The new species Minibiotus pentannulatus sp. nov., is described from the Sierra Nevada de Santa Marta, Colombia. It differs from the other species of the genus by star-shaped pores, the presence of a peculiar egg with a smooth shell, and small conical processes with 5 annulations. Other differences include the distribution and size of the pores, and other morphometric details regarding the size of the body and buccal tube, and the pt of the stylet support insertion point, macroplacoids and claws. To date, the genus Minibiotus R.O. Schuster, 1980, comprises 48 species, including the species described here.
Se describe la nueva especie Minibiotus pentannulatus sp. nov., proveniente de la Sierra Nevada de Santa Marta, Colombia. Ésta difiere de las otras especies del género por los poros en forma de estrella, la presencia de huevos característicos con cáscara lisa y procesos cónicos con 5 anillos. Otras diferencias incluyen la distribución y talla de los poros y otros detalles morfométricos. Hasta la fecha, el género Minibiotus R.O. Schuster, 1980, comprende 48 especies, incluyendo la especie aquí descrita.
The genus Minibiotus R.O. Schuster, 1980, currently comprises 47 species (Degma, Bertolani, & Guidetti, 2017). This genus is characterized by the presence of an antero-ventral mouth with 10 peribucal papulae but without lamellae; short, rigid, and narrow buccal tube usually with 2 bends; relative cephalic stylet support insertion point; and an extra thickening on the wall of the buccal tube immediately below the stylet support insertion point (Claxton, 1998; Michalczyk & Kaczmarek, 2004).
In Colombia, tardigrades have been poorly studied. To date, 52 species have been recorded for the country (Caicedo, Londoño, & Quiroga, 2014; Kaczmarek, Michalczyk, & McInnes, 2015; Lisi, Londoño, & Quiroga, 2014; Lisi, Daza, Londoño, & Quiroga, 2017; Londoño, Daza, Caicedo, Quiroga, & Kaczmarek, 2015; Melo, Beltrán-Pardo, Bernal, & Kaczmarek., 2015; Stec, Roszkowska, Kaczmarek, & Michalczyk, 2017), from which only 2 belong to the genus Minibiotus. The genus was recorded for the first time in Colombia, by Heinis (1914) with the species Minibiotus intermedius (Plate, 1888) that should be referred to as “sensu lato” according to modern taxonomic achievements. A century later in 2014, Minibiotus cf. pilatus Claxton, 1988 (Lisi et al., 2014), was reported in the Department of Magdalena, more precisely in the Sierra Nevada de Santa Marta (SNSM). This is an isolated costal mountain with high biological richness of species; 39.6% of the species of tardigrades recorded in Colombia have been found in this region. Herein, we describe the new species Minibiotus pentannulatus sp. nov. from SNSM, Colombia.
Materials and methodsA single sample of lichens growing on a tree trunk was collected on March 21st 2015 from El Campano, Department of Magdalena at 1,334masl in the Sierra Nevada de Santa Marta. This sample, included in the framework of the research project “Composición taxonómica de flora y fauna anhidrobiótica en microdoseles de la Sierra Nevada de Santa Marta” has been legally collected under the permit “Permiso Marco de Recolección de Especímenes de Especies Silvestres de la Diversidad Biológica con fines de investigación científica no comercial”, resolution 1293 (2013), expedited by the Autoridad Nacional de Licencias Ambientales (ANLA) granted to the Universidad del Magdalena. The sample was kept in a paper bag, then rehydrated for 48h with bottled water and examined using a Zeiss Stemi DV4 dissecting stereoscope. Tardigrades and eggs were extracted with micropipettes and mounted directly in PVA mounting media (BioQuip #6371A) for examination under a Phase Contrast Microscope (PCM) Zeiss Axiolab A1. Identification, using taxonomic keys, was based on morphological characters. Literature used in the identification included: Pilato and Binda (2010) for genus identification, and descriptions of several species (Binda & Pilato, 1992; Claxton, 1998; Meyer & Hinton, 2009; Michalczyk & Kaczmarek, 2003, 2004; Murray, 1910; Pilato, Binda, & Lisi, 2003; Rossi, Claps, & Ardohain, 2009; Roszkowska, Stec, Ciobanu, & Kaczmarek, 2016).
Photos were taken with CCD camera Zeiss AxioCam ERc 5s. All measurements are given in micrometers (μm) and were acquired with the software Zeiss AxioVision SE64. For the description of the new species, measurements of the taxonomically important structures were provided only when they were undamaged and in a suitable position. Buccal tube length was measured according to Pilato (1981). Lengths of primary and secondary branches of claws were measured from base to apex, including accessory points. The pt ratio is the ratio of the length of a given structure to the length of the buccal tube, expressed as a percentage (Pilato, 1981). All pt values are in square brackets.
