We report for the first time the presence of a rare salp Helicosalpa komaii (Ihle and Ihle-Landenberg, 1936) on the west coast of Baja California peninsula (26°52.7' N, 117°09.2' W). The single specimen recorded was a solitary zooid of 200mm length and 90ml of displacement volume. It was recognized by having a distinctive highly convoluted heart-shaped dorsal tubercle. The total number of muscle fibers in the solitary zooid (MI to MVII=517) surpassed that of the other 2 known congeners supporting its identity as H. komaii. H. komaii increases to 29 the total number of salps species reported in coastal and oceanic waters off north-central Baja California (gamma diversity). We hypothesize that the presence of H. komaii in the study area may be the result of onshore intrusions of subtropical waters originating west-southwest of the California Current domain.
Se registra por primera vez la presencia de una especie rara de salpa Helicosalpa komaii (Ihle y Ihle Landenberg, 1936) en la costa oeste de la península de Baja California (26°52.7' N, 117°09.2' O). El ejemplar registrado fue un zooide solitario de 200mm longitud total y una biomasa de 90ml (volumen desplazado). El ejemplar fue reconocido por su característico tubérculo dorsal en forma de corazón. El número total de fibras musculares en el zooide solitario (MI a MVII= 517) superó el de los otros dos congéneres conocidos, apoyando su identificación como H. komaii. El registro de H. komaii incrementó a 29 el número total de especies de salpas registradas en aguas costeras y oceánicas del centro-norte de Baja California (diversidad gamma). Nuestra hipótesis es que la presencia de H. komaii en el área de estudio se debe a su acarreo hacia la costa por agua subtropical procedente del oeste-suroeste del dominio de la corriente de California.
