aspects. In this paper, we describe the immature stages (larva and pupa) of Mycomya chilensis for the first time and we redescribe the adult male. We also include comments on the biology of the larva and pupa.
Materials and methodsSpecimens were collected in the months of November and December, 2015 in the Nothofagus forest of Central Chubut (Argentinean Patagonia). Mushroom fruiting bodies were separated together with part of the substrate (a piece of log) and taken into laboratory. Fungi were placed in jars with humid sand covered with a mesh, and moistened 3–4 times a week or as needed. Larvae were observed on a daily basis. Larvae and adults were fixed in 70% ethanol. Larval head and mouthparts were observed under microscope after being cleared with 10% KOH solution then neutralized with a weak solution of glacial acetic acid. Specimens were observed using a Leica DM 500 microscope equipped with a Leica EC3 camera. Measurements were taken using a Leitz Wetzlar Dialux compound microscope equipped with a micrometer. Drawings were prepared with a drawing mirror on a Leica MZ6 at 40× magnification. General terminology for Diptera follows that of McAlpine (1981), while specific morphological terminology for Mycetophilidae follows Väisänen (1984) for adults and Courtney, Sinclair, and Meier (2000) for larvae.
Material studied: 7 larvae, Argentina, Chubut Province, “Los Alerces” National Park, Nothofagus forest near Arrayanes campsite, path to the “Lahuan”, 42°44′41″ S, 71°44′33″ W, Leg. P. Pessacq & C. Pardo, 14.xi.2015; 2 male adults, reared in laboratory, same data as above, pupated on 27.xi.2015 and emerged on 3.xii.2015; 3 pupae reared in laboratory, same data as above, pupated on 18.xi.2015, 24.xi.2015, and 28.xi.2015, but were partially eaten by Coleoptera larvae.
DescriptionMycomya chilensis (Blanchard, 1852) (Figs. 1–8)
Sciophila chilensis Blanchard in Gay, 1852: 533–534 (male description, type locality: Coquimbo, Chile); Philippi, 1865: 624 (record for Coquimbo, Chile); Bigot, 1888: DV. 13 (first record for Tierra del Fuego); Lynch-Arribálzaga, 1892: 421 (male redescription); Johannsen, 1909: 38 (mentions that S. chilensis could belong to Mycomya).
Mycomya chilensisFreeman, 1951: 32, Figs. 10, 12, 75 (male redescription, genitalia figures, new records for Argentina in Río Negro and Neuquén provinces, and Chile in X Region); Oliveira & Amorim, 2014: 27 (included in Neotropical catalog).
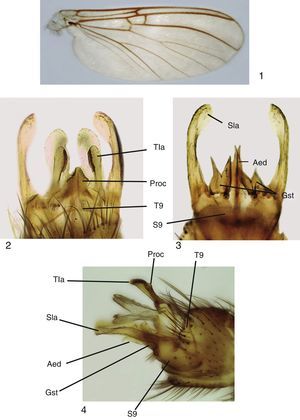
Adult male. Head: vertex brownish, with small scattered setae. Two clearly visible ocelli located in middle of vertex. Frons and clypeus yellowish. Palpus yellowish, with 5 segments, first 2 very short, last palpomere longer than remaining ones, approximately 2× longer than fourth palpomere. Antenna with scape and pedicel rounded, shorter than first flagellomere, both with ring of small setae on basal margin. Flagellum brownish, with 14 flagellomeres, each slightly longer than wide; last flagellomere thinner than remaining ones. Antenna 2× longer than thorax. Thorax: pronotum pale yellow, with long setae over its surface. Scutum light brown, covered with small setae and long bristles scattered at the sides and organized in a mid-dorsal line and 2 lateral lines on dorsum. Proepisternum bare, yellowish; proepimeron bare, light brown; proepisternum bare, pale yellow; anepisternum bare, light yellow, turning brownish at the central portion; katepisternum bare, dorsal half pale yellow turning light brown at the ventral portion; mesepimeron bare, pale yellow; laterotergite bare, light brown; mediotergite bare, pale yellow; scutellum yellowish, with a few bristles and several small setae. Legs pale yellow, fore coxa with scattered bristles and small setae on its anterior margin, with a row of apical small setae; mid coxa with scattered bristles and small setae on anterior side of distal half; hind coxae with a lateral row of small setae and a few bristles and scattered small setae on its posterior margin. Trochanter with scattered small setae. Femur with a row of thick short setae on flexor margin and scattered small setae on its surface. Tibiae with 2 rows of thick short setae on extensor margin and several rows of longitudinal setae on its surface. Wing venation and color pattern as shown in Figure 1. Abdomen: Tergites brownish, sternites light yellow. Terminalia (Figs. 2–4): tergite 9 well developed, processus short and relatively wide, with triangular apex (Fig. 2); tergal lateral appendages with rounded apex and a comb-like row of small teeth at the inner margin (Fig. 2); sternite 9 well developed, gonocoxite wide, with 4 or 5 setae at gonostylus base; gonostylus bifid, each lobe slender, with acute apex (Fig. 3); aedeagus bifid, slender, longer than gonostylus (Fig. 3). Sternal lateral appendages slightly curved (Figs. 3 and 4), with rounded and spatulated apex.