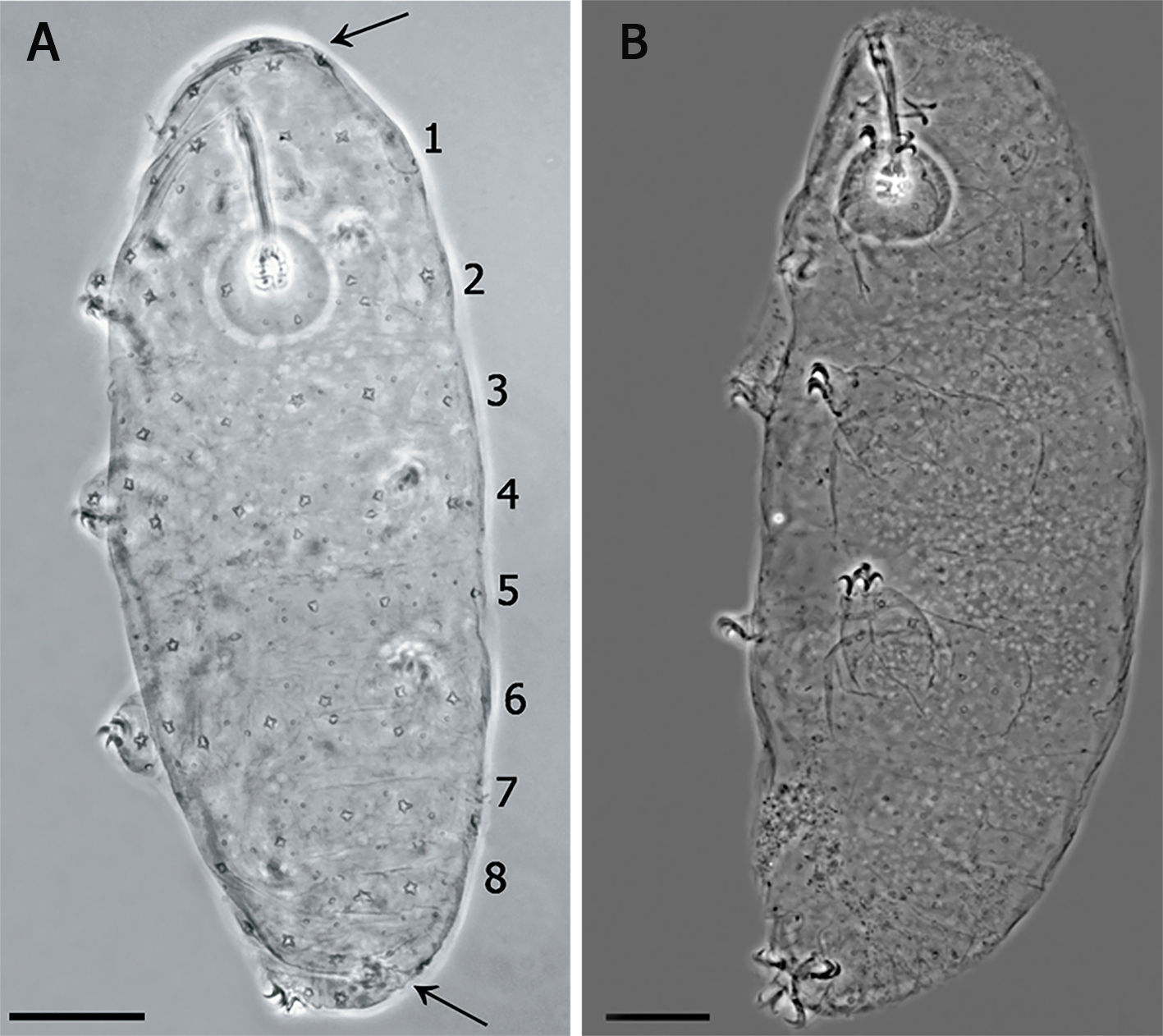
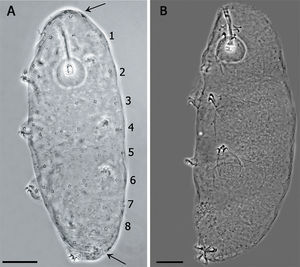
The Minibiotus population was composed by specimens between 144μm and 258μm body length. Therefore, the new Minibiotus specimens were assigned to 3 different life stages. The smallest specimens were attributed to the first life stage and correspond to those with buccal tube and claw lengths similar to the embryo inside the egg (about 19μm and 4.5–5μm respectively); these also showed a remarkable arrangement of the star-shaped pores in 8 single transverse rows along the body. The specimens placed in the second life stage were those of intermediate size (approx. 180–203μm). Specimens in this group maintain the 8 single transverse rows of pores still recognizable, but less evident than the first stage specimens due to the presence of some extra pores randomly distributed between the rows. Finally, the third life stage corresponds to the biggest specimens (about 205–258μm), which did not show any trace of the initial arrangement of the pores, being randomly distributed in the entire body.
DescriptionClass: Eutardigrada Ritchers, 1926
Order: Parachela Schuster, Nelson, Grigarick & Christenberry, 1980
Superfamily: Macrobiotoidea Thulin, 1928 in Marley et al. 2011
Family: Macrobiotidae Thulin, 1928
Genus: Minibiotus R.O. Schuster, 1980
Minibiotus pentannulatus sp. nov. (Figs. 1–3, Table 1)
(A) Juvenile in the first stage of life, showing the pattern of 8 bands of pores on its cuticle (scale bar 20μm). Arrows indicate the cluster of pores in the cephalic (anterior to the row 1) and caudal region (posterior to the row 8). (B) Specimen in third life stage showing the pores scattered on the cuticle.