Salps are pelagic tunicates widely distributed in all oceans, comprising the most diverse order among the thaliaceans (Van Soest, 1998). The order Salpida contains 1 family with 2 subfamilies: Cyclosalpinae Yount, 1954 including 2 genera, and Salpinae Yount, 1954 with eleven genera. Salps have wide zoogeographic distribution and the discovery of new species is considerably rare. Only a few new species have been added in the last decades to the worldwide 44 known species (Van Soest, 1998; Govindarajan et al., 2010).
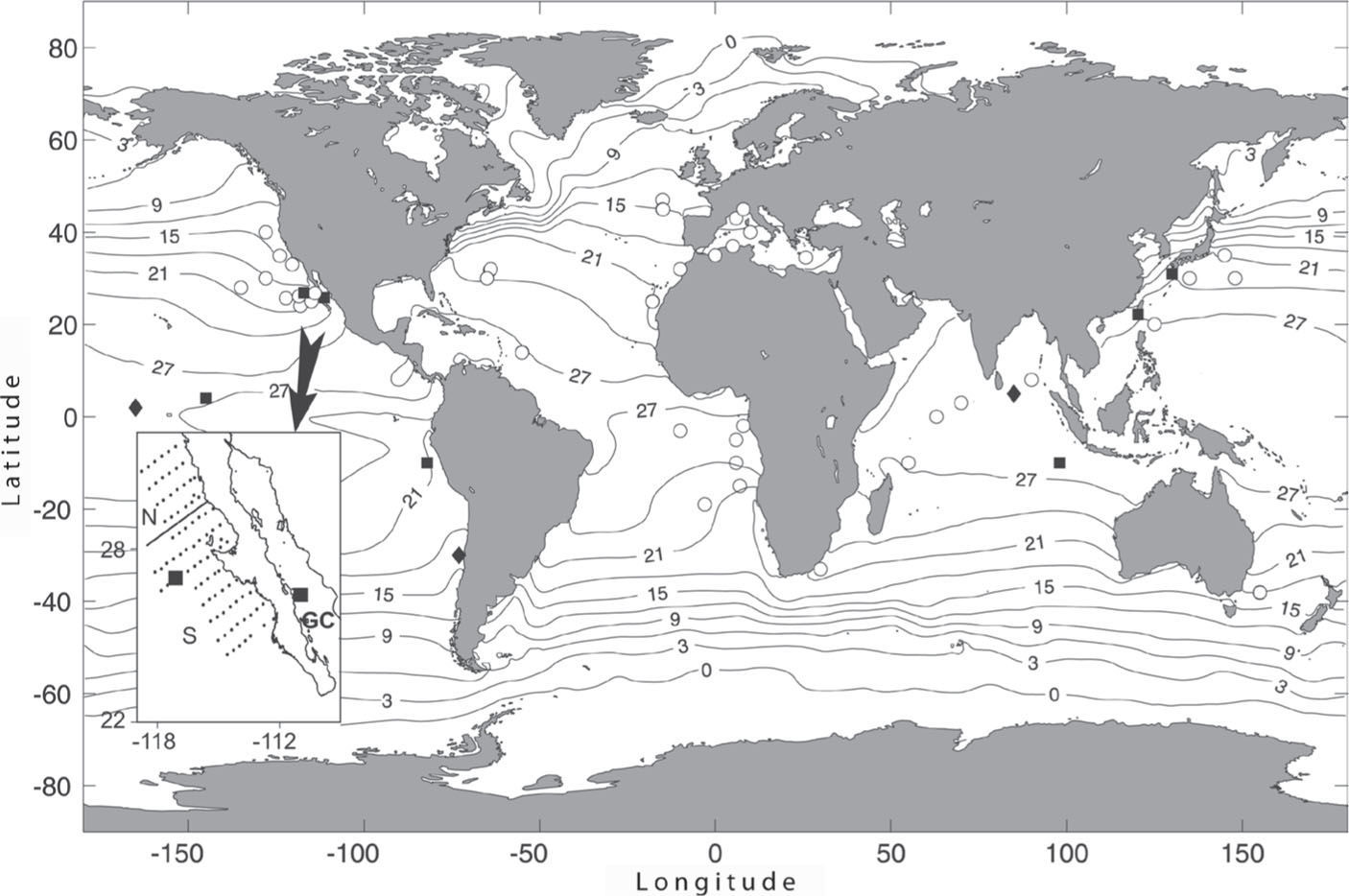
Cyclosalpinae salps of the genus Helicosalpa (Todaro, 1902) are among the most rarely collected. Currently, the genus contains 3 species: Helicosalpa virgula (Vogt, 1854), H. younti Kashkina 1973, and H. komaii (Ihle and Ihle-Landenberg, 1936), a species originally described as Cyclosalpa komaii (Van Soest, 2013). Helicosalpa virgula has the widest zoogeographic distribution and is the most frequent species reported in the genus. This large species has been recorded from different localities in the Mediterranean, the Atlantic Ocean and also from the Indian and the Pacific Oceans (Fig. 1). Fewer H. younti records exist, known only from the central Pacific, off India (Van Soest, 1974), and from Chilean waters; its aggregate zooid was recently described by Esnal et al. (1998). Helicosalpa komaii is the rarest species with only 1 solitary and 30 aggregates zooids recorded in the western Pacific (Komai, 1932), a single solitary zooid reported in Central Pacific waters (Yount, 1954), a chain of 8 aggregates in the Gulf of California (Madin, 1968) and 14 aggregates zooids collected in the Indian Ocean (Van Soest, 1974). There are 2 recent additional records of the species, from southwest Taiwan (Tew and Lo, 2005) and off the coast of Peru (Ayón et al., 2008). Unfortunately, neither figures nor zooid specifications were provided by these authors. Here we report the extension range of H. komaii in the Northeast Pacific, particularly off the coast of Baja California peninsula, Mexico.