Mycomya chilensis, male adult. (1) Wings; (2) terminalia, ventral view; (3) terminalia, dorsal view; (4) terminalia, lateral views. Abbreviations: Aed: aedeagus; Gst: gonostylus; Proc: central processus; Sla: sternal lateral appendages; S9: sternite 9; Tla: tergal lateral appendages; T9: tergite 9.
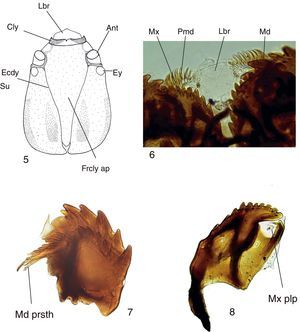
Mycomya chilensis, larva. (5) Head, dorsal view; (6) detail of mouthparts, ventral view; (7) mandible, dorsal view; (8) maxilla, dorsal view. Abbreviations: Ant: antenna; Cly: clypeus; Ecdy su: ecdysial suture; Ey: stemmata; Frcly ap: frontoclypeal apotome; Lbr: labrum; Md: mandible; Md prsth: prostheca; Mx: maxilla; Mx plp: maxilary palp; Pmd: premandible.
Mature larva description (Figs. 5–8). Probably fourth instar larva, length: 13.87±0.41mm (n: 5), general body shape cylindrical, with no projections, creamy white in color, with 12 segments, segments 5–8 wider and longer than remaining ones. Head capsule (Fig. 5) bare, well sclerotized, ovoid. Ecdysial suture V-shaped, lightly sinuous (forming a very shallow “S”). Frontoclypeal apoteme almost reaching the posterior border of the head capsule. Eye postero-lateral to the antenna (Fig. 5). Mouthparts well-sclerotized, occupying 1/3 of anterior head capsule in ventral view. Labrum broad and fleshy, ventrally with 3 small, rounded sensory papillae on each side (Fig. 6), clypeum with acute anterolateral margins and medial “V” emargination (Fig. 5). Premandible with row of poorly sclerotized teeth (Fig. 6). Mandible (Fig. 7) semicircular, with 2 rows of teeth, 1 apical, with 7 wide teeth, the central ones larger than the remaining ones; second row dorsal, subapical, with 5 teeth, smaller than those of the first row, in a few specimens an additional outer small tooth or an additional small tooth basal to the innermost tooth, prostheca with many seta, the apical ones short and acute, progressively longer and with bifurcated apex (Fig. 7). Maxilla (Fig. 8) with inner border bearing 13 small teeth; outer border straight, without teeth, with 3 sensory papillae.
Body with 1 pair of prothoracic, and 7 pairs of abdominal spiracles; prothoracic spiracle slightly larger than abdominal ones. Locomotor pads translucent, slightly visible.
Pupa. Body nearly cylindrical, light brown, free. Antenna curved over eyes, reaching middle of the wing. Wing sheath extending beyond half of abdomen. Legs held together along ventral part of the body. Seven pairs of small lateral abdominal spiracles; ten apparent abdominal segments. Entire body bare.