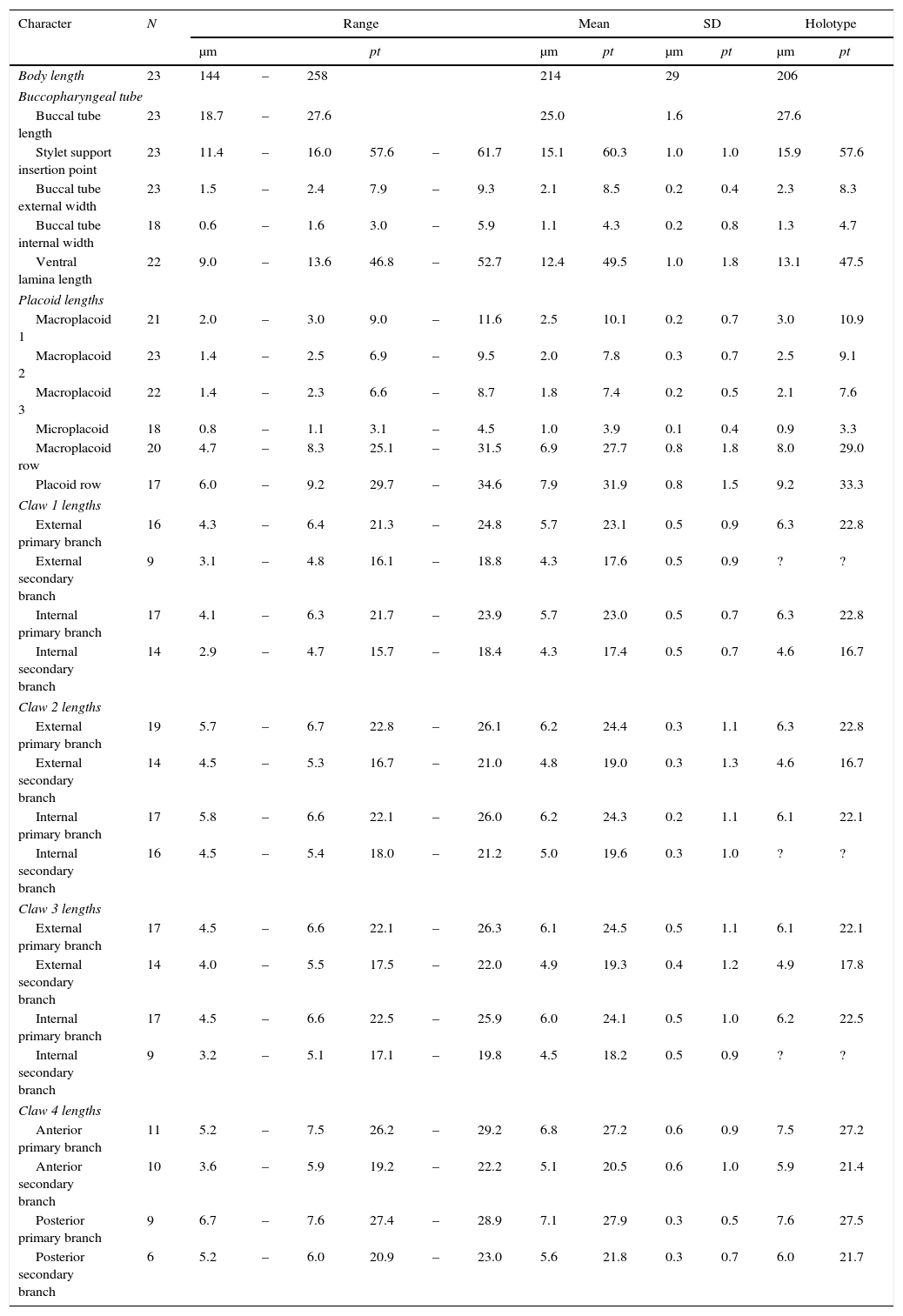
Measurements and pt values of selected morphological structures of the holotype and paratypes of Minibiotus pentannulatus sp. nov.
| Character | N | Range | Mean | SD | Holotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μm | pt | μm | pt | μm | pt | μm | pt | ||||||
| Body length | 23 | 144 | – | 258 | 214 | 29 | 206 | ||||||
| Buccopharyngeal tube | |||||||||||||
| Buccal tube length | 23 | 18.7 | – | 27.6 | 25.0 | 1.6 | 27.6 | ||||||
| Stylet support insertion point | 23 | 11.4 | – | 16.0 | 57.6 | – | 61.7 | 15.1 | 60.3 | 1.0 | 1.0 | 15.9 | 57.6 |
| Buccal tube external width | 23 | 1.5 | – | 2.4 | 7.9 | – | 9.3 | 2.1 | 8.5 | 0.2 | 0.4 | 2.3 | 8.3 |
| Buccal tube internal width | 18 | 0.6 | – | 1.6 | 3.0 | – | 5.9 | 1.1 | 4.3 | 0.2 | 0.8 | 1.3 | 4.7 |
| Ventral lamina length | 22 | 9.0 | – | 13.6 | 46.8 | – | 52.7 | 12.4 | 49.5 | 1.0 | 1.8 | 13.1 | 47.5 |
| Placoid lengths | |||||||||||||
| Macroplacoid 1 | 21 | 2.0 | – | 3.0 | 9.0 | – | 11.6 | 2.5 | 10.1 | 0.2 | 0.7 | 3.0 | 10.9 |
| Macroplacoid 2 | 23 | 1.4 | – | 2.5 | 6.9 | – | 9.5 | 2.0 | 7.8 | 0.3 | 0.7 | 2.5 | 9.1 |
| Macroplacoid 3 | 22 | 1.4 | – | 2.3 | 6.6 | – | 8.7 | 1.8 | 7.4 | 0.2 | 0.5 | 2.1 | 7.6 |
| Microplacoid | 18 | 0.8 | – | 1.1 | 3.1 | – | 4.5 | 1.0 | 3.9 | 0.1 | 0.4 | 0.9 | 3.3 |
| Macroplacoid row | 20 | 4.7 | – | 8.3 | 25.1 | – | 31.5 | 6.9 | 27.7 | 0.8 | 1.8 | 8.0 | 29.0 |
| Placoid row | 17 | 6.0 | – | 9.2 | 29.7 | – | 34.6 | 7.9 | 31.9 | 0.8 | 1.5 | 9.2 | 33.3 |
| Claw 1 lengths | |||||||||||||
| External primary branch | 16 | 4.3 | – | 6.4 | 21.3 | – | 24.8 | 5.7 | 23.1 | 0.5 | 0.9 | 6.3 | 22.8 |
| External secondary branch | 9 | 3.1 | – | 4.8 | 16.1 | – | 18.8 | 4.3 | 17.6 | 0.5 | 0.9 | ? | ? |
| Internal primary branch | 17 | 4.1 | – | 6.3 | 21.7 | – | 23.9 | 5.7 | 23.0 | 0.5 | 0.7 | 6.3 | 22.8 |
| Internal secondary branch | 14 | 2.9 | – | 4.7 | 15.7 | – | 18.4 | 4.3 | 17.4 | 0.5 | 0.7 | 4.6 | 16.7 |
| Claw 2 lengths | |||||||||||||
| External primary branch | 19 | 5.7 | – | 6.7 | 22.8 | – | 26.1 | 6.2 | 24.4 | 0.3 | 1.1 | 6.3 | 22.8 |