Worldwide records of the genus Helicosalpa: H. komaii (squares), H. younti (diamonds) and H. virgula (circles). The sampling grid off Baja California by IMECOCAL Program is detailed in lower left corner showing northern (N) and southern (S) sectors and the location of records of H. komaii off Baja California and in the Gulf of California (GC). Locations were obtained from: Thompson, 1948; Yount, 1954; Madin, 1968; Hubbard and Pearcy, 1971; Van Soest, 1974; Blackburn, 1979; Madin et al., 1996; Esnal et al., 1998; Tew and Lo, 2005; Hereu et al., 2006; Wiebe et al., 2006; Ayón et al., 2008; Weikert and Godeaux, 2008; and Lavaniegos (unpublished data). Average Sea Surface Temperature taken from WOA09 at NOAA's “Ocean Climate Laboratory” free access server (Locarnini et al., 2010).
The zooplankton sample containing the specimen of Helicosalpa komaii reported here was obtained during a nighttime trawl carried out on February 14, 2004 at station 120.70 (26°52.7' N, 117°9.2' W; 17.19°C and 33.44 PSU at 10m depth) of the research program “Investigaciones Mexicanas de la Corriente de California” (IMECOCAL, Fig. 1) (Lavaniegos et al., 2006). The zooplankton sample was collected with a Bongo net (71cm mouth diameter, 0.5mm mesh size) obliquely towed from 200m depth to the surface. The zooplankton sample was preserved in a 4% formaldehyde solution buffered with sodium borate. The The specimen of H. komaii was deposited in the collection of zooplankton at El Colegio de la Frontera Sur, Unidad Chetumal, in Chetumal, Quintana Roo, Mexico (specimen catalog number ECO-CH-Z-6300).
The solitary zooid specimen of H. komaii collected and examined was physically damaged, since a section of the outer gelatinous tunic from the body wall was partially broken off. However, a careful handling of the organism in a wide glass container allowed a detailed observation of the main taxonomic features that distinguishes the solitary form of H. komaii from the other species known of the same genus: H. virgula and H. younti. Distinctive H. komaii taxonomic features are: the presence of 2 ventral longitudinal muscles (found only in Helicosalpa, absent in Cyclosalpa) stretching between the intermediate muscles and muscle VII, 1 dorsal longitudinal muscle, and a highly convoluted heart-shaped dorsal tubercle (Figs. 2, 3).
Schematic representation of general morphology and muscle band distribution of the solitary zooid of Helicosalpa komaii: A, lateral view; B, dorsal view. Muscle numbers in Roman (I to VIII) from anterior (oral) to posterior (atrial) position. Oral musculature reconstructed following Yount (1954).
Another specimen corresponding to the aggregate form of the genus Helicosalpa was also found in the same zooplankton sample, bearing a horn-like antero-dorsal projection, which is also present in H. virgula and H. younti. The 8mm length aggregate zooid resembled the sinistral individual of H. komaii (figure 6B in Komai, 1932) in the presence of a roundish postero-ventral protrusion (not tapering posteriorly into a narrow point). The dorsal tubercle was not as convoluted as described by Van Soest (1974) for the aggregate zooid of H. komaii. However, Esnal et al. (1998) mention that this character is variable with size, being more convoluted in larger individuals. The disposition of body muscles and number of muscles fibers were difficult to observe in this small aggregate specimen, therefore its definitive identity remains dubious and emphasis is given on the collected solitary specimen.
Only 2 other studies provide biometric measurements of the solitary form of H. komaii. Komai (1932) described the largest specimen known (230mm) from Seto, Japan, and Yount (1954) described another solitary (94mm) collected south of Hawaii, outlining only slight differences between his and Komai's specimen. According to Nakamura and Yount (1958), this species is among the largest salps known in the Pacific Ocean, together with Tethys vagina and Salpa maxima. We compared our specimen based on the taxonomic characters used in the 2 previous morphometric descriptions, particularly taking care of the muscle arrangement and the presence/absence of other relevant taxonomic characters.