Taxonomic summaryBiology. Fourth stage larvae pupated after 4–14 days in the laboratory. Five specimens reached the stage of pupa, but only 2 emerged as adults. The remaining ones were killed by within-sample predatory coleopteran larvae. Adults emerged after 6 days. Larvae of Mycomya chilensis were observed in the field on the ventral surface of Trametes versicolor fresh fruiting bodies and are thus categorized as macromycobionts, as designated by Krivosheina and Zaitzev (2008). In the laboratory they were found both in the dorsal and ventral surfaces of the fungus, sometimes hiding deep between adjacent fructiferous bodies. No production of silk or tubules was observed in the field or laboratory. Larvae were observed free on the surfaces of the fruiting bodies, moving over the substrate sometimes along slime strands. Larvae pupated on the dorsal surface of mushrooms or in the sand used as substrate, without any kind of silk associated to it.
Distribution. Nothofagus forests at both slopes of the Andes, in the provinces of Chubut, Río Negro, Neuquén, and Tierra del Fuego (Argentina) and the X Region of Chile (Freeman, 1951). This is the first record for the species in Chubut province.
RemarksThe original description of Mycomya chilensis (Blanchard, 1852) is very brief and does not include figures. Fortunately, Freeman (1951) includes figures of the genitalia of this species in ventral and dorsal view. In order to improve species recognition, we have included the description of the thoracic sclerites, figures of the wings, and other characters that were missing in the original and subsequent descriptions.
The major contribution of our study, however, is the description of the immature stages and their biological aspects. The larva of Mycomya, with a few exceptions, inhabit the fruiting bodies of bark fungi or the mycelia under bark, spinning slimy webs (Jakovlev, 2011). Krivosheina and Zaitzev (2008) also mention Mycomya as epibionts living in the surface of fruiting bodies inside slime or silk tubules. These tubules are also reported by Madwar (1937) in Mycomya marginata, a species which lives under bark covered by Poria vaporaria and attached by silk threads. Madwar (1937) also reported silk threads suspending the pupae, mentioning that these threads probably provide moisture from the substrate, and that removal of them is fatal for the pupa. However, in our study no webs of silk or the construction of slime tubules were observed associated with larvae or pupa and the larvae moved freely on the substrate, sometimes over a slime strand. Regarding this, Krivosheina and Zaitzev (2008) mention Neoempheria as living in the open and not inside tubes and states that “the choice between living in the open or inside the slime tubules appears to depend not on the taxonomic group but rather on substrate moisture” and that “the larvae of some Limoniidae species live inside the tubules only on insufficiently moistened substrates”.
Krivosheina and Zaitzev (2008), based on literature, attribute a mixed feeding habitat for the genus (zoophages or mycophages), but conclude that most epibiontic species, including Mycomya, are micromycophages or sporomycophages. The mouthparts of M. chilensis larvae resemble those of other Mycomyiini, such as Neoempheria puncticoxa Edwards and M. marginata Meigen. These species bear wide and slightly curved mandibles, carrying 6 or 7 massive teeth on the outer margin, with a second row of smaller dorsal teeth. According to Krivosheina and Zaitzev (2008) the structure of mandibles in this group is closest to Mycetophilinae (but differs in the number of marginal teeth), and this indicate that, in the case of Mycomya, larvae are more likely to feed on mycelium rather than other microorganisms.
Larval descriptions of Mycomya are rare and therefore, there are few useful characters to facilitate species identification. Nevertheless, the larva of Mycomya chilensis shows notable differences with other larva of the genus, mainly in the head structures. In M. chilensis the ecdysial suture is approximately V-shaped and sinuous, forming a very shallow “S”, while in two other species (e.g., M. marginata, M. wankowiczii (Dziedzicki)), the suture is straight or slightly concave. Also, the shape of the sclerotized portion of the labrum seems to be variable among species. Mycomya chilensis has acute anterolateral margins and a medial “V” emargination, different from other known congenerics. The relative size of the eyes and their position also seems to be variable. In M. chilensis, the eyes are comparatively larger with a more posterior position. On the other hand, other head structures such as the mandible and maxilla, seem to only vary slightly in morphology.
This work was supported by the “Consejo Nacional de Investigaciones Científicas y Técnicas” (CONICET-Argentina). This is the contribution number 88 of the LIESA.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.