| External secondary branch | 14 | 4.5 | – | 5.3 | 16.7 | – | 21.0 | 4.8 | 19.0 | 0.3 | 1.3 | 4.6 | 16.7 |
| Internal primary branch | 17 | 5.8 | – | 6.6 | 22.1 | – | 26.0 | 6.2 | 24.3 | 0.2 | 1.1 | 6.1 | 22.1 |
| Internal secondary branch | 16 | 4.5 | – | 5.4 | 18.0 | – | 21.2 | 5.0 | 19.6 | 0.3 | 1.0 | ? | ? |
| Claw 3 lengths | |||||||||||||
| External primary branch | 17 | 4.5 | – | 6.6 | 22.1 | – | 26.3 | 6.1 | 24.5 | 0.5 | 1.1 | 6.1 | 22.1 |
| External secondary branch | 14 | 4.0 | – | 5.5 | 17.5 | – | 22.0 | 4.9 | 19.3 | 0.4 | 1.2 | 4.9 | 17.8 |
| Internal primary branch | 17 | 4.5 | – | 6.6 | 22.5 | – | 25.9 | 6.0 | 24.1 | 0.5 | 1.0 | 6.2 | 22.5 |
| Internal secondary branch | 9 | 3.2 | – | 5.1 | 17.1 | – | 19.8 | 4.5 | 18.2 | 0.5 | 0.9 | ? | ? |
| Claw 4 lengths | |||||||||||||
| Anterior primary branch | 11 | 5.2 | – | 7.5 | 26.2 | – | 29.2 | 6.8 | 27.2 | 0.6 | 0.9 | 7.5 | 27.2 |
| Anterior secondary branch | 10 | 3.6 | – | 5.9 | 19.2 | – | 22.2 | 5.1 | 20.5 | 0.6 | 1.0 | 5.9 | 21.4 |
| Posterior primary branch | 9 | 6.7 | – | 7.6 | 27.4 | – | 28.9 | 7.1 | 27.9 | 0.3 | 0.5 | 7.6 | 27.5 |
| Posterior secondary branch | 6 | 5.2 | – | 6.0 | 20.9 | – | 23.0 | 5.6 | 21.8 | 0.3 | 0.7 | 6.0 | 21.7 |
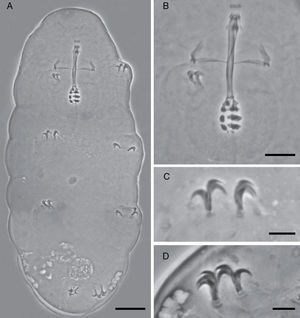
Species diagnosis. Eye-spots absent after slide mounting. Body colorless with smooth cuticle, only with a fine granulation on legs IV. Entire cuticle including the legs, with numerous pores variable in shape: rounded, oval, multi-lobated (3–4 tips), and star-shaped (5–6 tips). The rounded and oval are smaller (0.49–1.79μm) than the multi-lobated and star-shaped pores (1.35–2.84μm). The multi-lobated and star-shaped pores are bigger at the cephalic and caudal extremities of the body and on the legs. In the specimens found in early life stages, these pores form 8 transverse single rows, and a cluster of pores in the cephalic and caudal region, on the dorsal and ventral cuticle, which are no longer recognizable in older specimens due to the apparition of other pores among the rows (Fig. 3A and B).