Solitary formThe solitary specimen from Baja California was approximately 200mm length and 60mm height, with a biovolume of 90mL with 6 years of preservation after collection. Body muscles with variable number of muscles fibers (from 50 to 90). Total number of muscles fibers from M I to M VII=517. Muscles I and II conjoined near mid-dorsal line and continuing as 1 dorsal longitudinal muscle that joins M VII; it stretches backward ventrally at both sides of the body to form the paired ventral longitudinal muscles. Muscle VIII, named muscle Y in Yount (1954) description, emerges from the union of ventral longitudinal muscle with MVII, continuing dorsally as a single broad muscle (i.e., non-branched as in Komai's description). Body muscles III-VI free dorsally, but fused ventrally to longitudinal muscles (Fig. 2). Oral musculature was similar to description in Yount (1954), but there is a muscle joining the posterior dorsal lip with retractor (intermediate) muscle before the latter fuses to M I dorsally, as described in Komai (1932). A conspicuous heart-shaped dorsal tubercle highly convoluted (Fig. 3). This was the main morphological feature to identify H. komaii at first sight. The alimentary canal had generic characteristics, extending obliquely and dorsally to gill-bar, bending posteriorly at level of M I. Two prominent caeca are present at the opposite extreme of the intestine. A stolon is formed between muscles VI and VII and extends forward in the mid-ventral line, reaching the anterior extreme of the salp. It was not possible to determine the place where the stolon emerged to the body surface because of loosening of the tunic in this section of the body. Strobilation was evident along the stolon; however, the forming aggregates were small and their arrangement pattern along the stolon was more irregular than the one typically observed in H. virgula or other salp species. Atrial languets were absent, similar to the specimen described in Yount (1954), but this is an ambiguous morphological feature because atrial languets were present in the specimen described by Komai (1932).
According to previous descriptions, the species is characterized by the presence of luminous organs forming a continuous line on each side of the body. This feature was not observed in the Baja California collected specimen, but vestiges of the luminous organs were detected. Overall, these structures are not as conspicuous in H. komaii as they are in the other 2 species of the genus. Yount (1954) noted that they apparently are formed by sparse masses of cells. Chemical preservation and time elapsed after collection may distort the appearance of this structure (Esnal et al., 1998).
The total number of muscle fibers (M I-M VII) appears to be a taxonomic significant character to distinguish H. komaii from the other 2 species of the genus. This character is size and age independent and remains practically unaffected by chemical preservation (Esnal et al., 1998). Latitudinal variation in the total number of muscle fibers within a species has been reported, with an increasing number of muscle fibers from tropical to higher latitudes (Van Soest, 1975). The high number of muscles fibers determined for our specimen (517) surpasses the range known for H. younti (with 343 and 346 determined in 2 solitary zooids) (Esnal et al., 1998). It is also 2-fold higher than that reported in H. virgula whose solitary zooids have on average 264 and 218 total muscles fibers in cold and warm waters, respectively (Van Soest, 1975). Unfortunately, information on the total number of muscle fibers was not provided in the 2 previous descriptions of the solitary form of H. komaii and no intra-species comparison of this character is possible. We propose that this is another significant taxonomic character to identify the solitary specimen of H. komaii. For the aggregate zooid of the species, the average total number of muscle fibers from MI to MIV is 90 (range=81–97), doubling that of H. virgula aggregates (range=27–51; Van Soest, 1974) and similar to the upper limit of the range (65–90) reported for H. younti (Esnal et al., 1998).
Salp biogeography is relevant because several species have been useful indicators of intrusions of warm oceanic waters into colder zones (Fraser, 1962; Blackburn, 1979; McAlice, 1986; Sims, 1996; Iguchi and Kidokoro, 2006). A recent study on the variability of different zooplankton groups' abundance off Baja California within the period 1998–2007 denoted a marked seasonality in several taxa including salps. This seasonality is more notorious at the southern eco-region (Fig. 1) due to a stronger influence of a tropical oceanic influx in that sector of the IMECOCAL area (Durazo et al., 2010; Lavaniegos et al., 2010). This tropical influence is more pronounced during El Niño warm years and it is reflected in shifts in the species arrangement and geographic range extensions (Jiménez-Pérez and Lavaniegos, 2004; Linacre, 2005; Lavaniegos and Ambriz-Arreola, 2012). The winter 2004, when the specimen of Helicosalpa komaii was collected, was followed by the moderate 2002–2003 El Niño that resulted in a zooplankton community rich in tropical species off Baja California (Lavaniegos and Ambriz-Arreola, 2012). Helicosalpa komaii is distributed in low and mid latitudes in the Pacific Ocean, therefore its occurrence in the offshore station in the southern Baja California waters may have resulted from intrusion of warm water from the west-southwest toward the coast. Similarly, the presence of H. virgula and other warm water salp species (e.g. Thetys vagina, Ritteriella amboinensis, Thalia rhomboides) off Oregon (Hubbard and Pearcy, 1971) and Baja California (Berner, 1967; Hereu et al., 2006) were associated to the onshore intrusion of tropical waters of west-southwest origin and the weakening of the cold California Current during anomalous El Niño years. The high abundance of salps and other pelagic tunicates (i.e, doliolids) in the winter of 2004, mainly in the southern eco-region, reinforces the idea of a stronger influx of tropical oceanic waters in that area (Lavaniegos et al., 2006; Durazo, 2009). The determination of salp taxonomic identity in the remaining stations off Baja California in the winter 2004 cruise will help to reinforce the hypothesis.
This is the first report of Helicosalpa komaii in the California Current System (CCS) and the second report in the Northeast Pacific. With this new record, the number of salp species (gamma diversity) detected in Baja California waters is increased to 29 and to 30 in the Mexican Pacific (Berner, 1967; Esnal, 1976; Hereu et al., 2006; Lavaniegos and Hereu, 2009; Hereu et al. 2010). This report provides valuable information regarding the distribution of salps in the CCS given that there are no historical records of H. komaii previously identified in the area, including the long term CALCOFI sampling program of more than 50 years (Berner, 1967; Blackburn, 1979Lavaniegos and Ohman, 2003). Ecosystem changes were detected within the CCS from changes in the assemblages of pelagic tunicates over the period 1951–2002 in Southern California waters (Lavaniegos and Ohman, 2003). No Helicosalpa species were listed among the salps reported in Lavaniegos and Ohman (2003), hence it is still unclear if the occurrence of H. komaii in this sector of the northeast Pacific is new, or if it was not detected due to other factors. The pooling of samples before enumeration of organisms, as performed in that study, affects abundance estimates of rare organisms (Ohman and Lavaniegos, 2002). Also, their study based on samples from one season (spring) and was restricted to an area north to the IMECOCAL area where H. komaii was found. Interestingly, most recent records of H. virgula off California (2003, 2004 and 2005; Lavaniegos, unpublished data) coincide with a warming trend along most part of the CCS (Mackas et al., 2006; Lavaniegos, 2009). The increased presence of Helicosalpa in the CCS and the process underlying this deserves further attention.
Rosa María Hernández (ECOSUR-Chetumal) deposited the specimen in the Collection of Zooplankton and provided the catalogue number. Humberto Bahena Basave (ECOSUR-Chetumal) provided the photograph of the specimen. Valuable comments from Pablo Jorgensen improved an earlier version of the manuscript. The analysis of this additional material from the IMECOCAL cruises was possible with the aid of Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (E.S-M. grant number HC-020, 2009) and the Conacyt grant CB-129611 (for B.E. Lavaniegos). This work was completed during a post-doctoral research stay of the first author in ECOSUR-Chetumal. Two anonymous reviewers provided useful, constructive comments to improve a previous version of this contribution.