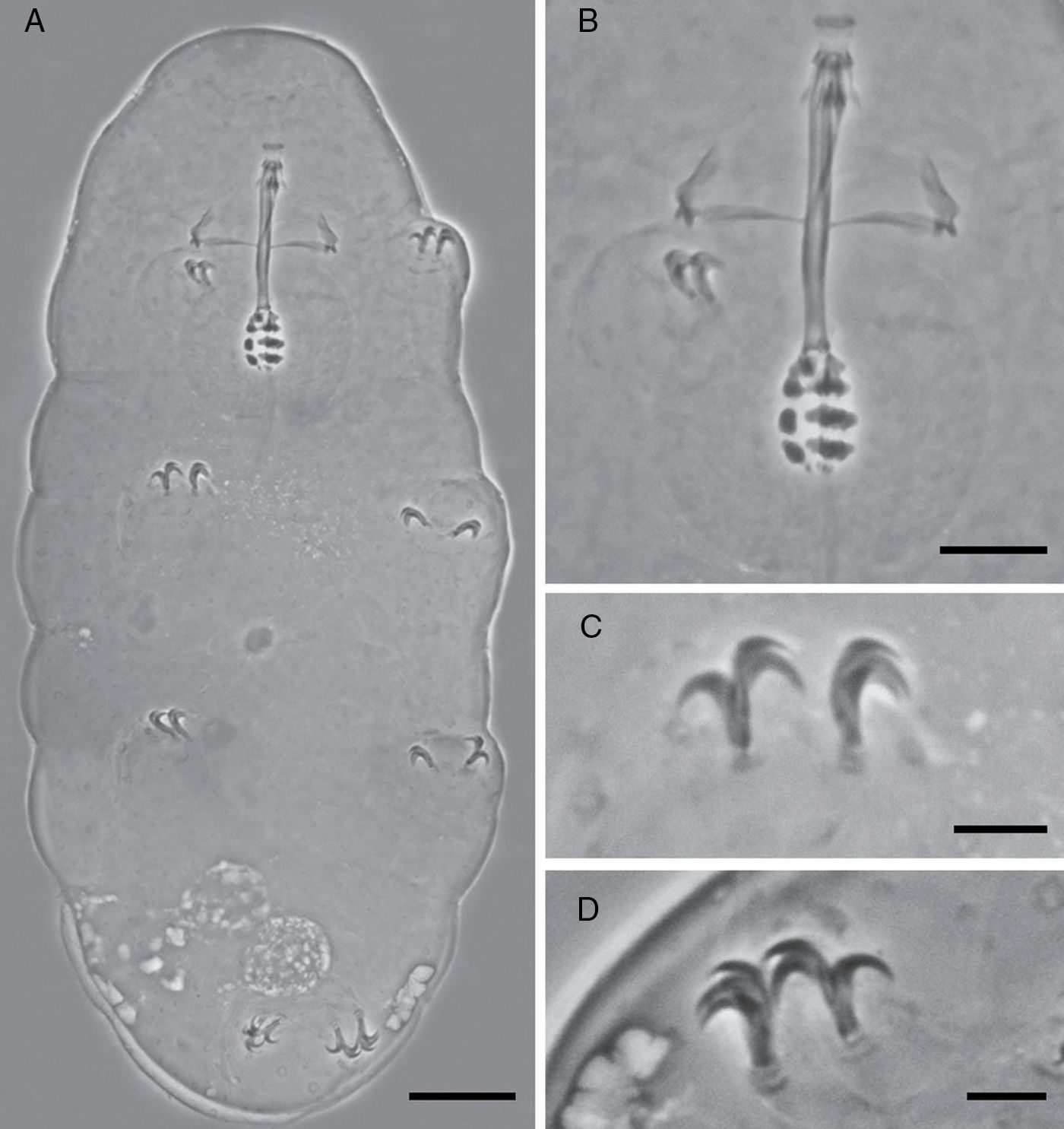
Teeth in the oral cavity absent or not visible under PCM. Buccal apparatus of the Minibiotus type. Buccal tube with 2 bends which are only evident in lateral view; well-developed ventral lamina, 3 granular macroplacoids and a microplacoid, pharyngeal bulb rounded. Claws of the hufelandi type with well-developed accessory points. Smooth lunules. Eggs laid freely, egg surface smooth, with numerous short conical processes with 5 transverse concentrical ridges appearing as thickened annulations.
Description of the holotype: Colorless, body length 206μm, although this measurement should be higher because of the retraction of the mouth (Fig. 1A). Without eye-spots after slide mounting. Smooth cuticle with only a fine, and slightly evident granulation on the hind legs. Cuticle covered with numerous pores distributed irregularly, variable in shape and size, rounded, and oval (0.49–1.57μm), and multi-lobated and star-shaped (1.35–2.66μm). The biggest star-shaped pores are more commonly present on the cephalic and caudal extremities of the body, and on the legs. Pores around the mouth seem to be absent.
Mouth antero-ventral. Peribuccal papulae are present, peribuccal lamellae absent. Buccopharyngeal apparatus of the Minibiotus type (Fig. 1B). Teeth in the oral cavity absent or not visible under PCM. Rigid buccal tube, 27.6μm long and 2.3μm of external width [8.3] with anterior and posterior bends; well-developed ventral lamina 13.1μm [47.5]. Stylet support inserted on the buccal tube at 15.9μm [57.6]. Rounded pharyngeal bulb 32.29μm, with triangular apophyses close to the first row of macroplacoids. Three rows of macroplacoids decreasing in size, with granular appearance and a row of small microplacoids. First macroplacoid 3.0μm [10.9], second macroplacoid 2.5μm [9.1], third macroplacoid 2.1μm [7.6]. Microplacoid, 0.9μm [3.3]. Macroplacoid row is 8.0μm [29.0], entire placoid row is 9.2μm.
Claws of hufelandi type. Primary branches with well-developed accessory points (Fig. 1C and D). Claw lengths: leg I, external primary branch 6.3μm [22.8]; internal primary branch 6.3μm [22.8], internal secondary branch 4.6μm [16.7]. Leg II, external primary branch 6.3μm [22.8], external secondary branch 4.6μm [16.7]; internal primary branch 6.1μm [22.1]. Leg III, external primary branch 6.1μm [22.1], external secondary branch 4.9μm [17.8]; internal primary branch 6.2μm [22.5]. Leg IV, anterior primary branch 7.5μm [27.2], anterior secondary branch 5.9μm [21.4]; posterior primary branch 7.6μm [27.5], posterior secondary branch 6.0μm [21.7]. Smooth lunules. Cuticular bars present.
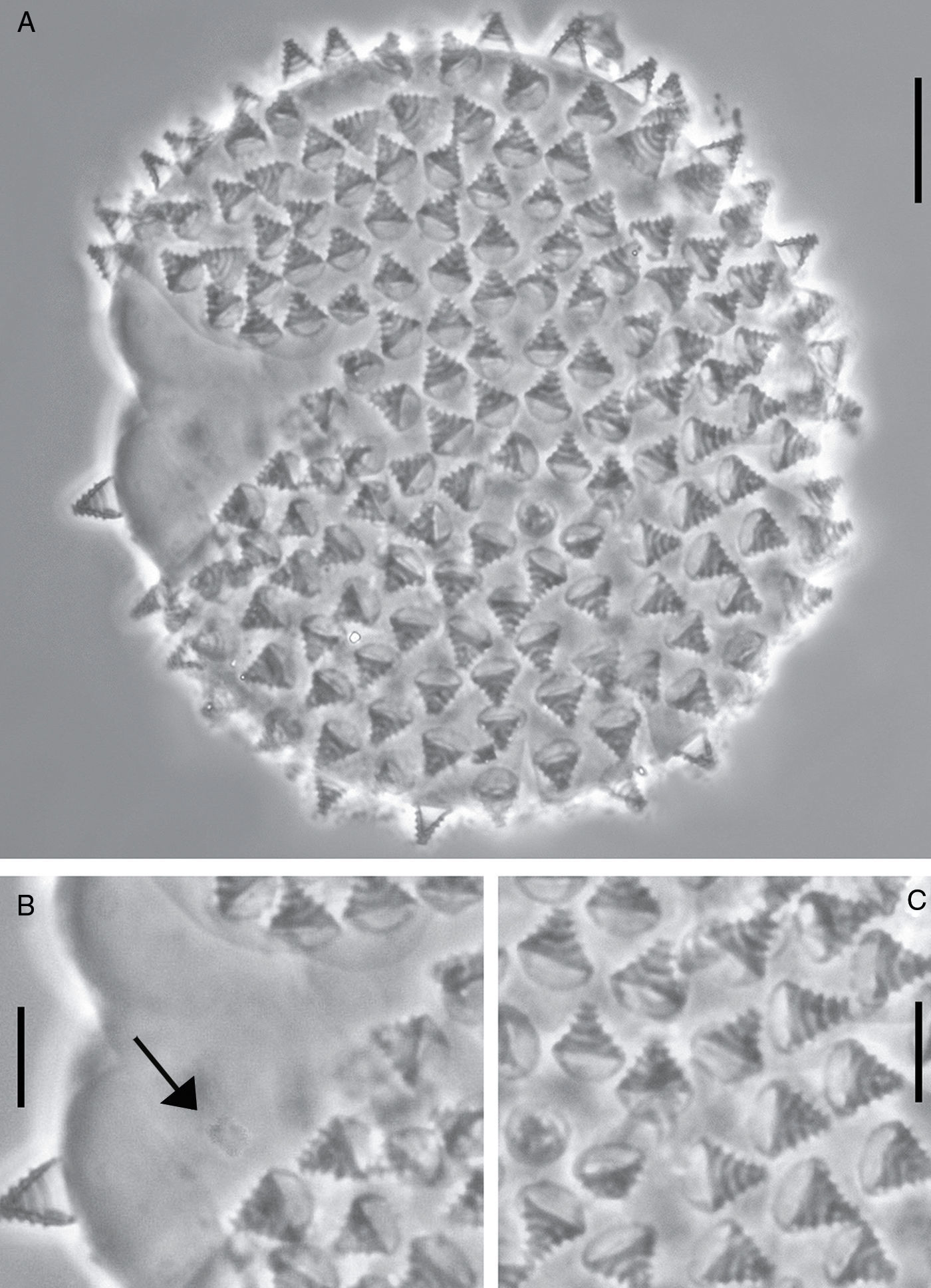
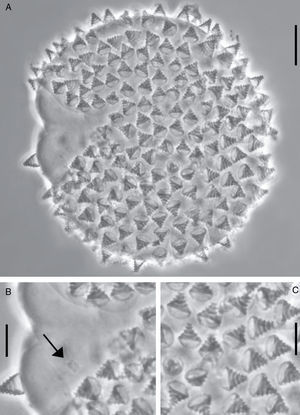
Egg: only 1 embryonated egg was found (Fig. 2B). White, spherical, and laid freely. Diameter 74.5μm (Fig. 2A). Processes without reticulation, but with 5 transverse concentrical ridges which appear as thickened annulations. Egg shell among processes smooth (Fig. 2C).
The measurements of selected morphological structures of the holotype and the ranges within the population are given in Table 1.
Taxonomic summaryMaterial examined: holotype, 37 paratypes, 7 simplex specimens, and 1 egg in PVA mounting media (BioQuip #6371A). All specimens were found in a single sample, which was a mixture of lichens from the genera Hypotrachyna, Heterodermia, and Parmotrema, growing on a tree trunk. Locality: El Campano 11°06′32.2″N, 74°05′31.1″W, 1,334masl, Sierra Nevada de Santa Marta, Department of Magdalena, Colombia.
Type repositories: the holotype, paratypes, and egg are deposited in the Centro de Colecciones Biológicas de la Universidad del Magdalena (CBUMAG), Santa Marta, Colombia. Slide numbers: holotype: CBUMAG:TAR:00447; paratypes: CBUMAG:TAR:00437 (6 specimens), TAR:00438 (2 specimens), TAR:00439 (6 specimens), TAR:00440 (5 specimens), TAR:00441 (5 specimens), TAR:00442 (3 specimens), TAR:00443 (1 specimen), TAR:00444 (2 specimens), TAR:00445 (1 specimen), TAR:00446 (3 specimens), TAR:00447 (1 specimen), TAR:00448 (7 specimens), TAR:00449 (2 specimens); egg: CBUMAG:TAR:00450.
Etymology: the specific epithet refers to the presence of 5 (penta) rings (annuli) on the egg processes: “pentannulatus”=provided with 5 annuli.
Taxonomic remarksThe pt ranges of the placoid lengths appear quite high within a species. However, we would like to emphasize that such small and peculiarly shaped (i.e., typical of Minibiotus species with 3 macroplacoids) placoids were often difficult to measure with total certainty. Additionally, our population was composed of hatchlings and specimens in the second, and most likely third stages. Although allometry was not verified, we cannot exclude the fact that some metric differences were due to allometric growing of structures in the first life stages.
Apart of the distribution of the smallest pores, the paratypes showed the same characters of the holotype. The specimens in which the multi-lobated/star-shaped pores formed the 8 transverse single rows, and a cluster of pores in the cephalic (anterior to the row 1) and caudal region (posterior to the row 8) (Fig. 3A), were those in the first life stage, body length between 144μm and 169μm and/or buccal tube length similar to that of the embryo in the egg. In specimens about 200μm, in the second life stage, the bands are no longer made of a single row of pores and the pattern is less defined. Larger specimens (third life stage) do not show any pattern, having a multi-lobated and star-shaped pores randomly distributed (Fig. 3B).
The egg of M. pentannulatus sp. nov. is unique not only within the genus Minibiotus but also compared to all eutardigrades. It resembles the eggs of the species Calcarobiotus (Calcarobiotus) digeronimoiPilato, Binda & Lisi, 2004a, C. (Calcarobiotus) imperialisAbe & Takeda, 2000, and C. (Discrepunguis) tetrannulatusPilato, Binda & Lisi, 2004b, because of the presence of annulations on the processes; however, the egg shell of M. pentannulatus sp. nov. is smooth, while in those species it is reticulated (Abe & Takeda, 2000; Pilato, Binda, & Lisi, 2004a, 2004b). Macrobiotus occidentalis striatusDastych, 1974, and Macrobiotus kristenseniGuidetti, Peluffo, Rocha, Cesari & Moly de Peluffo, 2013, also have eggs with annulations on the processes; however, in the first species their bases are surrounded with a wreath of several small points (Dastych, 1974), and in M. kristenseni the annulation is only visible in SEM.
Differential diagnosis by the presence of similar star-shaped pores in the cuticle, this species is most similar to Minibiotus pseudostellarusRoszkowska, Stec, Ciobanu & Kaczmarek, 2016, Minibiotus constellatusMichalczyk & Kaczmarek, 2003, Minibiotus eichhorniMichalczyk & Kaczmarek, 2004, Minibiotus sidereusPilato, Binda & Lisi, 2003, Minibiotus claxtonaeRossi, Claps & Ardohain, 2009 and Minibiotus aculeatus (Murray, 1910).
Minibiotus pentannulatus sp. nov. differs from M. pseudostellarus by having a smaller body size (144–258μm in M. pentannulatus sp. nov. and 241–320μm in M. pseudostellarus), an absence of eye-spots, and the presence of 4 types of pores. In contrast, M. pseudostellarus possesses only 2 types, rounded and pseudo-star shaped. Rounded/oval pores reach bigger sizes (0.49–1.57μm in the holotype of M. pentannulatus sp. nov. and 0.3–0.9μm in the holotype of M. pseudostellarus). Despite the overlapping in the size of the star-shaped pores between the 2 species, the smallest pores of M. pentannulatus sp. nov. are much larger than the smallest M. pseudostellarus (1.35–2.66μm and 0.6–2.5μm, respectively, in the holotypes). Star-shaped pores with 5–6 points are grouped on the cephalic and caudal portion and on the legs, while in M. pseudostellarus, these pores are present only on the legs. Shorter and narrower buccal tube, [7.9–9.3] in M. pentannulatus sp. nov. vs. [10.2–11.6] in M. pseudostellarus, respectively. Level of the stylet support insertion point in a more anterior position (11.4–16.0μm [57.6–61.7] in M. pentannulatus sp. nov. and 16.1–19.3μm [65.6–68.8] in M. pseudostellarus). Macroplacoid length sequence 1>2>3 while in M. pseudostellarus is 3>2≥1. Microplacoid smaller (0.8–1.1μm [3.1–4.5] in M. pentannulatus sp. nov. vs. 1.1–1.5μm [4.5–5.7] in M. pseudostellarus). Macroplacoid row and entire placoid row shorter (4.7–8.3μm [25.1–31.5] and 6.0–9.2μm [29.7–34.6] in M. pentannulatus sp. nov. vs. 8.7–11.0μm [35.5–38.9] and 10.4–13.0μm [42.2–46.2] in M. pseudostellarus, respectively). Slightly smaller claws and cuticular bar absent in M. pentannulatus sp. nov. It was not possible to compare the eggs between these 2 species, because in M. pseudostellarus eggs are unknown.
Minibiotus pentannulatus sp. nov. differs from M. constellatus by having the star-shaped pores with up to 6 tips (M. constellatus up to 7) grouped on the cephalic and caudal portion, and on the legs in the new species while in M. constellatus they are distributed randomly, but 2 rows are arranged along the main axis of the body. Fine granulation only present on leg IV while in M. constellatus, it is present on all legs. It was not possible to compare the eggs between these 2 species because there is no information about M. constellatus eggs.
Minibiotus pentannulatus sp. nov. differs from M. eichhorni by the absence of eye-spots. Star-shaped pores more common in the new species, with 5–6 tips, while rare in M. eichhorni, in which they are mostly trilobated and quadrilobated, and with no more than 5 tips. In the new species, the multi-lobated and star shaped pores are distributed on the cephalic and caudal extremities of the body, and in 8 transverse rows in juveniles (randomly in subsequent life stages), while in M. eichhorni those pores are arranged on the dorsal cuticle in 6 transverse bands as well as on the cephalic and caudal part of the body. However, the number of ‘stars’ rises on the caudal extremity. Stylet support insertion point more anteriorly (11.4–16.0μm [57.6–61.7] in M. pentannulatus sp. nov. and 16.2–23.8μm [65.4–70.6] in M. eichhorni). Macroplacoid length sequence 1>2>3, while in M. eichhorni it is 1>3>2. Microplacoid smaller (0.8–1.1μm [3.1–4.5] in M. pentannulatus sp. nov. vs. 1.1–1.9μm [4.6–6.1] in M. eichhorni). Macroplacoid row and placoid row shorter (4.7–8.3μm [25.1–31.5] and 6.0–9.2μm [29.7–34.6] vs. 7.6–12.8μm [29.3–39.7] and 9.0–14.3μm [36.2–44.1] in M. eichhorni, respectively). Smaller claws on all pairs of legs (e.g., claw I primary branch [21.3–24.8] in M. pentannulatus sp. nov. vs. [25.9–31.0] in M. eichhorni). Fine granulation only present on leg IV whereas in M. eichhorni it is present on all legs. It was not possible to compare the eggs between these 2 species because the eggs of M. eichhorni have not been described.
Minibiotus pentannulatus sp. nov. differs from M. sidereus by the absence of eye-spots. Multi-lobated and star-shaped pores with 3–6 tips (3–8 tips in M. sidereus). Multi-lobated and star-shaped pores distributed randomly (except from the first life stage); in M. sidereus the star-shaped pores are absent in the intersegmental folds and form transversal bands. The largest star-shaped pores in M. pentannulatus sp. nov. are smaller, up to 2.8μm, while in M. sidereus they are up to 3.9μm. They are grouped on the cephalic and caudal portion and on all the legs while in M. sidereus the large pores are present on the head and legs, and the largest are present on the IV pair of legs. Buccal tube wider (1.5–2.4μm [7.9–9.3] in M. pentannulatus sp. nov. and 1.0–1.7μm [4.7–6.4] in M. sidereus). Macroplacoid length sequence 1>2>3, whereas M. sidereus 1>3>2. Egg without filaments on their tips (longer conical, sharpened, with a flexible terminal portion rarely bifurcate in M. sidereus). Shorter processes (up to 4.9μm in M. pentannulatus sp. nov. and 9.5μm in M. sidereus) with smaller basal width (up to 4.5μm in M. pentannulatus sp. nov. and 5.3μm in M. sidereus). Different type and number of annulations on the processes (5 annulations in M. pentannulatus sp. nov.which are actually protruding ridges and 6–7 in M. sidereus which are weakly outlined folds appearing as dark lines).
Minibiotus pentannulatus sp. nov. differs from M. claxtonae by the absence of eye-spots. Rounded/oval pores may be above 1μm in diameter (nearly 1μm in M. claxtonae). In the new species the pores are randomly distributed in adult specimens; unlike in M. claxtonae the pores form 10 transverse bands and specifically the star-shaped are aligned in longitudinal rows. Fine granulation only present on pair leg IV (on all legs in M. claxtonae). Macroplacoid length with sequence 1>2>3, while 3>1>2 in M. claxtonae. Smaller eggs (74.2μm in M. pentannulatus sp. nov. and 82–86μm in M. claxtonae, including the processes) with more processes (circumference 27 and hemisphere 147 in M. pentannulatus sp. nov. vs. circumference ca. 22 and hemisphere nearly 62 in M. claxtonae). Trunco-conical processes (egg cup shape in M. claxtonae), smaller (4.6–4.9μm in M. pentannulatus sp. nov. and 7μm in M. claxtonae) and narrower in the base (4.1–4.5μm in M. pentannulatus sp. nov. and ca. 5μm in M. claxtonae).
Minibiotus pentannulatus sp. nov. differs from M. aculeatus by the absence of eye-spots. Without processes or appendices over cuticle (with 2–6 soft conical dorsal processes in pairs on the segments over II-IV pairs of legs in M. aculeatus). Teeth in the oral cavity present in M. aculeatus. Eggs with trunco-conical processes while in M. aculeatus they have slender conical processes with flexible points.
We thank Kevin Ramírez Roncallo for the lichen identifications, and Dr. Marcela Bolaños and Joseph Dunn who kindly improved the English of the manuscript. This work was carried out in the framework of the research project “Composición taxonómica de flora y fauna anhidrobiótica en micro-doseles de la Sierra Nevada de Santa Marta”, supported by Departamento Administrativo de Ciencia, Tecnología e Innovación “COLCIENCIAS” (#0091288) and the Universidad del Magdalena (BIO-659/2014). This is a scientific contribution number 5 from the Centro de Colecciones Biológicas de la Universidad del Magdalena.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.